The polyploid nature of Cenchrus ciliaris L. (Poaceae) has been overlooked: new insights for the conservation and invasion biology of this species – a review
Amina Kharrat-Souissi A E , Sonja Siljak-Yakovlev B , Spencer C. Brown C , Alex Baumel D , Franck Torre D and Mohamed Chaieb AA Université de Sfax, Faculté des Sciences, Unité de recherche, Biodiversité et Ecosystèmes en Milieu Aride, UR11ES71, Sfax, Tunisia.
B Université Paris-Sud, UMR 8079 CNRS-AgroParisTech-UPS, Laboratoire Ecologie, Systématique et Evolution, Université Paris-Sud, Bâtiment 360, 91405 Orsay Cedex, France.
C Compartimentation Cellulaire, Institut des Sciences du Végétal, CNRS UPR2355 and Imagif, 91198 Gif-sur-Yvette, France.
D Aix-Marseille Université, Institut Méditerranéen de la Biodiversité et d’Ecologie marine et continentale, IMBE, UMR CNRS 7263-IRD 237, Campus de l’Etoile, Saint Jérôme, case 421, 13397 Marseille Cedex 20, France.
E Corresponding author. Email: kharratsouissi@yahoo.fr
The Rangeland Journal 36(1) 11-23 https://doi.org/10.1071/RJ13043
Submitted: 12 May 2013 Accepted: 14 November 2013 Published: 13 January 2014
Abstract
Climate change, associated with increased aridity, and high grazing pressure by livestock results in the scarcity and loss of perennial Poaceae in arid ecosystems. The species threatened by this include Cenchrus ciliaris L., a native perennial grass of the tropical and sub-tropical arid rangelands of Africa and Western Asia and now introduced in Central and South America, and Australia. This species reproduces predominantly through aposporous apomixis although sexual individuals have been occasionally identified. Cenchrus ciliaris is characterised by a significant, heritable, phenotypic polymorphism and three ploidy levels including tetraploids (2n = 4x = 36), pentaploids (2n = 5x = 45) and hexaploids (2n = 6x = 54). Under water-deficit conditions, C. ciliaris shows plasticity in growth characteristics and aboveground biomass. This phenotypic plasticity has led to the identification of genotypic-associated responses conferring more productivity. This underlines the importance of conserving the genetic diversity of C. ciliaris in order to ensure the persistence of the vegetation cover in the arid ecosystems in which it occurs. Observations from cytogenetic and molecular data converge to underline the possibility of sexual reproduction, recombination and gene flow within and between populations of C. ciliaris. Genetic mechanisms, such as polyploidy, hybridisation between ploidy levels and apomixes, are generating and then maintaining the diversity of C. ciliaris. This review emphasises the role of polyploidy in the evolutionary development of C. ciliaris and how it may be a crucial factor for its conservation in some countries and its weedy nature in others.
Additional keywords: buffelgrass, facultative apomixis, genetic diversity, phenotypic variation, water stress.
Introduction
Climatic change, associated with increasing aridity, leads to a high loss of vegetation cover in arid and semiarid ecosystems (Reynolds et al. 2007; Gornall et al. 2010; Hoffmann and Sgrò 2011; Sowers et al. 2011). Because perennial herbaceous plants result in higher levels of ground cover and persist for longer than annual species, they are the most intensively grazed by cattle (Chaieb et al. 1996). They are the species most affected by dryland degradation and constitute one factor contributing to desertification (Whitford 2002; Jauffret and Visser 2003).
Cenchrus ciliaris L. [Poaceae syn. Pennisetum ciliare (L.) Link, buffelgrass] is a native perennial grass of tropical and sub-tropical arid rangelands of Africa and Western Asia. Because C. ciliaris is highly valued as a pastoral species (Arshadullah et al. 2011) and suited to a range of harsh conditions (Mansoor et al. 2002), it has been widely introduced as a pasture species and for erosion control in Australia, Mexico and South America (Marshall et al. 2012). Its establishment in Australia has been associated with a loss of native species (Clarke et al. 2005; Jackson 2005; Eyre et al. 2009; Smyth et al. 2009) and alteration of fire regimes (Miller et al. 2010).
Adaptive characteristics
This C4 perennial is the predominant grass in many arid lands (Marshall et al. 2012). Despite its wide distribution, the expansion of overgrazing associated with the severity of climatic factors in arid ecosystems has led to a continuous thinning of stands and the threat of extinction of this species throughout its natural area of occurrence (Chaieb et al. 1996; Whitford 2002). Considering the ecological interest offered by C. ciliaris: drought tolerance (Bhatt et al. 2007; De La Barrera and Castellanos 2007; Kharrat-Souissi et al. 2012a), high fodder production and nutritive value to livestock (Kharrat-Souissi et al. 2010; Arshadullah et al. 2011), rapid regeneration in response to rainfall (Bose and Balakarishnan 2001), tolerance of heavy grazing (M’seddi et al. 2004a) and soil stabilisation abilities because of a deep root system (Chaieb et al. 1996; Mnif and Chaieb 2009), the persistence of this species in its native range in arid ecosystems is a crucial factor for restoration ecology of degraded arid rangelands. For these reasons, C. ciliaris has been the subject of numerous applied studies, which have focussed on its phenotypic polymorphism, its ecological significance (M’seddi et al. 2002; Arshad et al. 2007; Jorge et al. 2008; Visser et al. 2008; Kharrat-Souissi et al. 2010; Arshadullah et al. 2011), resistance to drought (Mansoor et al. 2002; Mnif and Chaieb 2010; Kharrat-Souissi et al. 2012a), biological invasion capacity (Friedel et al. 1996; Franklin et al. 2006; Gutierrez-Ozuna et al. 2009; Marshall et al. 2012) and its genotypic diversity (Nisar et al. 2010; Al-Soqeer 2011; Kharrat-Souissi et al. 2011).
For effective conservation and management of C. ciliaris, it is important to understand intra-specific genetic diversity and its relationship to other traits. The association between biological and ecological characteristics, such as mode of reproduction (apomixis or sexuality), genetic characterisation (polyploidy and genotypic diversity), ability to cope with environmental disturbance and the capacity for colonising new habitats, will contribute considerably. One option for increasing availability of this species in its native range is through the development of an optimal seed collection strategy. The basic tenet of native seed production is to provide seed of local origin and of the widest adaptive genetic diversity possible. This review, based on an analysis of published data, focuses on the conservation, invasion biology and use of C. ciliaris in restoration of degraded arid and semiarid ecosystems. The specific objectives of this paper are, therefore, to:
-
Document the facultative nature of apomixis in C. ciliaris;
-
Underline neglected aspects concerning the invasion biology of C. ciliaris;
-
Understand the intra-specific phenotypic variation;
-
Emphasise the polyploid nature of C. ciliaris and its ecological implications;
-
Underline the adaptive response of the species to water deficit in arid environments, and
-
Consider the implications of molecular genotypic data for conservation and management of C. ciliaris.
Facultative nature of apomixis in Cenchrus ciliaris
Cenchrus ciliaris is one of few pastoral species that are apomictic, producing clones from seeds. This asexual reproduction provides the advantage of rapid multiplication allowing the development of a significant aboveground yield and consequently the formation of an extremely dense ground cover by C. ciliaris (Ozias-Akins et al. 2003; Akiyama et al. 2005; Miles 2007; Ozias-Akins and Van Dik 2007; Singh et al. 2007). Asexual reproduction may have benefits in stable environments, while sexual reproduction offers a net advantage by allowing more rapid generation of genetic diversity allowing adaptation to a changing environment. Cenchrus ciliaris was considered for many years as an obligate apomict, with apospory followed by pseudogamy (Savidan 2000; Visser et al. 2000). Apospory involves the formation of an unreduced embryo sac and the development of the unreduced egg cell without fertilisation. The discovery of an obligatory sexual plant in south Texas in 1958 (Bashaw 1962) provided the possibility to breed new genotypes since it has been used as a female parent and was pollinated with pollen from apomictic accessions in order to produce a wide range of hybrids possessing many different characters (Taliaferro and Bashaw 1966). After the discovery of this sexual plant, other authors determined that some other genotypes of C. ciliaris were also facultative apomicts (Bray 1978; Sherwood et al. 1980; Visser et al. 2000; Gupta et al. 2001). The use of sexual female parents of C. ciliaris in hybridisation programs may be advantageous for breeders by producing new genotypes from local germplasm (Bashaw 1980). This breeding approach has been used successfully to produce high-yielding, apomictic hybrids (Bashaw 1980).
Agamospermy is frequently associated with polyploidy (Roche et al. 2001) and almost all apomictic plants are polyploid (Asker and Jerling 1992). The C. ciliaris complex contains three ploidy levels including tetraploids (2n = 4x = 36), pentaploids (2n = 5x = 45) and hexaploids (2n = 6x = 54) (Fisher et al. 1954; Bashaw and Hignight 1990; Kharrat-Souissi et al. 2013). The high phenotypic polymorphism (Arshad et al. 2007; Jorge et al. 2008; Kharrat-Souissi et al. 2011) in the presence of polyploidy suggests that apomixis in C. ciliaris may be facultative. The flexibility in reproductive behaviour that is frequently associated with an increase in ploidy might be highly relevant for colonising species by increasing the ability to reproduce under suboptimal conditions and allowing for asexual reproduction in the absence of suitable mates (Bessa-Gomes et al. 2003). The mode of reproduction of each ploidy level of C. ciliaris was investigated using Flow Cytometric Seed Screening (FCSS) (Kharrat-Souissi et al. 2013). The same profiles of nuclear DNA histograms were found for all three cytotypes, with a dominant peak of 2C somatic embryonic nuclei and a smaller 3C peak corresponding to the endosperm (Kharrat-Souissi et al. 2013). Although many reports have shown the utility of FCSS for assessing modes of reproduction (Matzk et al. 2000; Hörandl et al. 2008; Hörandl and Temsch 2009; Krahulcová and Rotreklová 2010), these results obtained for native populations of C. ciliaris (Kharrat-Souissi et al. 2013) did not clarify the reproductive mode, because the endosperm cells of both the aposporous and sexual plants yield 3C values. Likewise, Matzk et al. (2000) concluded that for facultative apomictic species like Panicum maximum and C. ciliaris, FCSS could not differentiate sexual from apomictic genotypes. Despite these profiles with 2C-, 3C- and 4C-seed nuclei (Kharrat-Souissi et al. 2013), it is presumed that the pentaploid populations of C. ciliaris are maintained by apomixis. It would be more appropriate to detect aposporous apomixis in the case of C. ciliaris using the traditional cytological technique of dissecting immature ovaries. In this case the aposporous plants of the Panicum type, which produce a 4-nucleate sac(s) per ovule, can be easily differentiated from sexually reproducing plants which display a solitary 8-nucleate sac (Savidan 1982; Koltunow 1993; Visser et al. 2000).
In C. ciliaris, apospory inherits as a dominant trait under genetic control of a single locus and transmits with an apospory-specific genomic region (ASGR) located on a single chromosome (Roche et al. 1999; Jessup et al. 2002; Ozias-Akins and Van Dik 2007). Cytogenetic investigations of tetraploid C. ciliaris have demonstrated that the ASGR is located near the centromere in a genomic region that is hemizygous and heterochromatic (condensed part of chromatin genetically inactive) (Goel et al. 2003, 2006; Ozias-Akins et al. 2003; Akiyama et al. 2005). The ASGR-carrier chromosome has a unique morphological characteristic when compared with other chromosomes in the genome (Akiyama et al. 2005) and is ~20 Mb larger than its presumed homologous chromosomes (Goel et al. 2003; Akiyama et al. 2005). Goel et al. (2003) suggest that chromosome structure, proximity to centromere and hemizygosity (the state of having only one copy of a gene in the diploid cell instead of the usual two copies) may all play a role in the low recombination rates in the ASGR.
Neglected aspects concerning invasion biology of Cenchrus ciliaris
Invasive species constitute a major threat to natural ecosystems, and conservation strategies generally involve elimination of these species. Whereas C. ciliaris is endangered in its native habitat, e.g. drylands in Tunisia, it has invaded extensive areas in Australia, Mexico and South America (Jackson 2005; Gutierrez-Ozuna et al. 2009; Miller et al. 2010), constituting a threat to natural and managed ecosystems (Marshall et al. 2012). Factors that affect the capacity of plants to be rapid or efficient colonisers include wide environmental tolerance, high relative growth rate, effective dispersal, high competitive ability and high levels of phenotypic plasticity (Levin 2000). In addition, the ploidy level of the invasive alien species constitutes an important determinant of invasiveness in plants (te Beest et al. 2012). Genome duplication has played a major role in the evolutionary history of plants and can drastically alter a plant’s genetic make-up, morphology, physiology and ecology within only one or a few generations (te Beest et al. 2012). Polyploidy results in increased leaf and flower size, cell size and chloroplast count (Dhawan and Lavania 1996). These phenomena are collectively referred to as the gigas effect (Acquaah 2007). This allows increasing growth rate, photosynthesis, survival and, therefore, conferring a competitive advantage of polyploids to succeed in strongly fluctuating environments. Polyploidy could give introduced species an important advantage in negotiating novel habitats during the colonisation process (Richardson et al. 2000). In this context, Pandit et al. (2011) and te Beest et al. (2012) suggest that the success of invasive plants can be enhanced by polyploidisation (whole genome duplication), where polyploids have a higher survival rate and fitness than diploids. Until now, the polyploid nature of C. ciliaris has been neglected in the programs of conservation and restoration in the native range of the species and also in the context of biological invasion. Because polyploidy is a decisive factor of genome evolution and genotypic variation of plants (Ainouche and Jenczewski 2010), the conservation strategies for a polyploid complex should consider the frequency of each ploidy level, origin and size of their distribution area and their functional role in diversity. Accordingly, with respect to the C. ciliaris complex, further assessment of ploidy levels should be undertaken in order to understand the ecology, distribution, and the biodiversity impact of this invasive species.
Intra-specific phenotypic variation
Although several studies of the morphological variability of C. ciliaris have been conducted (M’seddi et al. 2002, 2004b; Mnif et al. 2003, 2005a; Arshad et al. 2007; Jorge et al. 2008; Visser et al. 2008; Arshadullah et al. 2011), few comparisons between populations and studies of the heritable nature of important traits have been undertaken. In one rare study, the progeny from different populations was tested. Here, variability among populations, within populations and among individuals of the same offspring was examined (Kharrat-Souissi et al. 2011). Multiple morphometric variables mainly linked to productivity (tiller number, spike number and aboveground biomass) were examined at the vegetative and reproductive stages. These measurements were quantitative, while previous studies concerning the phenotypic diversity of C. ciliaris were merely semiquantitative, using a visual scoring method (M’seddi et al. 2002; Visser et al. 2008).
In these studies the phenotypic variables measured showed considerable differences among populations of C. ciliaris and among maternal parent plants within the same population (Kharrat-Souissi et al. 2011). This was a novel result compared with previous studies on the morphological variability of C. ciliaris. High similarity among individuals belonging to the same offspring was found, indicating that the measured attributes were highly heritable. This no doubt results from the apomictic reproductive mode of C. ciliaris that leads to the formation of seeds genetically identical to the mother plant (Ozias-Akins et al. 2003; Akiyama et al. 2005). As stated above, apomixis is a heritable trait (Grimanelli et al. 2001; Jessup et al. 2002; Curtis and Grossniklaus 2007; Ozias-Akins and Van Dik 2007; Yadav et al. 2012), but is rarely obligatory, and such is the case for C. ciliaris. In mixed breeding systems (sexual and apomictic), the genetic factors controlling apomixis can be potentially transferred from an apomictic individual to the offspring of a sexual plant via pollen. In turn, the pollen of the sexual individuals does not fertilise an apomict plant and will not result in the inheritance of the sexual traits because the egg cell develops parthenogenetically (Hörandl and Temsch 2009). Because of this unidirectional hybridisation, apomicts have a superior colonising ability. The faster moving apomicts will build up a barrier against the slower moving sexual populations because the latter will always be pollinated mainly by the more abundant apomictic individuals. Consequently, sexuality would be replaced finally by apomixis (Mogie et al. 2007) and is thought to be a causal factor for the wide geographical distribution of apomictic complexes (Hörandl and Temsch 2009). This model is based on a simple heritability mechanism of apomixis via a single genetic factor and all progeny expressing the trait.
Kharrat-Souissi et al. (2011) showed that heritable phenotypic traits mainly associated with productivity (e.g. aboveground biomass) were highly correlated: number of tillers, number of spikes, plant height and tuft diameter. This has implications in breeding programs as selection for one trait will have an effect on another. In addition, selection could be practiced on a highly heritable trait which correlates with a more complex trait such as yield (Majidi et al. 2009). However, according to our results, the aboveground biomass is an efficient discriminating variable for identifying the most productive genotypes (Kharrat-Souissi et al. 2010, 2011). M’seddi et al. (2004b) has established that tuft diameter and tuft height are also good indicators which can be used to increase the productivity of C. ciliaris. Generally, increase in plant size has been proposed as an explanation for invasion success, inducing increased competitiveness and vigour (Jakobs et al. 2004; Stastny et al. 2005). Indeed, the variability in productivity in C. ciliaris could be an important factor in the invasion biology of this species, because the population growth rate is known to affect the speed of invasion (Elliott and Cornell 2012). These last authors showed that the presence of phenotypic polymorphism within a species can result in faster range expansions than if only a single phenotype is present in the landscape. This has implications for predicting the speed of biological invasions, suggesting that speeds cannot just be predicted from looking at a single phenotype and that the full community of phenotypes needs to be taken into consideration.
The phenotypic polymorphism, measured in C. ciliaris, did not show any clear relationship with the eco-geographical features of the source population (Kharrat-Souissi et al. 2010, 2011). These results accord with those of M’seddi et al. (2002, 2004b) and Jorge et al. (2008) demonstrating a generally poor correlation between environmental data and agro-morphological data of C. ciliaris. This lack of correlation can be explained by the colonising ability by its seeds, which are light and surrounded by stiff bristles, making them widely dispersed by wind over long distances (Pitt 2004). This frequent gene flow via seed dispersal contributes to a certain level of diversity among populations of C. ciliaris. These findings are in line with data from Gutierrez-Ozuna et al. (2009) showing that seeds were widely dispersed between C. ciliaris populations introduced into north-western Mexico. The invasiveness of C. ciliaris in arid and semiarid areas of Australia (Friedel et al. 2006) has also been attributed predominantly to propagation/dispersal mechanisms (Eyre et al. 2009). The speed of a species’ invasion depends upon its dispersal ability (Elliott and Cornell 2012).
Polyploidy of Cenchrus ciliaris
Before the discovery of the sexual individual in C. ciliaris, the knowledge of ploidy was not important, because the breeders were looking to select the most productive apomictic ecotypes. But once the sexual individuals were used in breeding programs and crosses between sexual and apomictic plants were made, knowledge of the ploidy level of the plants used in hybridisation became more important, because breeding success depends on using meiotically stable parental lines with compatible chromosome numbers (Burson et al. 2012).
Flow cytometry for ploidy level determination
The use of flow cytometry to determine nuclear DNA content and hence to screen for ploidy level in C. ciliaris permitted the prediction of the chromosome number for each population. The 2C DNA values were assessed for 28 natural populations collected from northern to southern Tunisia (Kharrat-Souissi et al. 2013). Genome size ranged from 2C = 3.03–4.61 pg, revealing three ploidy levels corresponding to 4x (tetraploid 2n = 36), 5x (pentaploid) and 6x (hexaploid), and mean 2C DNA amounts were of 3.03, 3.70 and 4.48 pg, respectively (Kharrat-Souissi et al. 2013). A very low variation in nuclear DNA content between plants of the same ploidy level (Kharrat-Souissi et al. 2013) supports a theory of stable genome size. The 1Cx values (monoploid genome size for n = 9) were similar for tetraploid, pentaploid and hexaploid cytotypes (0.757, 0.746 and 0.745 pg, respectively), (733 M bp). This stability is in contrast with the frequent occurrence of genome downsizing (decrease in monoploid genome size, 1Cx, according to Greilhuber (2005), in high polyploids. This intra-cytotype stability in nuclear DNA amount in C. ciliaris indicates that there was very little change during the life history of these cytotypes, which suggests a recent origin of pentaploids and hexaploids.
In a recent study, Burson et al. (2012) determined the DNA content of many accessions of C. ciliaris from the USDA National Plant Germplasm System by flow cytometry. This germplasm collection is dominated by tetraploids (54%), with some pentaploids (25%) and a few hexaploids (4%). This contrasts with the relative frequencies found in natural Tunisian populations: 4, 11, and 82%, respectively (Kharrat-Souissi et al. 2013). According to several authors (Hignight et al. 1991; Visser et al. 1998, 2000; Burson et al. 2012), the most common chromosome number reported for C. ciliaris corresponds to the tetraploid cytotype (2n = 4x = 36). However, at least for Tunisia, representing a small portion of the native range of C. ciliaris, the hexaploid is the most frequent cytotype (Kharrat-Souissi et al. 2013). In Tunisia, populations were characterised by assessing 5–14 individuals per population. A stable genome size within and between populations of each ploidy level was found, falling clearly into distinct classes corresponding to the three different cytotypes (Kharrat-Souissi et al. 2013). Unfortunately, Burson et al. (2012) tested only one individual plant of each accession and failed to test if different ploidy levels were associated with certain populations or with mixed populations.
In addition to the existence of different ploidy levels in C. ciliaris, several authors (Visser et al. 1998; Mnif et al. 2005a; Burson et al. 2012) reported frequent aneuploidy [gains (hyper-aneuploidy) or loss (hypo-aneuploidy) of chromosome, in one chromosome set] resulting from unequal segregation of chromosomes. Aneuploidy has a greater effect on phenotype than polyploidy and causes developmental abnormalities, which can reduce organismal fitness in species (Birchler and Veitia 2007). One natural population in arid southern Tunisia was uniformly hyper-aneuploid (2n = 2x = 55), probably apomictic (Kharrat-Souissi et al. 2013). Agamospermy must play an important role in formation and preservation of chromosomal abnomalies in C. ciliaris. As stated above, C. ciliaris reproduces mainly by apospory and the unreduced egg cell develops into an embryo parthenogenetically, requiring pollination/fertilisation for endosperm development via pseudogamy. Because early pollination tends to increase the frequency of 2n + n fertilisation in apomictic grasses (Martinez et al. 1994), the chances for early pollination and 2n + n fertilisation are increased in C. ciliaris as a protogynous species (i.e. shedding of pollen occurs after the stigma has stopped being receptive) (Burson et al. 2002).
Chromosome number confirmation of ploidy level
Although flow cytometry indicated ploidy levels in C. ciliaris, determination of chromosome number at the mitotic stage was required to confirm these putative ploidy levels. Cenchrus ciliaris has small chromosomes that make them difficult to count using traditional cytological methods. However, a protoplast dropping technique (Geber and Schweizer 1987) was appropriate for counting on complete mitotic cells with satisfactory spreading of chromosomes (Kharrat-Souissi et al. 2013). Thus, the three inferred cytotypes were confirmed by chromosome counting to be tetraploids (2n = 4x = 36), pentaploids (2n = 5x = 45) and hexaploids (2n = 6x = 54) (Kharrat-Souissi et al. 2013). The hexaploid chromosome number has already been reported for Tunisian populations of C. ciliaris (Mnif et al. 2005b) but tetraploid and pentaploid are new for this area (Kharrat-Souissi et al. 2013). These chromosome counts corroborate other researchers’ findings (Fisher et al. 1954; Hignight et al. 1991; Visser et al. 1998, 2000). Burson et al. (2012) even reported the existence of two septaploids (2n = 7x = 63) in C. ciliaris.
The existence of pentaploid cytotypes in C. ciliaris suggests ability for hybridisation between tetraploids and hexaploids (Kharrat-Souissi et al. 2013). This is yet one more argument that this species exhibits a mixed breeding system (apomixes and sexual) and that sexuality offers a potential mechanism for hybridisation and creation of intermediate cytotypes. However, polyploidisation may play an important role by restoring sexual reproduction after hybridisation. Hybridisation and polyploidisation events should increase adaptation to a variable climate due to a larger genetic diversity and may also assist the ‘evolution of invasiveness’.
Molecular cytogenetic evidence of polyploidy and ecological implications
In order to characterise the three ploidy levels of C. ciliaris, a physical mapping of chromosomes was carried out using fluorescence in situ hybridisation (Kharrat-Souissi et al. 2012b). Using double fluorescence in situ hybridisation, it was shown that the two rDNA gene families, 5S and 18S-5.8S-26S (18S), displayed intra-specific variation in the number of loci, following the different ploidy levels of C. ciliaris (Kharrat-Souissi et al. 2012b). The number of 5S and 18S rDNA sites corresponded to the ploidy level displaying four signals for tetraploid, five for pentaploids and six for hexaploids (Kharrat-Souissi et al. 2012b), showing a proportional increase of ribosomal loci number during polyploidisation (Kharrat-Souissi et al. 2012b). The tetraploid cytotype has been previously studied by Akiyama et al. (2005) showing four signals for both 5S and 18S. The positions of rRNA gene loci were consistent and their numbers were proportional in the three ploidy levels, observations which further support a recent origin for pentaploid and hexaploid cytotypes (Kharrat-Souissi et al. 2012b).
Although different ploidy levels for C. ciliaris have been recorded by several authors (Fisher et al. 1954; Hignight et al. 1991; Visser et al. 2000; Burson et al. 2012), the geographical distribution of these ploidy levels has not been studied. The distribution of C. ciliaris cytotypes in Tunisia (Fig. 1) seems to follow a north–south bioclimatic gradient (Kharrat-Souissi et al. 2013). A single tetraploid population was found in the semiarid north, the three pentaploid populations appeared in the centre of the country, particularly in a region where rainfall is between 100 and 200 mm, while the 24 hexaploid populations occupied the semiarid to the Saharan region where annual average rainfall is below 100 mm (Fig. 1). The wider distribution of hexaploids may suggest that these populations are better adapted to a broader range of climatic conditions (Kharrat-Souissi et al. 2013).
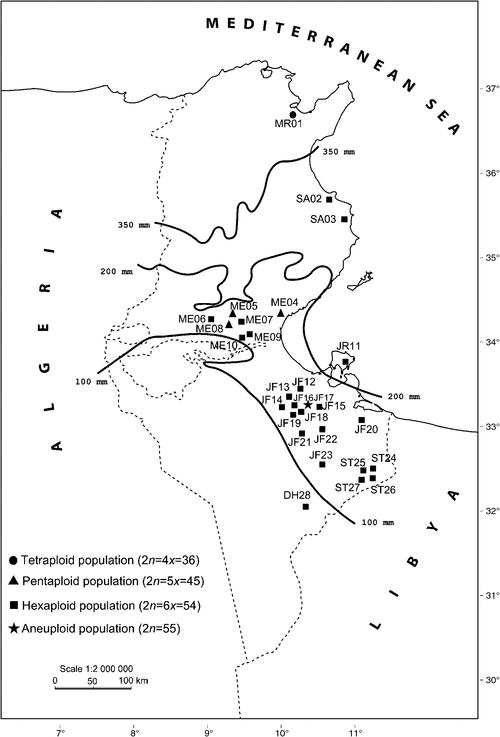
|
Several cytogeographical studies have showed that diploids and polyploids often occupy different parts of the landscape and that polyploids replace their diploid relatives along ecological gradients. Gradients in rainfall have shown that polyploids generally occupy drier habitats than their diploid relatives (Chen 2007; Hegarty and Hiscock 2008; Maherali et al. 2009; Treier et al. 2009; Paun et al. 2011). This clear distinction between cytotypes was not shown by Waters et al. (2010), who found a coexistence of different cytotypes of some Australian Danthonieae. Polyploids are also often less likely to become extinct than related diploids in disturbed habitats (Stebbins 1985; Otto and Whitton 2000; Fawcett et al. 2009). These authors concluded that polyploids have a greater tolerance of extreme ecological conditions and are often better adapted to dry conditions than their diploid counterparts. A further argument comes from the observation that increasing environmental stress (temperature variation and drought) increases the production of unreduced gametes and thus polyploidy (Fawcett et al. 2009; Parisod et al. 2010).
Molecular diversity
Genetic resource management of C. ciliaris and its use in programs of rehabilitation of degraded rangelands requires documentation of both levels and structure of its genetic diversity to optimise the sourcing of seeds with respect to potential existence of local adaptations. Due to the polyploidy nature of C. ciliaris and their relatively low cost, AFLP markers were developed to investigate the structure of variation at several loci among and within 11 Tunisian populations and among the three ploidy levels of C. ciliaris (Kharrat-Souissi et al. 2011). In other regions of the world (north-western Mexico, Pakistan, central Saudi Arabia), genotypic diversity of C. ciliaris has been studied (Gutierrez-Ozuna et al. 2009; Nisar et al. 2010; Al-Soqeer 2011) unfortunately without taking into account possible different ploidy levels. Although these authors sampled numerous individuals per population, they did not examine individuals descending from the same mother plants, and thus failed to investigate the frequency of sexual reproduction within C. ciliaris.
In the study on Tunisian populations (Kharrat-Souissi et al. 2011), AFLP markers revealed C. ciliaris to have genotype diversity within populations and between individuals descending from the same mother plant (average polymorphism within offspring = 1%, max. = 5%). This variability for a predominantly agamospermous species shows that sexual reproduction may occur in some C. ciliaris populations (Kharrat-Souissi et al. 2011). This result supports the finding of Hignight et al. (1991) that C. ciliaris can reproduce via a process of facultative apomixes. In Tunisia, C. ciliaris has more genetic diversity than in populations from the Al-Qassim region of Saudi Arabia (Al-Soqeer 2011). This latter author surmised that the low level of genetic variation of Saudi (Al-Qassim) C. ciliaris populations was not surprising given the presumed predominantly apomictic reproductive system of this species. The mean G/N value (the proportion of distinct genotypes in a population) observed for C. ciliaris in Tunisia (G/N = 0.55; Kharrat-Souissi et al. 2011) was also higher than that observed for invasive Mexican populations (G/N = 0.29, Gutierrez-Ozuna et al. 2009).
Each of the Tunisian populations studied for their genotypic diversity was characterised by the presence of both unique genotypes and genotypes, which were common to several individuals (clones) (Kharrat-Souissi et al. 2011). The fact that the AFLP diversity (polymorphism and G/N values) varied across populations suggests that the rates of occurrence of the two reproduction modes (apospory and sexual) are not homogeneous in Tunisia. Combined clonal/sexual reproduction processes enable species to disperse to new territories and to form relatively long-lived populations by replicating the best-adapted individuals (Grant 1971; Bayer 1983, 1990; Novak and Mack 2000).
A neighbour-joining tree, based on AFLP markers, performed for the Tunisian C. ciliaris populations revealed three major groups of genotypes: tetraploid, pentaploid and a mix of pentaploid and hexaploids (Kharrat-Souissi et al. 2011). The existence of pentaploids in two groups with different genotypes suggests that this cytotype may have two origins. This result highlights the evolutionary potential in the C. ciliaris complex and supports the statement that gene exchanges between different ploidy levels occur (Chapman and Abbott 2010). The groups of genotypes from the neighbour-joining tree do not correspond completely to the geographical origin of samples (Kharrat-Souissi et al. 2011). Gutierrez-Ozuna et al. (2009) also showed, in north-western Mexican populations of C. ciliaris, that genetic differentiation had no geographic pattern and apparently was not associated with environmental gradients. The lack of geographical structure of genotypic diversity of C. ciliaris could be attributed to the dispersal capacity of seeds by wind over a long distance (Pitt 2004). Gutierrez-Ozuna et al. (2009) concluded that invasion success of C. ciliaris in north-western Mexico is not directly related to genotypic variation and other factors, such as phenotypic plasticity and propagule pressure, could be major determinants of the invasion success of this species.
There are no previous studies examining the relationship between morphological and genotypic variability for C. ciliaris. In this context, there was a lack of correlation between genotypic distances from AFLP and phenotypic distances from the morphological study (Kharrat-Souissi et al. 2011). This result suggests that neutral markers (AFLP) and complex heritable traits (morphological traits) do not involve the same processes. The significant morphological differences found among populations of C. ciliaris may result from the high selection pressure rather than genetic drift, indicating the local adaptation of certain genotypes. In similar studies, species (Cichorium intybus L. and Cichorium spinosum L.), separated on the basis of morphological characters, could not be distinguished using molecular tools (Gemeinholzer and Bachmann 2005). Estimation of genetic diversity based on morphological characters is very difficult and is influenced by environmental effects (Afghan et al. 2005). By contrast, molecular markers offer an efficient measure of genetic relationship without any influence of environmental factors (Ubi et al. 2006).
To summarise the importance of cytogenetic and molecular data of C. ciliaris, we note that different observations support the notion that apomixis is not an absolute reproductive mode for this species. First, the pentaploids most probably arose through an event of sexual reproduction, being hybrids from the fusion of 2x and 3x gametes. Second, the existence of pentaploids in two groups with different genotypes suggests that two hybridisations or introgression events may have occurred. Finally, the diversity of AFLP genotypes shows that recombination is more frequent than expected for a predominantly agamospermous species.
Adaptive response to water deficit in arid environments
The contribution of the phenotypic polymorphism of C. ciliaris in response to a water deficit in the ecological context of dry lands was studied in southern Tunisia (Kharrat-Souissi et al. 2012a). Although C. ciliaris is well known for its predominantly apomictic nature, previous studies on its drought resistance (Mansoor et al. 2002; Mnif and Chaieb 2010) did not compare numerous offspring from the same population nor maternal siblings, and thus failed to examine the heritable part of the response to drought stress. Recently, highly heritable variation in the responses to applied water deficit for several measured attributes (e.g. productivity, leaf area, leaf hairiness and stomata density) has been observed for C. ciliaris (Kharrat-Souissi et al. 2012a). Different groups of genotypes responded in different ways to the applied water deficit. Although all individuals of C. ciliaris grew more slowly and produced less aboveground biomass under water deficit, some genotypes were more strongly affected than others and their aboveground biomass showed the largest decrease (Kharrat-Souissi et al. 2012a). These same genotypes maintained their leaf area under water stress, which allowed them to maintain their photosynthetic tissue. In contrast, other genotypes reduced their leaf surface area. This latter strategy may be advantageous under hot conditions and unpredictable rainfall, such as in arid environments, because reducing the leaf surface limits water loss through transpiration (Blum 2005; Taiz and Zeiger 2006). The capacity of C. ciliaris genotypes to express different phenotypes adapted to water stress show the important degree of phenotypic plasticity of this species in arid climates.
Several studies have shown that drought-tolerant species reduce water loss by reduction of leaf area simultaneously with a reduction in biomass production (Lazaridou and Noitsakis 2003; Lazaridou and Koutroubas 2004). For C. ciliaris, the strong decrease in leaf area was correlated with only a small decrease in aboveground biomass (Kharrat-Souissi et al. 2012a). Cenchrus ciliaris belongs to the C4 group of grasses which adapt well to dry environments (Maroco et al. 2000). This species is one of many grasses that can produce forage for livestock with limited precipitation, which help it to underpin its invasive weedy potential in areas where it has been introduced (Cox et al. 1988; Ward et al. 2006). Cenchrus ciliaris can out-compete native vegetation for soil nutrients and moisture and has the potential to displace native plant communities in countries where it has been introduced. Accordingly, Eyre et al. (2009) has shown that this species can colonise successfully in south-west Queensland at the expense of native species. Indeed, most studies in Australia have reported a negative relationship between C. ciliaris occurrence and native species’ biomass, species richness and/or individual native plant species cover (Fairfax and Fensham 2000; Franks 2002; McIvor 2003; Clarke et al. 2005; Jackson 2005; Smyth et al. 2009).
Genotypes that have increased their leaf hair density under water deficit have also been found in C. ciliaris (Mansoor et al. 2002; Kharrat-Souissi et al. 2012a). This increase of leaf hair density appears to be beneficial for plants under water-deficit conditions, because it increases light reflectance from the leaf surface (Ehleringer and Werk 1986; Vogelmann et al. 1996; Farooq et al. 2009), which would decrease the overall leaf temperature by decreasing heat load. Higher leaf hair density could also increase the leaf boundary layer (Schuepp 1993), which affects leaf temperature by modifying the rate of heat transfer from the leaf (Ehleringer and Mooney 1978; Farooq et al. 2009). Leaf hair density increase was correlated with a greater decrease in aboveground biomass. These results show a multiplicity of responses of C. ciliaris genotypes under limited water availability. The capacity of this species to regulate its growth in different ways as an adaptive mechanism under water deficit indicates significant genetic variation that could affect its productivity and, in consequence, the vegetation cover of ecosystems during an increase of aridity (Kharrat-Souissi et al. 2012a). Furthermore, Mansoor et al. (2002) demonstrated that genotypes of C. ciliaris have not shown uniformity for any single phenotypic character in response to stressed environments and each genotype appears to present a unique suite of adaptations helping its survival under drought conditions.
The large amount of variation in several traits in C. ciliaris may enhance its potential for responding to new selection pressures in variable environments. Furthermore, the spatial heterogeneity in the environment affects the degree of phenotypic plasticity displayed by plants (Van Kleunen and Fischer 2005; Volis et al. 2005). Phenotypic plasticity may be adaptive with phenotypes that are matched to their environment having relatively higher fitness than other phenotypes (Ruiz-R. et al. 2006; Ofir and Kigel 2010). Cenchrus ciliaris is a particularly good candidate for phenotypic plasticity and local adaptation because (1) it is highly genetically differentiated, (2) it is very phenotypically plastic and (3) it can support extreme variation of rainfall and temperature in arid environments, and is likely to be under considerable selective pressure for local adaptation.
A key characteristic of invasive species is their ability to colonise and persist in a broad range of environmental conditions (Buswell et al. 2011). Plasticity in C. ciliaris can play an important role in biological invasions by allowing individuals to colonise and establish in environmentally diverse habitats. On the other hand, Kawecki and Ebert (2004) suggest that, if sufficient heritable variation is available and alternative genotypes are favoured in different habitats, natural selection in an introduced range may create a mosaic of locally adapted populations. Such local adaptation can lead to increased local abundance and, therefore, can be a key mechanism of invasion success (Parker et al. 2003). The invasive C. ciliaris, therefore, may also spread across diverse habitats by undergoing local adaptation, evolving ecotypes with distinctive traits and/or patterns of individual plasticity. Invasion biologists could use controlled experimental gardens or reciprocal transplant experiments to investigate whether C. ciliaris relies on phenotype plasticity to colonise new areas.
Conclusion: insights for conservation and invasion biology
According to Visser et al. (2008), dealing with seed collection strategies for restoration projects of degraded arid areas, it would be more appropriate to use a seed mixture of native genotypes of C. ciliaris than to use cultivars composed of a single genotype. Whalley et al. (2013) made a similar recommendation for restoration using Australian native grasses, which are predominantly inbreeding using a range of different mechanisms. This corresponds to the objective of maximising the possibility of local adaptation of restored populations by selecting the most adapted genotypes. According to our current knowledge, this strategy is insufficient particularly for C. ciliaris. Further molecular and cytogenetic investigations are needed to understand the genetic structure of C. ciliaris and, in particular, the relationship between environmental and genomic variation. Indeed, studies of genetic diversity of C. ciliaris underline the possibility of sexual reproduction, recombination and gene flow within and between populations (Kharrat-Souissi et al. 2011). This poses questions about the methods of collection, culture and harvest of seeds of C. ciliaris. For instance, in order to guarantee the genetic constitution of native seeds at the time of reseeding, we suggest that the following ground rules should be respected: (1) seeds should be collected from different mother plants, separately, in order to maximise the representation of different genotypes and, (2) when multiplying the genotypes of C. ciliaris, the possibility of genetic exchange in the nursery, the possible phenomena of homogenisation and reduction of the genetic diversity caused by the methods of cultivation and ex situ conservation, should be taken into account.
The association of morphological, cytological and molecular data is of particular interest at both local and broad scales for various aspects concerning C. ciliaris. Indeed, the lack of strong correlations observed between geographical structure and either the AFLP data or the heritable phenotypes, the existence of genotypic variations within offspring and the various origins of pentaploids, suggest that long-distance gene exchange, hybridisation and genomic evolution processes all occur in C. ciliaris. This new evidence provides insights into the genetic diversity of C. ciliaris supporting the complex and highly dynamic nature of polyploid plants (Soltis and Soltis 2000), which is a key element in restoration ecology, and also in the context of invasion biology (Pandit 2006; Ainouche et al. 2009; Hull-Sanders et al. 2009; Treier et al. 2009; Pandit et al. 2011). In this context, te Beest et al. (2012) demonstrated that polyploidy might increase the adaptive potential of invasive species in their novel habitat. Many studies have compared the growth of plants from native and invasive populations (Daehler 2003; Ehrenfeld 2003; Callaway and Ridenour 2004; Colautti and MacIsaac 2004; Hierro et al. 2005), but few have considered the role of ploidy.
We reiterate that C. ciliaris has been considered as an invasive species that poses serious threats to biodiversity conservation when introduced in Australia, America and Mexico (Marshall et al. 2012). Many studies have been undertaken to understand the behaviour of C. ciliaris as an invader for the purpose of managing invasion (Franklin et al. 2006; Friedel and Wycott 2007; Eyre et al. 2009; Smyth et al. 2009; Miller et al. 2010). However, none of these studies have taken into account the impact of ploidy on the invasive capacity of this species. We suggest, therefore, that knowledge of polyploidy of the germplasm of C. ciliaris introduced in these areas is essential in comparative studies of invasive and native populations. Critical analysis of the relationship between invasiveness and polyploidy will require study of the polyploid complex, in which one may directly compare polyploids and their diploid relatives under field conditions (Kubátová et al. 2008; Treier et al. 2009; Schlaepfer et al. 2010).
Finally, the likelihood of genome evolution in the polyploid C. ciliaris, shown in this review, underlines the potential for adaptation in this facultative apomictic species. We conclude that three genetic mechanisms may contribute to the evolution of C. ciliaris complex, namely hybridisation, polyploidy and apomixis. Hybridisation and polyploidy generate the genetic variability in this taxon and, thanks to apomixes, the new hybrid types adapted to different environments can be maintained and perpetuate. Since apomixis is facultative, natural selection would also act on sexually reproduced genotypes, accelerating the evolution process.
Perspective: from research on the apospory to restoration ecology
Field (morphometry) and laboratory work (cytogenetic and molecular analysis) has shed light on the genetic reasons for the variation and the potential of adaptation of C. ciliaris in response to increasing aridity. At a time of unprecedented speeds of climate change, the identification and preservation of such potential will be essential for maintaining biodiversity. Conservation and restoration programs of C. ciliaris continue to be developed and new questions and debates are to be expected. First, unequivocal confirmation of the degree of apomixis of each ploidy level requires simultaneous examination of both genetic and cytological evidence. Second, further genetic and phenotypic examinations of C. ciliaris focussed on genome evolution will require more comprehensive sampling throughout its distribution area, in order to increase our knowledge of variation. Ultimately, experimental studies of polyploidy series are needed to test rigorously a hypothesis about ecological adaptation of polyploids and its consequence for C. ciliaris invasiveness.
References
Acquaah, G. (2007). ‘Principles of Plant Genetics and Breeding.’ (Wiley-Blackwell: Malden, UK.)Afghan, S., Haider, M. S., Shah, A. H., Rashid, N., Iqbal, J., Tahir, M., and Akhtar, M. (2005). Detection of genetic diversity among sugarcane genotypes using RAPD markers. Sugarcane International 23, 15–19.
| 1:CAS:528:DC%2BD2MXhtleltrzL&md5=47c76c5d886664009cf0875c8cc66727CAS |
Ainouche, M. L., and Jenczewski, E. (2010). Focus on polyploidy. New Phytologist 186, 1–4.
| Focus on polyploidy.Crossref | GoogleScholarGoogle Scholar | 20409175PubMed |
Ainouche, M. L., Fortune, M., Salmon, A., Parisod, C., Grandbastien, A., Fukunaga, K., Ricou, M., and Misset, T. (2009). Hybridization, polyploidy and invasion: lessons from Spartina (Poaceae). Biological Invasions 11, 1159–1173.
| Hybridization, polyploidy and invasion: lessons from Spartina (Poaceae).Crossref | GoogleScholarGoogle Scholar |
Akiyama, Y., Hanna, W. W., and Ozias-Akins, P. (2005). High-resolution physical mapping reveals that the apospory-specific genomic region (ASGR) in Cenchrus ciliaris is located on a heterochromatic and hemizygous region of a single chromosome. Theoretical and Applied Genetics 111, 1042–1051.
| High-resolution physical mapping reveals that the apospory-specific genomic region (ASGR) in Cenchrus ciliaris is located on a heterochromatic and hemizygous region of a single chromosome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtV2ksbvL&md5=2570140f6f5760cb6fdd7c111c5a086cCAS | 16133318PubMed |
Al-Soqeer, A. (2011). Genotypic diversity among wild populations of Buffelgrass (Cenchrus ciliaris L. Link) in Al-Qassim region. Asian Journal of Biotechnology 3, 262–268.
| Genotypic diversity among wild populations of Buffelgrass (Cenchrus ciliaris L. Link) in Al-Qassim region.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVKjsbs%3D&md5=31d4be028ad4aaf30d29f98b75fd04feCAS |
Arshad, M., Ashraf, M. Y., Ahmad, M., and Zaman, F. (2007). Morpho-genetic variability potential of Cenchrus ciliaris L. from Cholistan Desert, Pakistan. Pakistan Journal of Botany 39, 1481–1488.
Arshadullah, M., Malik, M. A., Rasheed, M., Jilani, G., Zahoor, F., and Kaleem, S. (2011). Seasonal and genotypic variations influence the biomass and nutritional ingredients of Cenchrus ciliaris grass forage. International Journal of Agriculture and Biology 13, 120–124.
Asker, S. E., and Jerling, L. (1992). ‘Apomixis in Plants.’ (CRC Press: Boca Raton, FL.)
Bashaw, E. C. (1962). Apomixis and sexuality in Buffelgrass. Crop Science 2, 412–415.
| Apomixis and sexuality in Buffelgrass.Crossref | GoogleScholarGoogle Scholar |
Bashaw, E. C. (1980). Apomixis and its application in crop improvement. In: ‘Hybridization of Crop Plants’. (Eds W. R. Fehr and H. H. Hadley.) pp. 45–63. (ASA and CSSA: Madison, WI.)
Bashaw, E. C., and Hignight, K. W. (1990). Gene transfer in apomictic Buffelgrass through fertilization of an unreduced egg. Crop Science 30, 571–575.
| Gene transfer in apomictic Buffelgrass through fertilization of an unreduced egg.Crossref | GoogleScholarGoogle Scholar |
Bayer, R. J. (1983). Distribution of sexual and apomict populations of Antennaria parlinii. Evolution 37, 555–561.
| Distribution of sexual and apomict populations of Antennaria parlinii.Crossref | GoogleScholarGoogle Scholar |
Bayer, R. J. (1990). Patterns of clonal diversity in the Antennaria rosea (Asteraceae) polyploid agamic complex. American Journal of Botany 77, 1313–1319.
| Patterns of clonal diversity in the Antennaria rosea (Asteraceae) polyploid agamic complex.Crossref | GoogleScholarGoogle Scholar |
Bessa-Gomes, C., Clobert, J., Legendre, S., and Moller, A. P. (2003). Modelling mating patterns given mutual mate choice: the importance of individual mating preferences and mating system. Journal of Biological System 11, 205–219.
| Modelling mating patterns given mutual mate choice: the importance of individual mating preferences and mating system.Crossref | GoogleScholarGoogle Scholar |
Bhatt, R. K., Baig, M. J., and Tiwari, H. S. (2007). Growth, biomass production, and assimilatory characters in Cenchrus ciliaris L. under elevated CO2 condition. Photosynthetica 45, 296–298.
| Growth, biomass production, and assimilatory characters in Cenchrus ciliaris L. under elevated CO2 condition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktlSmsbc%3D&md5=0f91e96c24f46f90558f05a7ad54aaadCAS |
Birchler, J. A., and Veitia, R. A. (2007). The gene balance hypothesis: from classical genetics to modern genomics. The Plant Cell 19, 395–402.
| The gene balance hypothesis: from classical genetics to modern genomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktFGnsro%3D&md5=7f2612147b463cd419dfbe05ac1a4ec0CAS | 17293565PubMed |
Blum, A. (2005). Drought resistance, water-use efficiency, and yield potential are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research 56, 1159–1168.
| Drought resistance, water-use efficiency, and yield potential are they compatible, dissonant, or mutually exclusive?Crossref | GoogleScholarGoogle Scholar |
Bose, M. S. C., and Balakarishnan, V. (2001). ‘Forage Production Technologies.’ (South Asian Publishers: New Delhi, India.)
Bray, R. A. (1978). Evidence for facultative apomixis in Cenchrus ciliaris. Euphytica 27, 801–804.
| Evidence for facultative apomixis in Cenchrus ciliaris.Crossref | GoogleScholarGoogle Scholar |
Burson, B. L., Hussey, M. A., Actkinson, J. M., and Shafer, G. S. (2002). Effect of pollination time on the frequency of 2n + n fertilization in apomictic Buffelgrass. Crop Science 42, 1075–1080.
| Effect of pollination time on the frequency of 2n + n fertilization in apomictic Buffelgrass.Crossref | GoogleScholarGoogle Scholar |
Burson, B. L., Actkinson, J. M., Hussey, M. A., and Jessup, R. W. (2012). Ploidy determination of buffel grass accessions in the USDA National Plant Germplasm System collection by flow cytometry. South African Journal of Botany 79, 91–95.
| Ploidy determination of buffel grass accessions in the USDA National Plant Germplasm System collection by flow cytometry.Crossref | GoogleScholarGoogle Scholar |
Buswell, J. M., Moles, A. T., and Hartley, S. (2011). Is rapid evolution common in introduced plant species? Journal of Ecology 99, 214–224.
| Is rapid evolution common in introduced plant species?Crossref | GoogleScholarGoogle Scholar |
Callaway, R. M., and Ridenour, W. M. (2004). Novel weapons: invasive success and the evolution of increased competitive ability. Frontiers in Ecology and the Environment 2, 436–443.
| Novel weapons: invasive success and the evolution of increased competitive ability.Crossref | GoogleScholarGoogle Scholar |
Chaieb, M., Henchi, B., and Boukhris, M. (1996). Impact of clipping on root systems of three grass species in Tunisia. Journal of Range Management 49, 336–339.
| Impact of clipping on root systems of three grass species in Tunisia.Crossref | GoogleScholarGoogle Scholar |
Chapman, M. A., and Abbott, R. J. (2010). Introgression of fitness genes across a ploidy barrier. New Phytologist 186, 63–71.
| Introgression of fitness genes across a ploidy barrier.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvVOktrs%3D&md5=146eeda67fbca62715b2ecd36cea0f30CAS | 19912548PubMed |
Chen, Z. J. (2007). Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annual Review of Plant Biology 58, 377–406.
| Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsVahsLs%3D&md5=d74188dc054c29ca55a7ed69e0267ba2CAS | 17280525PubMed |
Clarke, P. J., Latz, P. K., and Albrecht, D. E. (2005). Long-term changes in semi-arid vegetation: invasion of an exotic perennial grass has larger effects than rainfall variability. Journal of Vegetation Science 16, 237–248.
| Long-term changes in semi-arid vegetation: invasion of an exotic perennial grass has larger effects than rainfall variability.Crossref | GoogleScholarGoogle Scholar |
Colautti, R. I., and MacIsaac, H. J. (2004). A neutral terminology to define “invasive” species. Diversity & Distributions 10, 135–141.
| A neutral terminology to define “invasive” species.Crossref | GoogleScholarGoogle Scholar |
Cox, J. R., Martin, M. H., Ibarra, F. A., Fourie, J. H., Rethman, N. F. G., and Wilcox, D. G. (1988). The influence of climate and soils on the distribution of four African grasses. Journal of Range Management 41, 127–139.
| The influence of climate and soils on the distribution of four African grasses.Crossref | GoogleScholarGoogle Scholar |
Curtis, M. D., and Grossniklaus, U. (2007). Amphimixis and apomixis: two sides of the same coin. In: ‘Apomixis: Evolution, Mechanisms and Perspectives’. (Eds E. Hörandl, U. Grossniklaus, P. J. Van Dijk and T. Sharbel.) pp. 37–62. (ARG-Gantner: Ruggell, Liechtenstein.)
Daehler, C. C. (2003). Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology, Evolution and Systematics 34, 183–211.
| Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration.Crossref | GoogleScholarGoogle Scholar |
De La Barrera, E., and Castellanos, A. E. (2007). High temperature effects on gas exchange for the invasive buffel grass (Pennisetum ciliare L. Link). Weed Biology and Management 7, 128–131.
| High temperature effects on gas exchange for the invasive buffel grass (Pennisetum ciliare L. Link).Crossref | GoogleScholarGoogle Scholar |
Dhawan, O., and Lavania, U. (1996). Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87, 81–89.
| Enhancing the productivity of secondary metabolites via induced polyploidy: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xitlaisbk%3D&md5=291b5e8ba945599e16335ae912173ed7CAS |
Ehleringer, J. R., and Mooney, H. A. (1978). Leaf hairs: effects on physiological activity and adaptive value to a desert shrub. Oecologia 37, 183–200.
| Leaf hairs: effects on physiological activity and adaptive value to a desert shrub.Crossref | GoogleScholarGoogle Scholar |
Ehleringer, J. R., and Werk, K. S. (1986). Modifications of solar-radiation absorption patterns and implications for carbon gain at the leaf level. In: ‘On the Economy of Plant Form and Function’. (Ed. T. J. Givnish.) pp. 57–82. (Cambridge University Press: Cambridge, UK.)
Ehrenfeld, J. G. (2003). Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6, 503–523.
| Effects of exotic plant invasions on soil nutrient cycling processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptFKrsr4%3D&md5=177edc866d87f15d5ddc9a0e509f827eCAS |
Elliott, E. C., and Cornell, S. J. (2012). Dispersal polymorphism and the speed of biological invasions. PLoS ONE 7, e40496.
| Dispersal polymorphism and the speed of biological invasions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSkt7jI&md5=ca0bfbf0c4f6126838584e74fbc5b812CAS | 22911701PubMed |
Eyre, T. J., Wang, J., Venz, M. F., Chilcott, C., and Whish, G. (2009). Buffel grass in Queensland’s semi-arid woodlands: response to local and landscape scale variables, and relationship with grass, forb and reptile species. The Rangeland Journal 31, 293–305.
| Buffel grass in Queensland’s semi-arid woodlands: response to local and landscape scale variables, and relationship with grass, forb and reptile species.Crossref | GoogleScholarGoogle Scholar |
Fairfax, R. J., and Fensham, R. J. (2000). The effect of exotic pasture development on floristic diversity in central Queensland, Australia. Biological Conservation 94, 11–21.
| The effect of exotic pasture development on floristic diversity in central Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., and Basra, S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29, 185–212.
| Plant drought stress: effects, mechanisms and management.Crossref | GoogleScholarGoogle Scholar |
Fawcett, J. A., Maere, S., and Van de Peer, Y. (2009). Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proceedings of the National Academy of Sciences of the United States of America 106, 5737–5742.
| Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkvFGhu7s%3D&md5=0f108fb26174ca59053c72632c468213CAS | 19325131PubMed |
Fisher, W. D., Bashaw, E. C., and Holt, E. C. (1954). Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agronomy Journal 46, 401–404.
| Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus.Crossref | GoogleScholarGoogle Scholar |
Franklin, K. A., Lyons, K., Nagler, P. L., Lampkin, D., Glenn, E. P., Molina-Freaner, F., Markow, T., and Huete, A. R. (2006). Buffelgrass (Pennisetum ciliare) land conversion and productivity in the plains of Sonora, Mexico. Biological Conservation 127, 62–71.
| Buffelgrass (Pennisetum ciliare) land conversion and productivity in the plains of Sonora, Mexico.Crossref | GoogleScholarGoogle Scholar |
Franks, A. J. (2002). The ecological consequences of buffel grass Cenchrus ciliaris establishment within remnant vegetation of Queensland. Pacific Conservation Biology 8, 99–107.
Friedel, M., and Wycott, M. (2007). The dispersal, impact and management of Buffel grass in desert Australia. Project Fact Sheet. Desert Knowledge CRC. Available at: http://wildlife.lowecol.com.au/files/DKACRC-Buffel-Grass-Control-Factsheet.pdf (accessed 5 November 2013).
Friedel, M. H., Muller, W. J., and Kinloch, J. E. (1996). The potential of some mechanical treatments for rehabilitating arid rangelands I. Identifying indicators from between-site comparisons. The Rangeland Journal 18, 165–178.
| The potential of some mechanical treatments for rehabilitating arid rangelands I. Identifying indicators from between-site comparisons.Crossref | GoogleScholarGoogle Scholar |
Friedel, M., Puckey, H., O’Malley, C., Waycott, M., Smyth, A., and Miller, G. (2006). Buffel grass: both friend and foe: an evaluation of the advantages and disadvantages of Buffel grass use and recommendations for future research. Desert Knowledge CRC. Available at: www.nintione.com.au/resource/DKCRC-Report-17-Buffel-Grass.pdf (accessed 5 November 2013).
Geber, G., and Schweizer, D. (1987). Cytochemical hetero-chromatin differentiation in Sinapis alba (Cruciferae) using a simple air-drying technique for producing chromosome spreads. Plant Systematics and Evolution 158, 97–106.
| Cytochemical hetero-chromatin differentiation in Sinapis alba (Cruciferae) using a simple air-drying technique for producing chromosome spreads.Crossref | GoogleScholarGoogle Scholar |
Gemeinholzer, B., and Bachmann, K. (2005). Examining morphological and molecular diagnostic character states in Cichorium intybus L. (Asteraceae) and Cichorium spinosum L. Plant Systematics and Evolution 253, 105–123.
| Examining morphological and molecular diagnostic character states in Cichorium intybus L. (Asteraceae) and Cichorium spinosum L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXls1Sntbg%3D&md5=77a667a0d80a6af51ef5aeb7e216882cCAS |
Goel, S., Chen, Z., Conner, J. A., Akiyama, Y., Hanna, W. W., and Ozias-Akins, P. (2003). Delineation by fluorescence in situ hybridization of a single hemizygous chromosomal region associated with aposporous embryo sac formation in Pennisetum squamulatum and Cenchrus ciliaris. Genetics 163, 1069–1082.
| 1:CAS:528:DC%2BD3sXjtlOgs7c%3D&md5=361f36385d1cc35e87a785256dbdc611CAS | 12663545PubMed |
Goel, S., Chen, Z., Akiyama, Y., Conner, J. A., Basu, M., Gualtieri, G., Hanna, W. W., and Ozias-Akins, P. (2006). Comparative physical mapping of the apospory-specific genomic region in two apomictic grasses: Pennisetum squamulatum and Cenchrus ciliaris. Genetics 173, 389–400.
| Comparative physical mapping of the apospory-specific genomic region in two apomictic grasses: Pennisetum squamulatum and Cenchrus ciliaris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvF2it78%3D&md5=447d560dbf244b4556ddc9381f9b487aCAS | 16547108PubMed |
Gornall, J., Betts, R., Burke, E., Clark, R., Camp, J., Willett, K., and Wilshire, A. (2010). Implications of climate change for agricultural productivity in the early twenty-first century. Philosophical Transactions of the Royal Society B, Biological Sciences 365, 2973–2989.
| Implications of climate change for agricultural productivity in the early twenty-first century.Crossref | GoogleScholarGoogle Scholar | 20713397PubMed |
Grant, V. (1971). ‘Plant Speciation.’ (Colombia University Press: New York.)
Greilhuber, J. (2005). Intraspecific variation in genome size in angiosperms: identifying its existence. Annals of Botany 95, 91–98.
| Intraspecific variation in genome size in angiosperms: identifying its existence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1KitbY%3D&md5=14f6c8b46c3ee22558688a30365b9ea4CAS | 15596458PubMed |
Grimanelli, D., Leblanc, O., Perotti, E., and Grossniklaus, U. (2001). Developmental genetics of gametophytic apomixis. Trends in Genetics 17, 597–604.
| Developmental genetics of gametophytic apomixis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnt1Wju7Y%3D&md5=b269e55b8f1db65fde40140b9beab0f5CAS | 11585667PubMed |
Gupta, S., Gupta, M. G., Bhat, B. V., and Bhat, V. (2001). Status of apomixis and sexuality in four species of Cenchrus. Journal of Plant Biology 28, 153–159.
Gutierrez-Ozuna, R., Eguiarte, L. E., and Molina-Freaner, F. (2009). Genotypic diversity among pasture and roadside populations of the invasive buffelgrass (Pennisetum ciliare L. Link) in north-western Mexico. Journal of Arid Environments 73, 26–32.
| Genotypic diversity among pasture and roadside populations of the invasive buffelgrass (Pennisetum ciliare L. Link) in north-western Mexico.Crossref | GoogleScholarGoogle Scholar |
Hegarty, M. J., and Hiscock, S. J. (2008). Genomic clues to the evolutionary success of polyploid plants. Current Biology 18, R435–R444.
| Genomic clues to the evolutionary success of polyploid plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVaktbY%3D&md5=08df55e7b82ad1c7fb79d8838e6e30eaCAS | 18492478PubMed |
Hierro, J. L., Maron, J. L., and Callaway, R. M. (2005). A bio-geographical approach to plant invasions: the importance of studying exotics in their introduced and native range. Journal of Ecology 93, 5–15.
| A bio-geographical approach to plant invasions: the importance of studying exotics in their introduced and native range.Crossref | GoogleScholarGoogle Scholar |
Hignight, K. W., Bashaw, E. C., and Hussey, M. A. (1991). Cytological and morphological diversity of native apomictic Buffel grass, Pennisetum ciliare (L.) Link. Botanical Gazette 152, 214–218.
| Cytological and morphological diversity of native apomictic Buffel grass, Pennisetum ciliare (L.) Link.Crossref | GoogleScholarGoogle Scholar |
Hoffmann, A. A., and Sgrò, C. M. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485.
| Climate change and evolutionary adaptation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVags7o%3D&md5=035440bd183eec8dee3362b193f7badfCAS | 21350480PubMed |
Hörandl, E., and Temsch, E. M. (2009). Introgression of apomixis into sexual species is inhibited by mentor effects and ploidy barriers in the Ranunculus auricomus complex. Annals of Botany 104, 81–89.
| Introgression of apomixis into sexual species is inhibited by mentor effects and ploidy barriers in the Ranunculus auricomus complex.Crossref | GoogleScholarGoogle Scholar | 19386790PubMed |
Hörandl, E., Cosendai, A. C., and Temsch, E. (2008). Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecology & Diversity 1, 309–320.
| Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach.Crossref | GoogleScholarGoogle Scholar |
Hull-Sanders, H. M., Johnson, R. H., Owen, H. A., and Meyer, G. A. (2009). Effects of polyploidy on secondary chemistry, physiology, and performance of native and invasive genotypes of Solidago gigantea (Asteraceae). American Journal of Botany 96, 762–770.
| Effects of polyploidy on secondary chemistry, physiology, and performance of native and invasive genotypes of Solidago gigantea (Asteraceae).Crossref | GoogleScholarGoogle Scholar | 21628231PubMed |
Jackson, J. (2005). Is there a relationship between herbaceous species richness and buffel grass (Cenchrus ciliaris)? Austral Ecology 30, 505–517.
| Is there a relationship between herbaceous species richness and buffel grass (Cenchrus ciliaris)?Crossref | GoogleScholarGoogle Scholar |
Jakobs, G., Weber, E., and Edwards, P. J. (2004). Polyploidy of Reynoutria japonica var. Japonica (Polygonaceae) in Japan. Journal of Phytogeography and Taxonomy 52, 137–142.
Jauffret, S., and Visser, M. (2003). Assigning life history traits to plant species to better qualify arid land degradation in Pre-Saharian Tunisia. Journal of Arid Environments 55, 1–28.
| Assigning life history traits to plant species to better qualify arid land degradation in Pre-Saharian Tunisia.Crossref | GoogleScholarGoogle Scholar |
Jessup, R. W., Burson, B. L., Burow, O., Wang, Y. W., Chang, C., Li, Z., Paterson, A. H., and Hussey, M. A. (2002). Disomic inheritance, suppressed recombination, and allelic interactions govern apospory in buffelgrass as revealed by genome mapping. Crop Science 42, 1688–1694.
| Disomic inheritance, suppressed recombination, and allelic interactions govern apospory in buffelgrass as revealed by genome mapping.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsFOhtrg%3D&md5=ca9b8ee3c4acf8b72686677901820874CAS |
Jorge, M. A. B., Van de Wouw, M., Hanson, J., and Mohammed, J. (2008). Characterisation of a collection of buffel grass (Cenchrus ciliaris). Tropical Grasslands 42, 27–39.
Kawecki, T. J., and Ebert, D. (2004). Conceptual issues in local adaptation. Ecology Letters 7, 1225–1241.
| Conceptual issues in local adaptation.Crossref | GoogleScholarGoogle Scholar |
Kharrat-Souissi, A., Baumel, A., Mseddi, K., Torre, F., and Chaieb, M. (2010). Polymorphism of Cenchrus ciliaris L.: a perennial grass of arid zones. African Journal of Ecology 49, 209–220.
| Polymorphism of Cenchrus ciliaris L.: a perennial grass of arid zones.Crossref | GoogleScholarGoogle Scholar |
Kharrat-Souissi, A., Baumel, A., Torre, F., Juin, M., Siljak-Yakovlev, S., Roig, A., and Chaieb, M. (2011). New insights into the polyploid complex Cenchrus ciliaris L. (Poaceae) show its capacity for gene flow and recombination processes despite its apomictic nature. Australian Journal of Botany 59, 543–553.
| New insights into the polyploid complex Cenchrus ciliaris L. (Poaceae) show its capacity for gene flow and recombination processes despite its apomictic nature.Crossref | GoogleScholarGoogle Scholar |
Kharrat-Souissi, A., Baumel, A., Torre, F., and Chaieb, M. (2012a). Genetic differentiation of the dominant perennial grass Cenchrus ciliaris L. contributes to response to water deficit in arid lands. The Rangeland Journal 34, 55–62.
| Genetic differentiation of the dominant perennial grass Cenchrus ciliaris L. contributes to response to water deficit in arid lands.Crossref | GoogleScholarGoogle Scholar |
Kharrat-Souissi, A., Siljak-Yakovlev, S., Pustahija, F., and Chaieb, M. (2012b). Physical mapping of the 5S and 18S–5.8S–26S rRNA genes in polyploid series of Cenchrus ciliaris L. Comparative Cytogenetics 6, 273–286.
| Physical mapping of the 5S and 18S–5.8S–26S rRNA genes in polyploid series of Cenchrus ciliaris L.Crossref | GoogleScholarGoogle Scholar | 24260668PubMed |
Kharrat-Souissi, A., Siljak-Yakovlev, S., Brown, S., and Chaieb, M. (2013). Cytogeography of Cenchrus ciliaris (Poaceae) in Tunisia. Folia Geobotanica 48, 95–113.
| Cytogeography of Cenchrus ciliaris (Poaceae) in Tunisia.Crossref | GoogleScholarGoogle Scholar |
Koltunow, A. M. (1993). Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. The Plant Cell 5, 1425–1437.
| 12271038PubMed |
Krahulcová, A., and Rotreklová, O. (2010). Use of flow cytometry in research on apomictic plants. Preslia 82, 23–39.
Kubátová, B., Trávnicek, P., Bastlová, D., Curn, V., Jarolimová, V., and Suda, J. (2008). DNA ploidy-level variation in native and invasive populations of Lythrum salicaria at a large geographical scale. Journal of Biogeography 35, 167–176.
Lazaridou, M., and Koutroubas, S. D. (2004). Drought effect on water efficiency of berseem clover at various growth stages. In: ‘New Directions for a Diverse Planet. Proceedings for 4th International Crop Science Congress’. Brisbane, Queensland, 26 Sept.–1 Oct. 2004. (Ed. R. A. Fischer.) (The Regional Institute Ltd, Online Community Publishing: Brisbane, Qld.)
Lazaridou, M., and Noitsakis, B. (2003). The effect of water deficit on yield and water use efficiency of lucerne. In: ‘Grassland Science in Europe. Vol. 8’. (Eds A. Kirilov, N. Todorov and I. Katerov.) pp. 344–347. (European Grassland Federation: Pleven, Bulgaria.)
Levin, D. A. (2000). ‘The Origin, Expansion and Demise of Plant Species.’ (Oxford University Press: Oxford, UK.)
M’seddi, K., Visser, M., Neffati, M., Reheul, D., and Chaieb, M. (2002). Seed and spike traits from remnant populations of Cenchrus ciliaris L., in South Tunisia: high distinctiveness, no ecotypes. Journal of Arid Environments 50, 309–324.
| Seed and spike traits from remnant populations of Cenchrus ciliaris L., in South Tunisia: high distinctiveness, no ecotypes.Crossref | GoogleScholarGoogle Scholar |
Maherali, H., Walden, A. E., and Husband, B. C. (2009). Genome duplication and the evolution of physiological responses to water stress. New Phytologist 184, 721–731.
| Genome duplication and the evolution of physiological responses to water stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVOgs7nP&md5=f0489556c6472588ddd795337882b573CAS | 19703115PubMed |
Majidi, M. M., Mirlohi, A., and Amini, F. (2009). Genetic variation, heritability and correlations of agro-morphological traits in tall fescue (Festuca arundinacea Schreb.). Euphytica 167, 323–331.
| Genetic variation, heritability and correlations of agro-morphological traits in tall fescue (Festuca arundinacea Schreb.).Crossref | GoogleScholarGoogle Scholar |
Mansoor, U., Hameed, M., Wahid, A., and Rao, A. R. (2002). Ecotypic variability for drought resistance in Cenchrus ciliaris L. germplasm from Cholistan Desert in Pakistan. International Journal of Agriculture and Biology 4, 392–397.
Maroco, J. P., Pereira, J. S., and Chaves, M. M. (2000). Growth, photosynthesis and water-use efficiency of two C4 Sahelian grasses subjected to water deficits. Journal of Arid Environments 45, 119–137.
| Growth, photosynthesis and water-use efficiency of two C4 Sahelian grasses subjected to water deficits.Crossref | GoogleScholarGoogle Scholar |
Marshall, V. M., Lewis, M. M., and Ostendorf, B. (2012). Buffel grass (Cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: a review. Journal of Arid Environments 78, 1–12.
| Buffel grass (Cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: a review.Crossref | GoogleScholarGoogle Scholar |
Martinez, E. J., Espinoza, F., and Quarin, C. L. (1994). BIII Progeny (2n+n) from apomictic Paspalum notatum obtained through early pollination. The Journal of Heredity 85, 295–297.
Matzk, F., Meister, A., and Schubert, I. (2000). An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal 21, 97–108.
| An efficient screen for reproductive pathways using mature seeds of monocots and dicots.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7hslGgtg%3D%3D&md5=c21b5e293c59f0d85a2cde8158fcfbbaCAS | 10652155PubMed |
McIvor, J. G. (2003). Competition affects survival and growth of Buffel grass seedlings: is Buffel grass a coloniser or an invader? Tropical Grasslands 37, 176–181.
Miles, J. (2007). Apomixis for cultivar development in tropical forage grasses. Crop Science 47, S-238–S-249.
| Apomixis for cultivar development in tropical forage grasses.Crossref | GoogleScholarGoogle Scholar |
Miller, G., Friedel, M., Adam, P., and Chewings, V. (2010). Ecological impacts of Buffel grass (Cenchrus ciliaris L.) invasion in central Australia: does field evidence support a fire-invasion feedback? The Rangeland Journal 32, 353–365.
| Ecological impacts of Buffel grass (Cenchrus ciliaris L.) invasion in central Australia: does field evidence support a fire-invasion feedback?Crossref | GoogleScholarGoogle Scholar |
Mnif, L., and Chaieb, M. (2009). Root growth and morphology of four provenances of a perennial grass (Cenchrus ciliaris L.) in rhizotron chamber. Acta Botanica Gallica 156, 273–282.
| Root growth and morphology of four provenances of a perennial grass (Cenchrus ciliaris L.) in rhizotron chamber.Crossref | GoogleScholarGoogle Scholar |
Mnif, L., and Chaieb, M. (2010). Net photosynthesis and leaf water potential of buffel grass (Cenchrus ciliaris L.) accessions growing in the arid zone of Tunisia. Journal of Biological Research - Thessaloniki 14, 231–238.
| 1:CAS:528:DC%2BC3cXhtVygurzL&md5=a3ad6372da633180e812d67bd4e08b9fCAS |
Mnif, L., Mseddi, K., Roux, M., and Chaieb, M. (2003). Genetic diversity of several provenances of Cenchrus ciliaris L. a perennial Poaceae of arid zone of Tunisia. Bocconea 16, 641–656.
Mnif, L., Ouled Belgacem, A., Cortina, J., and Chaieb, M. (2005a). A comparative analysis of the establishment of Cenchrus ciliaris provenances in arid zone of Tunisia. Arid Land Research and Management 19, 341–351.
| A comparative analysis of the establishment of Cenchrus ciliaris provenances in arid zone of Tunisia.Crossref | GoogleScholarGoogle Scholar |
Mnif, L., Mseddi, K., and Chaieb, M. (2005b). Cenchrus ciliaris L. in arid zone of Tunisia: chromosome number, germination and reproductive behaviour of several populations. Acta Botanica Gallica 152, 45–56.
| Cenchrus ciliaris L. in arid zone of Tunisia: chromosome number, germination and reproductive behaviour of several populations.Crossref | GoogleScholarGoogle Scholar |
Mogie, M., Britton, N. F., and Stewart-Cox, J. A. (2007). Asexuality, polyploidy and the male function. In: ‘Apomixis: Evolution, Mechanisms and Perspectives’. (Eds E. Hörandl, U. Grossniklaus, P. J. Van Dijk and T. Sharbel.) pp. 195–214. (ARG-Gantner: Ruggell, Liechtenstein.)
M’seddi, K., Chaieb, M., Visser, M., and Neffati, M. (2004a). Productivity and response to grazing simulated by cuts in different provenances of Cenchrus ciliaris L. Option Méditerranéennes 62, 349–352.
M’seddi, K., Mnif, L., Chaieb, M., Neffati, M., and Roux, M. (2004b). Above-ground phytomass productivity and morphological variability of Tunisian accessions Cenchrus ciliaris L. African Journal of Range & Forage Science 21, 49–55.
| Above-ground phytomass productivity and morphological variability of Tunisian accessions Cenchrus ciliaris L.Crossref | GoogleScholarGoogle Scholar |
Nisar, M. F., Iram, S., Akhtar, Y., Mageed, A., Ismail, S., and Lin, F. (2010). AFLP-based analysis of genetic diversity in Buffel grass. World Applied Sciences Journal 10, 560–567.
Novak, S. J., and Mack, R. N. (2000). Clonal diversity within and among introduced populations of the apomictic vine Bryonia alba (Cucurbitaceae). Canadian Journal of Botany 78, 1469–1481.
Ofir, M., and Kigel, J. (2010). Ecotypic variation of summer dormancy relaxation associated with rainfall gradient in the geophytic grass Poa bulbosa. Annals of Botany 105, 617–625.
| Ecotypic variation of summer dormancy relaxation associated with rainfall gradient in the geophytic grass Poa bulbosa.Crossref | GoogleScholarGoogle Scholar | 20156924PubMed |
Otto, S. P., and Whitton, J. (2000). Polyploid incidence and evolution. Annual Review of Genetics 34, 401–437.
| Polyploid incidence and evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFOjsg%3D%3D&md5=86db68152810e6f9f75fc18d8bc9366dCAS | 11092833PubMed |
Ozias-Akins, P., and Van Dik, P. J. (2007). Mendelian genetics of apomixis in plants. Annual Review of Genetics 41, 509–537.
| Mendelian genetics of apomixis in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1SitQ%3D%3D&md5=6c0ebf1dffe25c5defcbe63f99d0af89CAS | 18076331PubMed |
Ozias-Akins, P., Akiyama, Y., and Hanna, W. W. (2003). Molecular characterization of the genomic region linked with apomixis in Pennisetum/Cenchrus. Functional & Integrative Genomics 3, 94–104.
| Molecular characterization of the genomic region linked with apomixis in Pennisetum/Cenchrus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXls1yns7g%3D&md5=de34fc38f418402d90709de4cfa175a3CAS |
Pandit, M. K. (2006). Continuing the search for pattern among rare plants: are diploid species more likely to be rare? Evolutionary Ecology Research 8, 543–552.
Pandit, M. K., Pocock, M. J. O., and Kunin, W. E. (2011). Ploidy influences rarity and invasiveness in plants. Journal of Ecology 99, 1108–1115.
| Ploidy influences rarity and invasiveness in plants.Crossref | GoogleScholarGoogle Scholar |
Parisod, C., Holderegger, R., and Brochmann, C. (2010). Evolutionary consequences of autopolyploidy. New Phytologist 186, 5–17.
| Evolutionary consequences of autopolyploidy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvVOksbo%3D&md5=cfe3dc1af472953197eaf45c86d0827eCAS | 20070540PubMed |
Parker, I. M., Rodriguez, J., and Loik, M. E. (2003). An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conservation Biology 17, 59–72.
| An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus.Crossref | GoogleScholarGoogle Scholar |
Paun, O., Bateman, R. M., Fay, M. F., Luna, J. A., Moat, J., Hedren, M., and Chase, M. (2011). Altered gene expression and ecological divergence in sibling allopolyploids of Dactylorhiza (Orchidaceae). BMC Evolutionary Biology 11, 113.
| Altered gene expression and ecological divergence in sibling allopolyploids of Dactylorhiza (Orchidaceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXls1SmtL0%3D&md5=d349fdc285f6c182597d5a3a1cdd053dCAS | 21521507PubMed |
Pitt, J. (2004). Current distribution and strategic management options for Buffel grass (Cenchrus ciliaris L.) in South Australia. Plant Protection Quarterly 19, 73–74.
Reynolds, J. F., Stafford Smith, D. M., Lambin, E. F., Turner, B. L., Mortimore, M., Batterbury, S. P. J., Downing, T. E., Dowlatabadi, H., Fernández, R. J., Herrick, J. E., Huber-Sannvald, E., Leemans, R., Lynam, T., Maestre, F. T., Ayarza, M., and Walker, B. (2007). Global desertification: building a science for dryland development. Science 316, 847–851.
| Global desertification: building a science for dryland development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltVentb4%3D&md5=a2b30803485f45e76c739d1b7bca0181CAS | 17495163PubMed |
Richardson, D. M., Pysek, P., Rejmanek, M., Barbour, M. G., Panetta, F. D., and West, D. J. (2000). Naturalization and invasion of alien plants: concepts and definitions. Diversity & Distributions 6, 93–107.
| Naturalization and invasion of alien plants: concepts and definitions.Crossref | GoogleScholarGoogle Scholar |
Roche, D., Cong, P., Chen, Z., Hanna, W. W., Gustine, D. L., Sherwood, R. T., and Ozias-Akins, P. (1999). Short communication: an apospory specific genomic region is conserved between Buffel grass (Cenchrus ciliaris L.) and Pennisetum squamulatum fresen. The Plant Journal 19, 203–208.
| Short communication: an apospory specific genomic region is conserved between Buffel grass (Cenchrus ciliaris L.) and Pennisetum squamulatum fresen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmtlClsb4%3D&md5=035c1353c328878b18c6f6dd3ea69efeCAS | 10476067PubMed |
Roche, D., Hanna, W. W., and Ozias-Akins, P. (2001). Is supernumerary chromatin involved in gametophytic apomixis of polyploid plants? Sexual Plant Reproduction 13, 343–349.
| Is supernumerary chromatin involved in gametophytic apomixis of polyploid plants?Crossref | GoogleScholarGoogle Scholar |
Ruiz-R., N., Ward, D., and Saltz, D. (2006). Population differentiation and the effects of herbivory and sand compaction on the subterranean growth of a desert lily. The Journal of Heredity 97, 409–416.
| Population differentiation and the effects of herbivory and sand compaction on the subterranean growth of a desert lily.Crossref | GoogleScholarGoogle Scholar | 16793866PubMed |
Savidan, Y. H. (1982). Embryological analysis of facultative apomixis in Panicum maximum. Crop Science 22, 467–469.
| Embryological analysis of facultative apomixis in Panicum maximum.Crossref | GoogleScholarGoogle Scholar |
Savidan, Y. (2000). Apomixis: genetics and breeding. In: ‘Plant Breeding Reviews’. (Ed. J. Janick.) pp. 13–86. (Wiley: New York.)
Schlaepfer, D. R., Edwards, P., and Billeter, R. (2010). Why only tetraploid Solidago gigantea (Asteraceae) became invasive: a common garden comparison of ploidy levels. Oecologia 163, 661–673.
| Why only tetraploid Solidago gigantea (Asteraceae) became invasive: a common garden comparison of ploidy levels.Crossref | GoogleScholarGoogle Scholar | 20238128PubMed |
Schuepp, P. (1993). Tansley Review No. 59. Leaf boundary layers. New Phytologist 125, 477–507.
| Tansley Review No. 59. Leaf boundary layers.Crossref | GoogleScholarGoogle Scholar |
Sherwood, R. T., Young, B. A., and Bashaw, E. C. (1980). Facultative apomixes in Buffel grass. Crop Science 20, 375–379.
| Facultative apomixes in Buffel grass.Crossref | GoogleScholarGoogle Scholar |
Singh, M., Burson, B. L., and Finlayson, S. A. (2007). Isolation of candidate genes for apomictic development in Buffel grass (Pennisetum ciliare). Plant Molecular Biology 64, 673–682.
| Isolation of candidate genes for apomictic development in Buffel grass (Pennisetum ciliare).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlajsLk%3D&md5=2b8f65a9139821d5015b518b964596b0CAS | 17541705PubMed |
Smyth, A., Friedel, M., and O’Malley, C. (2009). The influence of Buffel grass (Cenchrus ciliaris) on biodiversity in an arid Australian landscape. The Rangeland Journal 31, 307–320.
| The influence of Buffel grass (Cenchrus ciliaris) on biodiversity in an arid Australian landscape.Crossref | GoogleScholarGoogle Scholar |
Soltis, P. S., and Soltis, D. E. (2000). The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the United States of America 97, 7051–7057.
| The role of genetic and genomic attributes in the success of polyploids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksVKisbY%3D&md5=ea172f15c36478619c77ad8b8a317e23CAS | 10860970PubMed |
Sowers, J., Avner, V., and Weinthal, E. (2011). Climate change, water resources, and the politics of adaptation in the Middle East and North Africa. Climatic Change 104, 599–627.
| Climate change, water resources, and the politics of adaptation in the Middle East and North Africa.Crossref | GoogleScholarGoogle Scholar |
Stastny, M., Schaffner, U., and Elle, E. (2005). Do vigour of introduced populations and escape from specialist herbivores contribute to invasiveness? Journal of Ecology 93, 27–37.
| Do vigour of introduced populations and escape from specialist herbivores contribute to invasiveness?Crossref | GoogleScholarGoogle Scholar |
Stebbins, G. L. (1985). Polyploidy, hybridization, and the invasion of new habitats. Annals of the Missouri Botanical Garden 72, 824–832.
| Polyploidy, hybridization, and the invasion of new habitats.Crossref | GoogleScholarGoogle Scholar |
Taiz, L., and Zeiger, E. (2006). ‘Plant Physiology.’ 4th edn. (Sinauer Associates: Sunderland, MA.)
Taliaferro, C. M., and Bashaw, E. C. (1966). Inheritance and control of obligate apomixis in breeding Buffel grass, Pennisetum ciliare. Crop Science 6, 473–476.
| Inheritance and control of obligate apomixis in breeding Buffel grass, Pennisetum ciliare.Crossref | GoogleScholarGoogle Scholar |
te Beest, M. J., Le Roux, J. J., Richardson, D. M., Brysting, A. K., Suda, J., Kubešová, M., and Pyšek, P. (2012). The more the better? The role of polyploidy in facilitating plant invasions. Annals of Botany 109, 19–45.
| The more the better? The role of polyploidy in facilitating plant invasions.Crossref | GoogleScholarGoogle Scholar |
Treier, U. A., Broennimann, O., Normand, S., Guisan, A., Schaffner, U., Steinger, T., and Müller-Schärer, H. (2009). Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology 90, 1366–1377.
| Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa.Crossref | GoogleScholarGoogle Scholar | 19537556PubMed |
Ubi, B. E., Honda, C., Bessho, H., Kondo, S., Wada, M., Kobayashi, S., and Moriguchi, T. (2006). Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Science 170, 571–578.
| Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvVGmuw%3D%3D&md5=d6e71a88ad510991089421c20872cb6bCAS |
Van Kleunen, M., and Fischer, M. (2005). Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytologist 166, 49–60.
| Constraints on the evolution of adaptive phenotypic plasticity in plants.Crossref | GoogleScholarGoogle Scholar | 15760350PubMed |
Visser, N. C., Spies, J. J., and Venter, H. J. T. (1998). Aneuploidy in Cenchrus ciliaris (Poaceae, Panicoideae, Paniceae): truth or fiction? South African Journal of Botany 64, 337–345.
Visser, N. C., Spies, J. J., and Venter, H. J. T. (2000). Apomictic embryo sac development in Cenchrus ciliaris (Panicoideae). Bothalia 30, 103–110.
Visser, M., Mseddi, K., Chaïeb, M., and Neffati, M. (2008). Assessing yield and yield stability of remnant populations of Cenchrus ciliaris L. in arid Tunisia: developing a blueprint for initiating native seed production. Grass and Forage Science 63, 301–313.
| Assessing yield and yield stability of remnant populations of Cenchrus ciliaris L. in arid Tunisia: developing a blueprint for initiating native seed production.Crossref | GoogleScholarGoogle Scholar |
Vogelmann, T. C., Bornman, J. F., and Yates, D. J. (1996). Focusing of light by leaf epidermal cells. Physiologia Plantarum 98, 43–56.
| Focusing of light by leaf epidermal cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvV2nt7s%3D&md5=45ef67f6b959d4261c92e50ad909d69cCAS |
Volis, S., Yakubov, B., Shulgina, I., Ward, D., Zur, V., and Mendlinger, S. (2005). Distinguishing adaptive from non-adaptive genetic differentiation: comparison of Q ST and F ST at two spatial scales. Heredity 95, 466–475.
| Distinguishing adaptive from non-adaptive genetic differentiation: comparison of Q ST and F ST at two spatial scales.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1GisL3M&md5=d14005fc5e4e67f05b474f661272fd4aCAS | 16189543PubMed |
Ward, J. P., Smith, S. E., and McClaran, M. P. (2006). Water requirements for emergence of Buffel grass (Pennisetum ciliare). Weed Science 54, 720–725.
| Water requirements for emergence of Buffel grass (Pennisetum ciliare).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvF2ntbg%3D&md5=973c3130cf45ba3d5a10872ba2b3c620CAS |
Waters, C., Murray, B. G., Melville, G., Coates, D., Young, A., and Virgona, J. (2010). Polyploidy and possible implications for the evolutionary history of some Australian Danthonieae. Australian Journal of Botany 58, 23–34.
| Polyploidy and possible implications for the evolutionary history of some Australian Danthonieae.Crossref | GoogleScholarGoogle Scholar |
Whalley, R. D. B., Chivers, I. H., and Waters, C. M. (2013). Revegetation with Australian native grasses – a reassessment of the importance of using local provenances. The Rangeland Journal 35, 155–166.
| Revegetation with Australian native grasses – a reassessment of the importance of using local provenances.Crossref | GoogleScholarGoogle Scholar |
Whitford, W. G. (2002). ‘Ecology of Desert Systems.’ (Academic Press: London, UK.)
Yadav, C. B., Kumar, A. S., Gupta, M. G., and Bhat, V. (2012). Genetic linkage maps of the chromosomal regions associated with apomictic and sexual modes of reproduction in Cenchrus ciliaris. Molecular Breeding 30, 239–250.
| Genetic linkage maps of the chromosomal regions associated with apomictic and sexual modes of reproduction in Cenchrus ciliaris.Crossref | GoogleScholarGoogle Scholar |


