The History of Azulenyl Squaraines
Daniel E. Lynch A C and Darren G. Hamilton BA Exilica Limited, The Technocentre, Puma Way, Coventry CV1 2TT, UK.
B Department of Chemistry, Mount Holyoke College, 50 College Street, South Hadley, MA 01075, USA.
C Corresponding author. Email: d.lynch@exilica.co.uk

Daniel Lynch is a materials chemist, and received his B.App.Sc. (1990) and Ph.D. (1994) degrees from QUT in Brisbane (Australia). A post-doctoral fellowship at Cranfield University (UK) preceded a six-year university research fellowship at Coventry University (UK), becoming Senior Lecturer in 2001 and Reader of Applied Chemistry in 2007 at the same institution. He is the author of over 240 research publications, the principal inventor of Exilica's patented technology and became the full-time Technical Director of Exilica Limited in 2007, a post that he had held on a part-time basis since Exilica's incorporation in May 2005. He was on the 2005-2006 Enterprise Fellowship Scheme and was a 2004-2005 Midlands Medici Fellow. |

Darren Hamilton was born in southern England in 1969 and received his B.Sc. degree from Royal Holloway College, University of London, in 1990. A Ph.D. in organic chemistry from the University of Southampton (1994) was followed by several years as a post-doctoral researcher and Ramsay Memorial Research Fellow, principally in the research group of Jeremy Sanders CBE, FRS, at the University of Cambridge. He taught chemistry from 1997–1999 at Murray Edwards College (formerly New Hall), a college of the University of Cambridge, an experience that led him to accept a position on the faculty of Mount Holyoke College (Massachusetts, USA) in the summer of 1999. He continues to explore topics in supramolecular and materials chemistry as Professor of Chemistry at this institution. |
Australian Journal of Chemistry 70(8) 857-871 https://doi.org/10.1071/CH16383
Submitted: 2 July 2016 Accepted: 9 February 2017 Published: 3 March 2017
Abstract
The synthesis of the first azulenyl squaraine dye was reported in 1966 and the paucity of activity in the scientific literature since that time has given the false impression that they have remained relatively unexplored as organic dyes. In contrast, a wealth of research activity on azulenyl squaraines has been recorded in the patent literature and it is the purpose of this review to bring these reports to the attention of scientists that have an interest in the chemistry and applications of squaraine dyes. This review shows that it is possible to prepare, via targeted substitution of the azulene ring, a dye with an absorption maximum value ranging from 650 to 840 nm.
References
[1] H. E. Sprenger, W. Ziegenbein, Angew. Chem. 1966, 78, 937.[W. Ziegenbein, H. E. Sprenger, Angew. Chem. Int. Ed. Engl. 1966, 5, 893]| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXhvFyisQ%3D%3D&md5=13e7d096a494c409f9f463f91d7d8dbbCAS |
[2] T. Satoh, I. Shimizu, Y. Ito, U. S. Patent 5 085 909 1992.
[3] S. Yagi, H. Nakazumi, Top. Heterocycl. Chem. 2008, 14, 133.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXis12ms7c%3D&md5=df8443e935020ea656847597c93c8f08CAS |
[4] S. Sreejith, P. Carol, P. Chithra, A. Ajayaghosh, J. Mater. Chem. 2008, 18, 264.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVOlsw%3D%3D&md5=cbee1ff0a2ae00906dc047949ba49d45CAS |
[5] J. J. McEwen, K. J. Wallace, Chem. Commun. 2009, 6339.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1yisrnJ&md5=216a4ccd809869e2aac8baed39576349CAS |
[6] L. D. Patsenker, A. L. Tatarets, E. A. Terpetschnig, in Advanced Fluorescence Reporters in Chemistry and Biology I (Ed. A. Demchenko) 2010, pp. 65–104 (Springer: Berlin).
[7] L. Beverina, P. Salice, Eur. J. Org. 2010, 1207.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitlegt7s%3D&md5=a50aa92d1e3adac73033f165cc14feb7CAS |
[8] Y. Li, Q. Guo, Z. Li, J. Pei, W. Tian, Energy Environ. Sci. 2010, 3, 1427.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFSnsLfN&md5=9aa7492e0ea4a5df7927ecbc29cb2835CAS |
[9] C. Qin, W. Y. Wong, L. Han, Chem. Asian J. 2013, 8, 1706.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvFWrs7c%3D&md5=9f15868f6fbd99bb2f05fa1862ae9218CAS |
[10] L. Hu, Z. Yan, H. Xu, RSC Adv. 2013, 3, 7667.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVyru7c%3D&md5=ee068c83e127a2bdea1b786481426232CAS |
[11] L. Beverina, M. Sassi, Synlett 2014, 25, 477.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXptlShsr8%3D&md5=07759142418931c63e66466cb4ae92b5CAS |
[12] D. E. Lynch, Metals 2015, 5, 1349.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFegsLzE&md5=5266efb5fd000f516b71f3c8b76094eeCAS |
[13] A. Treibs, K. Jacob, Angew. Chem. Int. Ed. Engl. 1965, 4, 694.
| Crossref | GoogleScholarGoogle Scholar |
[14] H. E. Sprenger, W. Ziegenbein, Angew. Chem. Int. Ed. Engl. 1966, 5, 894.
| Crossref | GoogleScholarGoogle Scholar |
[15] G. Maahs, P. Hegenberg, Angew. Chem. Int. Ed. Engl. 1966, 5, 888.
| Crossref | GoogleScholarGoogle Scholar |
[16] H. E. Sprenger, W. Ziegenbein, Angew. Chem. Int. Ed. Engl. 1967, 6, 553.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXks1eru74%3D&md5=8915b597774adab357a2c32b6fcb50f0CAS |
[17] H. E. Sprenger, W. Ziegenbein, Angew. Chem. Int. Ed. Engl. 1968, 7, 530.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXltVOmurw%3D&md5=f4abe7526517f78e85c294da8758733bCAS |
[18] A. H. Schmidt, Synthesis 1980, 1980, 961.
| Crossref | GoogleScholarGoogle Scholar |
[19] P. H. Wöbkenberg, J. G. Labram, J. M. Swiecicki, K. Parkhomenko, D. Sredojevic, J. P. Gisselbrecht, D. M. De Leeuw, D. D. C. Bradley, J. P. Djukic, T. D. Anthopoulos, J. Mater. Chem. 2010, 20, 3673.
| Crossref | GoogleScholarGoogle Scholar |
[20] N. F. Haley, J. J. Krutak, R. J. Ott, U. S. Patent 4 175 956 1979.
[21] A. Treibs, K. Jacob, Liebigs Ann. Chem. 1968, 712, 123.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXktFWnsro%3D&md5=e9f99e53093612d4744177b7ba2098acCAS |
[22] T. Kubota, H. K. Wurster, U. S. Patent 4 481 270 1984.
[23] H. K. Wurster, U. S. Patent 4 500 621 1985.
[24] T. Sato, M. Umehara, M. Abe, H. Oba, Y. Ueda, U. S. Patent 4 656 121 1987.
[25] K. Katagiri, Y. Oguchi, Y. Takasu, U. S. Patent 4 548 886 1985.
[26] K. Katagiri, Y. Oguchi, Y. Takasu, U. S. Patent 4 565 761 1986.
[27] K. Katagiri, Y. Oguchi, T. Ohtake, K. Arao, Y. Takasu, U. S. Patent 4 629 670 1986.
[28] K. Katagiri, Y. Oguchi, T. Ohtake, K. Arao, M. Kitahara, Y. Takasu, U. S. Patent 4 673 630 1987.
[29] Y. Oguchi, K. Katagiri, Y. Takasu, U. S. Patent 4 738 908 1988.
[30] T. Urabe, U. S. Patent 4 606 613 1986.
[31] Y. Nagae, in Infrared Absorbing Dyes (Ed. M. Matsuoka) 1990, Ch. 11, pp. 144–147 (Springer: New York, NY).
[32] A. Kuroiwa, S. Asami, T. Aoi, K. Takahashi, K. Namba, U. S. Patent 4 752 820 1988.
[33] N. Makino, T. Hioki, Y. Inagaki, S. Horie, U. S. Patent 4 840 462 1989.
[34] Y. Inagaki, T. Hioki, N. Makino, K. Adachi, Y. Suzuki, U. S. Patent 4 851 322 1989.
[35] W. Schrott, B. Albert, P. Neumann, H. Benthack-Thoms, U. S. Patent 4 904 566 1990.
[36] W. Schrott, K. H. Beck, K. H. Etzbach, P. Neumann, U. S. Patent 5 071 588 1991.
[37] W. Schrott, P. Neumann, S. Brosius, H. Barzynski, H. Kuppelmaier, U. S. Patent 5 084 592 1992.
[38] The order parameter S here was determined at room temperature on the above mentioned solution in a commercial measuring cell with homogeneous edge orientation (polyimide, thickness 10–20 μm) in accordance with the known equation
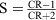 where the dichroic ratio CR was determined in accordance with the relationship
where the dichroic ratio CR was determined in accordance with the relationship 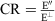 by measuring the absorbance A″ (measurement with light polarized parallel to the director of the nematic phase) and A┴ (measurement with light polarized perpendicular to the director of the nematic phase), the dye concentration having been chosen in such a way that A″ was within the range from 1 to 2.
by measuring the absorbance A″ (measurement with light polarized parallel to the director of the nematic phase) and A┴ (measurement with light polarized perpendicular to the director of the nematic phase), the dye concentration having been chosen in such a way that A″ was within the range from 1 to 2. [39] W. Schrott, P. Neumann, M. Schmidtt, S. Brosius, K. D. Schomann, H. Kuppelmaier, U. S. Patent 5 087 727 1992.
[40] M. Schmitt, B. Albert, S. Brosius, K. D. Schomann, H. Kuppelmaier, U. S. Patent 5 169 975 1992.
[41] M. Schmitt, B. Albert, S. Brosius, K. D. Schomann, H. Kuppelmaier, U. S. Patent 5 122 435 1992.
[42] B. Albert, F. Closs, J. Kipper, W. Kurtz, K. H. Beck, R. Griebel, U. S. Patent 5 282 894 1994.
[43] P. Neumann, J. Kipper, B. Albert, G. Wagenblast, U. S. Patent 5 554 318 1996.
[44] B. Albert, J. Kipper, F. Closs, H. Bellaire, U. S. Patent 5 607 762 1997.
[45] M. Schmitt, W. Schrott, P. Neumann, S. Brosius, K. D. Schomann, H. Kuppelmaier, U. S. Patent 5 728 867 1998.
[46] B. Albert, J. Kipper, C. Vamvakaris, K. H. Beck, G. Wagenblast, U. S. Patent 5 998 211 1999.
[47] Y. Oguchi, T. Santoh, U. S. Patent 4 921 780 1990.
[48] T. Santoh, C. Hioki, U. S. Patent 4 965 178 1990.
[49] T. Santoh, C. Mihara, H. Sugata, U. S. Patent 5 190 849 1993.
[50] K. Arai, T. Urabe, T. Morita, K. Miura, T. Ozawa, J. Iwanami, U. S. Patent 5 013 474 1991.
[51] I. Ando, M. Furuki, L. S. Pu, U. S. Patent 5 030 009 1991.
[52] K. Miura, T. Ozawa, J. Iwanami, U. S. Patent 5 037 575 1991.
[53] P. M. Kazmaier, G. K. Hamer, R. A. Burt, Can. J. Chem. 1990, 68, 530.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXltlWgt7w%3D&md5=f6eb5d45ee02701eb0878440b93406b7CAS |
[54] G. W. Scott, K. Tran, J. Phys. Chem. 1994, 98, 11563.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXms1Oms7Y%3D&md5=d981f1fec53c21e61fc89c6a3d1ad361CAS |
[55] G. W. Scott, K. Tran, D. J. Funk, D. S. Moore, J. Mol. Struct. 1995, 348, 425.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlsFGis74%3D&md5=de4760ee9ff0783eb02d212162476c50CAS |
[56] K. Tran, G. W. Scott, D. J. Funk, D. S. Moore, J. Phys. Chem. 1996, 100, 11863.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjvVart70%3D&md5=7cf635925729f44e9d7f104c4d2ebe3dCAS |
[57] F. Meyer, G. Wagenblast, K. H. Beck, C. Vamvakaris, U. S. Patent 6 312 958 2001.
[58] W. Pham, R. Weissleder, C. H. Tung, Angew. Chem. Int. Ed. 2002, 41, 3659.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotFymsL8%3D&md5=e5e7c07d7790840c3a746dd3dfe003a7CAS |
[59] W. Pham, R. Weissleder, C. H. Tung, Tetrahedron Lett. 2003, 44, 3975.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1Gru74%3D&md5=e9378cba8a7e5f9e06c26ff970307202CAS |
[60] C. H. Tung, R. Weissleder, W. Pham, U. S. Patent 2006/0147378 2006.
[61] Z. Y. Li, Z. H. Jin, K. Kasatani, H. Okamoto, Chin. Phys. Lett. 2005, 22, 2282.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFels7zM&md5=fde922efd410b1b854972dffb5f66108CAS |
[62] A. Weiss, U. S. Patent 2007/0196767 2007.
[63] E. C. P. Smits, S. Setayesh, T. D. Anthopoulos, M. Buechel, W. Nijssen, R. Coehoorn, P. W. M. Blom, B. De Boer, D. M. de Leeuw, Adv. Mater. 2007, 19, 734.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsFCitr4%3D&md5=7b6f35a7acba0d45fcff96ff56b19c05CAS |
[64] L. Beverina, R. Ruffo, G. Patriarca, F. De Angelis, D. Roberto, S. Righetto, R. Ugo, G. A. Pagani, J. Mater. Chem. 2009, 19, 8190.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlWht7nJ&md5=f0cffe5d6e1a83a20b20e3d3f5c9fbf7CAS |
[65] Y. Maeda, K. Shigeno, T. Ariyoshi, M. Okada, Y. Kanehara, U. S. Patent 2013/0147345 2013.
[66] Y. Maeda, T. Ishida, U. S. Patent 8 759 540 2014.
[67] M. Sramek, O. Hayden, U. S. Patent 8 614 440 2013.
[68] Y. Chen, Y. Zhu, D. Yang, S. Zhao, L. Zhang, L. Yang, J. Wu, Y. Huang, Z. Xu, Z. Lu, Chem. – Eur. J. 2016, 22, 14527.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xht12nsL%2FJ&md5=b84c89607254eed2073157aff5d6af45CAS |
[69] H. Ceymann, Synthesis and Optical Spectroscopic Properties of Squaraine Superchromophores 2016, Ph.D. thesis, pp. 42–51 (Universität Würzburg).
[70] S. Carret, A. Blanc, Y. Coquerel, M. Berthod, A. E. Greene, J. P. Deprés, Angew. Chem. Int. Ed. 2005, 44, 5130.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpslWqtr4%3D&md5=636b67d01e9ca7a3bda71453134feadaCAS |
[71] T. Nozoe, S. Seto, S. Matsumura, T. Terasawa, Chem. Ind. 1954, 1357.
| 1:CAS:528:DyaG2MXmvFSnuw%3D%3D&md5=e6c469de587271f9beea0ce1645c7628CAS |
[72] K. P. Grytsenko, Y. L. Slominski, A. I. Tolmachev, T. Tanaka, S. Schrader, L. Brehmer, A. Thierry, J. C. Wittmann, Proc. SPIE 2003, 4833, 482.
| Crossref | GoogleScholarGoogle Scholar |
[73] M. Tian, M. Furuki, I. Iwasa, Y. Sato, L. S. Pu, S. Tatsuura, J. Phys. Chem. B 2002, 106, 4370.(and references therein)
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVOhurs%3D&md5=2f0b490c361215e9ed313a17b6ad6d74CAS |
[74] A. M. Smith, M. C. Mancini, S. Nie, Nat. Nanotechnol. 2009, 4, 710.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlygsLbL&md5=f0f3f0a9e7b466f50e9ce963793133bfCAS |


