Molecular phylogeny reveals the true colours of Myeloconidaceae (Ascomycota: Ostropales)
Matthew P. Nelsen A B F , Robert Lücking B , Carrie J. Andrew B , André Aptroot C , Marcela E. S. Cáceres D , Joel A. Mercado-Díaz E , Eimy Rivas Plata B and H. Thorsten Lumbsch BA Committee on Evolutionary Biology, University of Chicago, 1025 E. 57th Street, Chicago, IL 60637, USA.
B Science & Education, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605, USA.
C ABL Herbarium, Gerrit van der Veenstraat 107, NL-3762 XK Soest, the Netherlands.
D Departamento de Biociências, Universidade Federal de Sergipe, CEP: 49.500-000, Itabaiana, Sergipe, Brazil.
E Herbario, Jardín Botánico, Universidad de Puerto Rico, 1187 Calle Flamboyán, San Juan, 00926-1177, Puerto Rico.
F Corresponding author. Email: mpnelsen@gmail.com
Australian Systematic Botany 27(1) 38-47 https://doi.org/10.1071/SB13040
Submitted: 11 September 2013 Accepted: 14 February 2014 Published: 30 June 2014
Abstract
The lichen-forming fungal family Myeloconidaceae, with the single genus Myeloconis, has been suggested to share affinities with Porinaceae (Lecanoromycetes: Ostropales). We examined its position relative to this family by using molecular data from the mitochondrial small-subunit and nuclear large-subunit rDNA. Our results revealed that Myeloconis forms a monophyletic group nested within Porinaceae, closely related to Porina farinosa. Neither Porina s.str. nor Clathroporina sensu Harris form monophyletic groups; instead, two strongly supported clades were recovered, which differ in ascospore septation (septate v. muriform), with the clade producing muriform ascospores including Myeloconis. We therefore reduce Myeloconidaceae to synonymy with Porinaceae; however, because generic delimitations within Porinaceae remain unclear, we retain Myeloconis as a separate genus within the family. The species concept currently used in the genus, based largely on secondary metabolites and ascospore measurements, is supported by the phylogeny.
Introduction
In their treatment of lichen-forming fungi in the Guianas in the family Porinaceae (as Trichotheliacae), Aptroot and Sipman (1993) noted collections identified as Clathroporina enteroxantha, with yellow medullary pigmentation. McCarthy (1995) re-examined some of these collections and concluded that they represented a new genus known also from Brazil, Australia, Melanesia and Malaysia. The genus Myeloconis (Fig. 1) was subsequently established to accommodate this group of four crustose species with yellow to orange medullary phenalenones (Ernst-Russell et al. 2000), producing perithecial ascomata with a dense perithecial wall (McCarthy and Elix 1996). In addition, these species lack an involucrellum and periphyses, but form basally anastomosing paraphyses that are simple above, asci that are thin-walled, unitunicate and lack an apical apparatus, and produce hyaline, muriform ascospores that are elongate in shape (McCarthy and Elix 1996; McCarthy 2001a, 2001b). The phenalenones often burst through the thallus (Fig. 1), together with fungal hyphae and algal cells, and these structures have been suggested to possibly fulfill a reproductive role similar to soredia (McCarthy 2001a). Species within the genus are distinguished on the basis of spore and perithecial size, as well as the presence or absence of various secondary metabolites. Myeloconis associates with trentepohlioid algae and is corticolous, occurring in lowland tropical rainforests (McCarthy and Elix 1996).

|
When describing Myeloconis, McCarthy and Elix (1996) noted that its thallus morphology and chemistry, along with the basally anastomosing hamathecium, suggested affinities with Trypetheliaceae, whereas its ascospore shape, ascus structure (unitunicate) and the otherwise mostly free paraphyses suggested a relationship with Porinaceae. They felt it more likely that Myeloconis was related to Porinaceae than Trypetheliaceae, but did not rule out the possibility of it belonging to an undescribed family, and left the genus as incertae sedis (McCarthy and Elix 1996). Later, McCarthy (2001a, 2001b) placed Myeloconis in a new family, Myeloconaceae (corrected to Myeloconidaceae by McCarthy 2003), and argued for its distinction from Porinaceae (as Trichotheliaceae) on the basis of its chemistry, lack of an involucrellum and the presence of a deeply pigmented wall of periclinal cells external to the exciple (McCarthy 2001a, 2001b).
Porinaceae is a medium-sized family of over 400 species world-wide (McCarthy 2001c, 2003, 2013), being most diverse in the tropics and decreasing in species richness toward higher latitudes, although it remains rich in New Zealand and Tasmania (McCarthy 1993, 2003; McCarthy and Kantvilas 2000). Taxa primarily occur on bark, rock and leaves, although some are known to grow over bryophytes (McCarthy and Kantvilas 2000; McCarthy 2003; Lücking 2008). All species associate with trentepohlioid photobionts (Santesson 1952; McCarthy 2003; Baloch and Grube 2006; Lücking 2008; Nelsen et al. 2011a). Porinaceae produce crustose thalli that are typically greenish, sometimes with a hypothallus, and often with calcium-oxalate crystals in the thallus that form a distinct layer (crystallostratum); in addition, they form immersed to sessile, variously coloured, perithecial ascomata with angiocarpous ascohymenial development, a distinct to vestigial, sometimes absent involucrellum, cylindrical to obclavate, thin-walled asci without tholus that are functionally unitunicate, paraphyses which are generally unbranched, variously developed or lacking periphyses, and hyaline, transversely septate to muriform ascospores (Swinscow 1962; Vězda 1968; Janex-Favre 1971; Henssen and Jahns 1974; Hafellner and Kalb 1995; Harris 1995; Lücking 2008).
The family Porinaceae (as Trichotheliaceae) was placed in the order Trichotheliales (Hafellner and Kalb 1995), and McCarthy (2001a, 2001b, 2003) suggested that also Myeloconidaceae belonged to that order. The first available molecular data, provided by Bhattacharya et al. (2000), suggested that Porina formed part of Lecanoromycetes, specifically being sister to Stereocaulon in the Lecanorales; this, however, stemmed from the sequences of the species used (P. guentheri) apparently being incorrectly labelled or from contaminants (the nuSSU appears to be part of Dothideomycetes, whereas the nuLSU is suggested to belong to Parmeliaceae and includes a rare intron). Nevertheless, the placement of Porinaceae in Lecanoromycetes was subsequently shown by Grube et al. (2004), who demonstrated that it belonged in Ostropomycetidae, not Lecanoromycetidae, close to taxa in Ostropales. Hibbett et al. (2007) later synonymised Trichotheliales with Ostropales, a change further supported by the phylogenetic analysis of Baloch et al. (2010), which placed Porinaceae in an expanded Ostropales (sensu Kauff and Lutzoni 2002).
Here we utilise molecular sequence data to (1) assess the monophyly of Myeloconis and (2) reconstruct the phylogenetic position of Myeloconidaceae relative to Porinaceae.
Materials and methods
Taxon selection
We sequenced fungal DNA from 10 neo- and paleotropical collections of Myeloconis representing three of the four known species, as well as 13 collections of Porina s.lat. representing species currently placed in either Porina s.str. or Clathroporina (Table 1).
Molecular methods
The Sigma REDExtract-N-Amp Plant PCR Kit (Sigma–Aldrich, St Louis, MO, USA) was used to isolate DNA, following the manufacturer’s instructions, except that 10–25 µL of extraction buffer and 10–25 µL dilution buffer were used, and a 20× DNA dilution was then utilised in subsequent polymerase chain reaction (PCR) reactions. Portions of the fungal mitochondrial small subunit (mtSSU) and nuclear ribosomal large subunit (nuLSU) were amplified using the mrSSU1, mrSSU2R, mrSSU3R (Zoller et al. 1999) and mrSSU-2/3–5′-mpn (Nelsen et al. 2011b) primers for the mtSSU, and the AL2R (Mangold et al. 2008), f-nu-LSU-0116–5′/ITS4A-5′ (Nelsen et al. 2011b, 2012; reverse complement of D. L. Taylor’s ITS4A in Kroken and Taylor 2001), LR3 (Vilgalys and Hester 1990) and LR3-Porina-mpn (the present study: CCA TTA CGC CMG CAT CCG TGC) primers for the nuLSU. The 10-µL PCR reactions consisted of 5 µM of each PCR primer, 2 µL diluted DNA and 5 µL REDExtract-n-Amp PCR Ready Mix (Sigma–Aldrich). The PCR cycling conditions were as follows: 95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 53°C (mtSSU) or 55°C (nuLSU) for 1 min, and 72°C for 1 min, followed by a single 72°C final extension for 7 min. Samples were visualised on a 1% ethidium bromide-stained agarose gel under UV light and bands were gel extracted, heated at 70°C for 5 min, cooled to 45°C for 10 min, treated with 1 µL GELase (Epicentre Biotechnologies, Madison, WI, USA) and incubated at 45°C for at least 24 h.
The 10-µL cycle sequencing reactions consisted of 1–1.5 µL of Big Dye version 3.1 (Applied Biosystems, Foster City, CA, USA), 2.5–3 µL of Big Dye buffer, 1–6 µM primer, 0.75–2 µL of GELase-treated PCR product and water. Samples were sequenced with PCR primers. The cycle sequencing conditions were as follows: 96°C for 1 min, followed by 25 cycles of 96°C for 10 s, 50°C for 5 s and 60°C for 4 min. Samples were precipitated and sequenced in an 3730 DNA Analyzer (Applied Biosystems), and sequences assembled in Sequencher 4.9 (Gene Codes Corporation, Ann Arbor, MI, USA) and sequences submitted to GenBank (Table 1).
Phylogenetic analyses
Initial BLASTn (Altschul et al. 1997) searches of Myeloconis sequences revealed close matches with Ostropales; consequently, we assembled a dataset (Ostropales dataset) focused on this order, including representatives of most families (Table 1). Lecanoromycetidae taxa were included as outgroup representatives. These initial analyses suggested that Myeloconidaceae was part of or closely related to Porinaceae; therefore, we constructed a second, more focused dataset (Porinaceae+Myeloconidaceae dataset), including representatives of these two families. We sequenced nuLSU and mtSSU from Porinaceae specimens and supplemented these with sequences from GenBank representing a further 16 OTUs in Porinaceae. Additionally, sequences of two Coenogoniaceae taxa were retrieved from GenBank and used as the outgroup (Table 1). Sequences were aligned in Mesquite v. 2.75 (Maddison and Maddison 2010) using a combination of manual and automated (MUSCLE 3.6: Edgar 2004) alignment in MAFFT v. 7.029b (Katoh et al. 2002) using the l-ins-i algorithm. Introns and manually delimited ambiguous regions were removed and the resulting alignment was deposited in TreeBase (Study ID 15391).
For both datasets, a maximum-likelihood (ML) analysis, which was partitioned by locus, was performed in RAxML 7.4.4 (Stamatakis 2006), using the GTRGAMMA model. Support was estimated by utilising 1000 fast-bootstrap pseudoreplicates (Stamatakis et al. 2008). A Bayesian analysis was also performed, using Markov chain Monte Carlo (MCMC) sampling (Larget and Simon 1999) in MrBayes 3.2.1 (Ronquist et al. 2012). We performed a reversible-jump MCMC analysis (Huelsenbeck et al. 2004), partitioning the dataset by gene and employing the time-reversible class of substitution models with a gamma distributed rate heterogeneity. This allowed for exploration of different submodels with the GTR+G model space, and liberated us from a priori model testing. Two parallel analyses were run at a temperature of 0.1 in MrBayes for 30 000 000 generations, with four chains each, sampling every 1000 generations. The program AWTY (Wilgenbusch et al. 2004; Nylander et al. 2008) was used to diagnose convergence between parallel runs by the creation of a bivariate plot of bipartitions. Furthermore, the average standard deviation of split frequencies (Lakner et al. 2008) dropped below 0.01, and the potential scale-reduction factor (Gelman and Rubin 1992) for all parameters was found to approach 1.0. Initial burn-in trees (initial 25%) were discarded for each run and a majority-rule consensus tree was constructed. Relationships were considered supported if they had ML bootstrap support (BS) values of 70 or greater and Bayesian posterior probabilities (PP) of 0.95 or greater. To assess potential conflict among loci, individual ML phylogenies were constructed for each locus as described above. We compared supported clades from the single-locus phylogenies using the python program compat.py 3.0 (Kauff and Lutzoni 2002, 2003); conflict among supported clades was taken as evidence for topological incongruence. All ML and Bayesian analyses were performed in the Cipres Web Portal 3.3 (Miller et al. 2010).
We also evaluated the monophyly of selected morphological and ecological characters used to define Myeloconis and genera in Porinaceae. These included substrate, ascospore septation, the presence or absence of yellow–orange medullary pigments, and the Clathroporina-type thallus and prothallus combination (shiny thallus with shiny, violet–black prothallus). Character states were retrieved from the literature and sequenced specimens and mapped onto the tips of the phylogeny.
Finally, we conducted a Shimodaira–Hasegawa (SH) test (Shimodaira and Hasegawa 1999) to assess whether the monophyly of Porina could be rejected. Using the Ostropales dataset, we conducted ML searches to obtain the best-known tree under the following two topological constraints: (1) monophyletic Porina and monophyletic Myeloconis; and (2) monophyletic Porina (but Myeloconis was left unconstrained). These trees were then compared to the best-known ML tree obtained from the unconstrained analysis using the SH test as implemented in RAxML. Because assumptions for the SH test are frequently violated (Goldman et al. 2000), we also performed 500 replicates of the Swofford–Olsen–Waddell–Hillis (SOWH) test (Swofford et al. 1996) for each constraint using the Sowhat Perl script (Church et al. 2014), RAxML 7.9.5 (Stamatakis 2006) and Seq-Gen 1.3.2x (Rambaut and Grassly 1997).
Results
The final Ostropales alignment consisted of 1816 sites (645 mtSSU, 1171 nuLSU) and conflict was detected among the placement of Fissurina insidiosa, which had a supported placement with the Graphidaceae subfamily Gomphilloideae in the nuLSU analysis, whereas in the mtSSU analysis, it formed a supported relationship with the Graphidaceae subfamily Graphidoideae. We retained F. insidiosa, because we assumed that its position was not germane to the groups of interest, and proceeded with further analyses. The more detailed Porinaceae+Myeloconidaceae dataset consisted of 1117 sites (651 mtSSU, 466 nuLSU), and no conflict was detected among loci. Bayesian analyses of both the Ostropales and Porinaceae+Myeloconidaceae datasets demonstrated that no single substitution model was found to achieve an exceptionally high posterior probability; instead, several GTR+G submodels were sampled. Analysis of the Ostropales dataset confirmed the placement of Myeloconis in this order with strong support (Fig. 2). The phylogenies of the combined Ostropales and Porinaceae+Myeloconidaceae datasets recovered a strongly supported, monophyletic Myeloconis that was embedded within Porinaceae (Figs 2, 3). Analyses of both datasets revealed that Myeloconis formed a strongly supported sister relationship with Porina farinosa (Figs 2, 3). The SH and SOWH tests rejected a topology in which Porina and Myeloconis each formed monophyletic groups, as well one in which monophyly of Porina was enforced (both rejected at P ≤ 0.01).

|

|
Analysis of the Porinaceae+Myeloconidaceae dataset further revealed that the Myeloconis–P. farinosa clade formed a sister group to a strongly supported clade containing the remainder of Porinaceae, including members of Trichothelium and the P. epiphylla (Phylloporina), P. nucula (Porina s.str.), P. dolichophora, P. radiata, P. nitidula and P. rufula groups (Pseudosagedia and Segestria) (Fig. 3). Within this clade, several well supported clades emerged, although support for relationships among these clades was weak (Fig. 3). In addition to relationships recovered in previous studies, our results highlight the non-monophyly of the P. imitatrix group (Clathroporina sensu Harris 1995, here represented by P. exasperatula, P. imitatrix, P. tetracerae), with P. imitatrix grouping with P. alba (P. epiphylla group) with strong support, whereas P. tetracerae grouped with P. karnatakensis and other members of the P. epiphylla group, and P. exasperatula with P. dolichophora. Trichothelium s.lat., including Pseudosagedia (P. atrocoerula, P. nitidula, P. papillifera, P. repanda, P. subnitidula), was recovered as a strongly supported, monophyletic clade, but there was not support for Trichothelium s.str. and Pseudosagedia being reciprocally monophyletic; instead, Trichothelium appears embedded within Pseudosagedia. Our analysis also revealed the lack of support for groups defined by substrate preferences (foliicolous Phylloporina (P. epiphylla and P. radiata groups, the latter not included in the present study)), suggesting that substrate shifts have occurred multiple times within this lineage. Notably, ascospore septation appeared correlated with phylogeny, with taxa forming muriform ascospores (Myeloconis, Porina farinosa) occurring in a clade separate from those with transversely septate ascospores (Fig. 3). Within the clade containing taxa producing transversely septate ascospores, taxa with very long, narrow, tapering, multiseptate ascospores (P. dolichophora, P. exasperatula) separated from those with broader, fusiform, mostly seven-septate ascospores (P. nucula and relatives).
We examined the P. farinosa collections (Fig. 4A–C) to determine whether their anatomy and morphology were more consistent with that of Porina or Myeloconis. Ascomatal sections revealed a narrow, pale yellow, prosoplectenchymatous, basally closed exciple, covered by a thick thalline, basally expanded layer resembling an involucrellum; that layer was composed of an inner, dark-pigmented layer adjacent to the exciple, a thick crystallostratum, a thin photobiont layer, and a thin, hyaline, corticiform layer. Close to the ostiole, the covering layer lacked a photobiont layer and crystals, as well as the inner, dark-pigmented layer, and instead was formed by an orange to dark brown (bordering the ostiole) tissue covered by a hyaline, corticiform layer, which in turn covered the narrow, true exciple reaching up to the ostiole. In addition, we observed periphysoids near the ostiolar region, and ellipsoid (to fusiform), distinctly muriform ascospores with thickened walls. McCarthy (1995) described the presence of an involucrellum for species of Porina (Clathroporina) with muriform ascospores; however, his illustrations (e.g. of C. eminentior and C. exocha) depicted a covering thalline layer, including algae and crystal clusters, similar to what we found in the two specimens here identified as P. farinosa. To confirm this, we also investigated the sequenced sample of P. nucula and found its perithecial sections very similar to those of the sequenced P. farinosa.
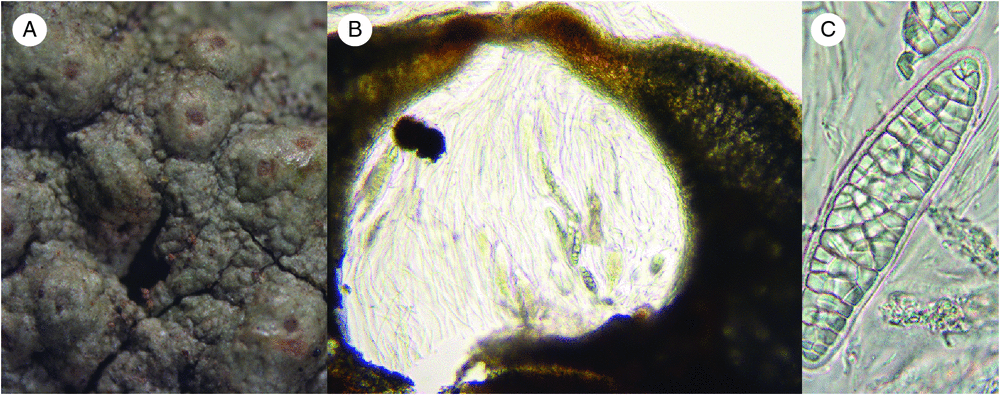
|
Within Myeloconis, our sampling included three of the four known species. Representatives of M. fecunda formed a strongly supported, monophyletic group. This taxon is characterised by the presence of myeloconone B, and ascospores 17–26 μm in length. Our data also showed a strongly supported, monophyletic M. erumpens, which is characterised by its large ascospores (145–207 μm long) and the presence of myeloconone A as a major component. However, this species is embedded within an unsupported, paraphyletic M. guyanensis, a taxon characterised by small ascospores (22–34 μm long) and the presence of myeloconone A as a major component.
Discussion
The present study confirmed that Myeloconidaceae is not related to Trypetheliaceae but belongs in Ostropales close to Porinaceae, as suggested by previous authors (McCarthy and Elix 1996; McCarthy 2001a, 2001b, 2003). However, instead of forming a separate family, our results suggested it is nested within Porinaceae. This poses a challenge to the currently accepted classification, because retaining Myeloconidaceae as a separate family would result in a paraphyletic Porinaceae. The critical taxon appears to be Porina farinosa (Fig. 4) which, although agreeing with P. nucula (the type of Porina) in all features except the muriform ascospores, forms a strongly supported sister relationship with Myeloconis (Fig. 3). The topology in Fig. 3 permits the retention of Myeloconidaceae with the inclusion of P. farinosa; however, we argue against this because the distinction between the two families, (perithecial anatomy and medullary chemistry) would be erased by the inclusion of P. farinosa in Myeloconidaceae. The only character in the present taxon sampling shared exclusively between Myeloconis and P. farinosa is the muriform ascospores, but their shape is very different (long-fusiform v. ellipsoid) and the ascospores of P. farinosa are more consistent with those of species currently placed in Porina s.lat. (McCarthy 1995). We, therefore, see no alternative to reducing Myeloconidaceae to synonymy with Porinaceae (see below).
Revision of the type material of P. farinosa and its synonyms (McCarthy 1995) revealed that possibly three different taxa are involved. The types of P. farinosa C.Knight, described from Australia, and Thelenella elaeophthalma Vain., described from the Caribbean, have thin, distinctly verrucose thalli and prominent, hemispherical to wart-shaped perithecia. In contrast, the type of Clathroporina superans Müll. Arg. from Africa has a non-verrucose thallus and prominent perithecia, whereas the types of Thelenella turgidula Vain. and T. irregularis Vain., both from the Caribbean (Vainio 1896, 1915, 1923), are more similar to Clathroporina eminentior in their slightly glossy thallus and largely immersed, lens-shaped perithecia. Perithecial anatomy and ascospore details in the types of P. farinosa and Thelenella elaeophthalma agree with the material sequenced here; hence, we are confident with our identification of this material as P. farinosa. In any case, the correct identification of this material does not affect the finding that its thallus and perithecial morphology and anatomy, as well as its chemistry, conforms to that of P. nucula and is different from Myeloconis, with which it clusters with strong support.
Generic delimitation within Porinaceae has long been debated (Santesson 1952; Hafellner and Kalb 1995; Harris 1995; Malcolm and Vězda 1995; McCarthy and Malcolm 1997; Lücking 1998, 2004, 2008), with none of the proposed classification schemes being supported by molecular data (Baloch and Grube 2006). Presently, Porina and Trichothelium are universally accepted in variable definitions, whereas the recognition of additional genera, such as Clathroporina, Polycornus, Pseudosagedia, Segestria and Zamenhofia, is disputed (McCarthy and Malcolm 1997; Lücking 1998, 2004, 2008; Hafellner and Türk 2001; Aptroot 2002; McCarthy 2003; Santesson et al. 2004; Harris 2005; Galloway 2007; Orange et al. 2009; Lumbsch and Huhndorf 2010). Our topology largely agrees with that of Baloch and Grube (2006), confirming the non-monophyly of several proposed segregate genera of Porina. With our increased taxon sampling, we were further able to confirm the non-monophyly of the P. imitatrix–P. eminentior group (Clathroporina). The largely unsupported backbone of our phylogeny would permit the possible recognition of the following five genus-level taxa: Myeloconis, the P. farinosa group, Segestria (species with red-walled perithecia lacking a crystallostratum), Porina s.str. (species with red-walled perithecia and crystallostratum, including Clathroporina and Phylloporina) and Trichothelium s.lat. (species with black-walled perithecia lacking a crystallostratum, including Pseudosagedia and Zamenhofia). To clarify this situation, further species of Segestria and Clathroporina must be sequenced, particularly the type species of the latter, C. eminentior. As Trichothelium appears embedded within Porina (Fig. 2; Baloch and Grube 2006), further work is needed to address its continued recognition or synonymisation.
In contrast to the non-monophyly observed for most other species groups or generic segregates in Porinaceae, specifically in the taxa with a crystallostratum, our data support the monophyly of Myeloconis. However, further work is needed to critically test species delimitation within this genus. Except for the medullary pigments, species of Myeloconis fit well within the family definition and are morphologically reminiscent of species currently placed in Clathroporina sensu Harris (1995). Although the medullary chemistry is unique for Myeloconis, it does not necessarily warrant separation at the family level, because similar variation is, for example, known from Graphidaceae, where species with pigmented medulla are concentrated in a single genus, Ocellularia s.str. (Rivas Plata et al. 2012, 2013). Therefore, synonymising Myeloconidaceae with Porinaceae and placing Myeloconis in the latter family, on the basis of molecular data, do not unduly conflict with morphological or anatomical characters. Whether increased sampling will uphold the monophyly of Myeloconis remains to be seen, but given these results, we argue for the retention of the genus Myeloconis while genera are re-delimited in Porinaceae. The situation is complicated by the close relationship of Myeloconis with P. farinosa, a taxon that shares the general morphology and anatomy with P. nucula and related species (McCarthy 1995, 2001c). If Myeloconis is retained in its current sense, then P. farinosa and other species clustering here would have to be placed in a genus different from Porina s.str. The only argument for this would be the muriform ascospores, a character otherwise shared with Myeloconis. Therefore, expanded sampling of other species with muriform ascospores, especially Clathroporina eminentior, is required to resolve this issue. Future work should also attempt to determine the position of the enigmatic genus Amphorothecium. This genus has been described as sharing many features with Myeloconis, and also produces large, thick-walled ascospores (McCarthy et al. 2001). Its apically thin-walled asci suggest it may occupy a position close to Porinaceae, Coenogoniaceae or Gyalectaceae in Ostropales.
Revised taxonomy
Porinaceae Rchb. [as ‘Porineae’], Consp. Regn. Veg.: 20 (1828)
Type. Porina Ach. [nom. cons.; conserved type: P. nucula Müll. Arg.].
Type: Myeloconis P. M. McCarthy & Elix.
Acknowledgements
This study was supported by a grant from the National Science Foundation: Neotropical Epiphytic Microlichens – An Innovative Inventory of a Highly Diverse yet Little Known Group of Symbiotic Organisms (DEB 0715660 to The Field Museum; PI R. Lücking) as well as the University of Chicago (Committee on Evolutionary Biology), American Society of Plant Taxonomists, Botanical Society of America and a Brown Family Fellowship through the Field Museum (all to M. P. Nelsen). In addition, the Caterpillar company provided funds for sequencing material from Panama. Collecting trips to the Amazon (Rondonia State) were supported by a grant from CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico (Sisbiota Processo 563342/2010-2) to M. E. S. Cáceres. The curators in G and TUR, Philippe Clerc and Seppo Huhtinen, provided valuable information and images on types of synonyms of Porina farinosa, and Patrick McCarthy is thanked for taxonomic advice. Leon Perrie and three anonymous reviewers are thanked for comments that improved the manuscript.
References
Altschul SF, Madden TL, Schäffer ZJ, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI–BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402.| Gapped BLAST and PSI–BLAST: a new generation of protein database search programs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvFyhu7w%3D&md5=76caa785641f1f84fc284efda12da3f4CAS | 9254694PubMed |
Aptroot A (2002) Porina. In ‘Lichen of the Greater Sonoran Desert region. Vol. 1’. (Eds TH Nash III, BD Ryan, C Gries, F Bungartz) pp. 402–405. (Lichens Unlimited: Tempe, AZ)
Aptroot A, Sipman HJM (1993) Trichotheliaceae (lichens). In ‘Flora of the Guianas Series E: Fungi and Lichens, Fascicle 2’. (Ed ARA Gorts-van Rijn) pp. 1–57. (Koeltz Scientific Books: Konigstein, Germany)
Baloch E, Grube M (2006) Evolution and phylogenetic relationships within Porinaceae (Ostropomycetidae), focusing on foliicolous species. Mycological Research 110, 125–136.
| Evolution and phylogenetic relationships within Porinaceae (Ostropomycetidae), focusing on foliicolous species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsFOrtr0%3D&md5=7199e01485109a2f2bae8c60ceeb5334CAS | 16376532PubMed |
Baloch E, Lücking R, Lumbsch HT, Wedin M (2010) Major clades and phylogenetic relationships between lichenized and non-lichenized lineages in Ostropales (Ascomycota: Lecanoromycetes). Taxon 59, 1483–1494.
Bhattacharya D, Lutzoni F, Reeb V, Simon D, Nason J, Fernandez F (2000) Widespread occurrence of spliceosomal introns in the rDNA genes of ascomycetes. Molecular Biology and Evolution 17, 1971–1984.
| Widespread occurrence of spliceosomal introns in the rDNA genes of ascomycetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXptVWrtro%3D&md5=aaee53871ab17f87ac98bec6458af76fCAS | 11110913PubMed |
Church SH, Ryan JF, Dunn C (2014) Sowhat. GitHub repository. Available at https://github.com/josephryan/sowh.pl [Verified 25 February 2014]
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797.
| MUSCLE: multiple sequence alignment with high accuracy and high throughput.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisF2ks7w%3D&md5=cb5c1add9aa11a9a28fb7566d4607ea8CAS | 15034147PubMed |
Ernst-Russell MA, Chai CLL, Elix JA, McCarthy PM (2000) Myeloconone A2, a new phenalenone from the lichen Myeloconis erumpens. Australian Journal of Chemistry 53, 1011–1013.
| Myeloconone A2, a new phenalenone from the lichen Myeloconis erumpens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktlSitrs%3D&md5=9c5a042d44e6d6a56c930dc1c439fc26CAS |
Galloway DJ (2007) ‘Flora of New Zealand: Lichens’, revised 2nd edn. (Manaaki Whenua Press: Lincoln, New Zealand)
Gelman A, Rubin D (1992) Inference from iterative simulation using multiple sequences. Statistical Science 7, 457–472.
| Inference from iterative simulation using multiple sequences.Crossref | GoogleScholarGoogle Scholar |
Goldman N, Anderson JP, Rodrigo AG (2000) Likelihood-based tests of topologies in phylogenetics. Systematic Biology 49, 652–670.
| Likelihood-based tests of topologies in phylogenetics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38zntVOjtw%3D%3D&md5=d6e4c1d0de9cb692c3e90fd92438e9b4CAS | 12116432PubMed |
Grube M, Baloch E, Lumbsch HT (2004) The phylogeny of Porinaceae (Ostropomycetidae) suggests a neotenic origin of perithecia in Lecanoromycetes. Mycological Research 108, 1111–1118.
| The phylogeny of Porinaceae (Ostropomycetidae) suggests a neotenic origin of perithecia in Lecanoromycetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXot1Clu7w%3D&md5=28612ce5fd0c9d9b66ba37781eadde20CAS | 15535063PubMed |
Hafellner J, Kalb K (1995) Studies in Trichotheliales ordo novus. Bibliotheca Lichenologica 57, 161–186.
Hafellner J, Türk R (2001) Die lichenisierten Pilze Österreichs – eine Checkliste der bisher nachgewiesenen Arten mit Verbretungsangaben. Stapfia 76, 1–167.
Harris RC (1995) ‘More Florida Lichens: Including the 10¢ Tour of the Pyrenolichens.’ (R. C. Harris: New York)
Harris RC (2005) Some name changes in Porina s.lat. Opuscula Philolichenum 2, 15–16.
Henssen A, Jahns HM (1974) ‘Lichenes.’ (Georg Thieme Verlag: Stuttgart, Germany)
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Lumbsch HT, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Beny GL, Castlebury LA, Crous PW, Dai Y-C, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson K-H, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo J-M, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüßler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao Y-J, Zhang N (2007) A higher-level classification of the fungi. Mycological Research 111, 509–547.
| A higher-level classification of the fungi.Crossref | GoogleScholarGoogle Scholar | 17572334PubMed |
Huelsenbeck JP, Larget B, Alfaro ME (2004) Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Molecular Biology and Evolution 21, 1123–1133.
| Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVymu70%3D&md5=93ee1aa7feda5f044947a8e9e4ae0493CAS | 15034130PubMed |
Janex-Favre MC (1971) Recherches sur l’ontogenie, l’organisation et les asques de quelques pyrenolichens. Revue Bryologique et Lichénologique 37, 421–469.
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066.
| MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlslOqu7s%3D&md5=ba90dc9439a36328ead61c836187a47fCAS | 12136088PubMed |
Kauff F, Lutzoni F (2002) Phylogeny of the Gyalectales and Ostropales (Ascomycota: Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution 25, 138–156.
| Phylogeny of the Gyalectales and Ostropales (Ascomycota: Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVeru7c%3D&md5=283ee10175964debe7b99fae9c84ebe9CAS | 12383757PubMed |
Kauff F, Lutzoni F (2003) Compat.py – a program to detect topological conflict between supported clades in phylogenetic trees. Available at http://www.lutzonilab.net/pages/download.shtml [Verified 25 February 2014].
Kroken S, Taylor JW (2001) A gene genealogical approach to recognize phylogenetic species boundaries in the lichenized fungus Letharia. Mycologia 93, 38–53.
| A gene genealogical approach to recognize phylogenetic species boundaries in the lichenized fungus Letharia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktVSgtro%3D&md5=ba78a72fafca4a1f7c6fa46df71efe1aCAS |
Lakner C, van der Mark P, Huelsenbeck J, Larget B, Ronquist F (2008) Efficiency of Markov chain Monte Carlo tree proposals in Bayesian phylogenetics. Systematic Biology 57, 86–103.
| Efficiency of Markov chain Monte Carlo tree proposals in Bayesian phylogenetics.Crossref | GoogleScholarGoogle Scholar | 18278678PubMed |
Larget B, Simon DL (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16, 750–759.
| Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjslGitb8%3D&md5=4d882594f0872de6d3e0f9daf914aad5CAS |
Lücking R (1998) Additions and corrections to the knowledge of the foliicolous lichen flora of Costa Rica. The genus Trichothelium (lichenized Ascomycetes: Trichotheliaceae). Nova Hedwigia 66, 375–417.
Lücking R (2004) A revised key to foliicolous Porinaceae (Ascomycota: Trichotheliales). In ‘Bibliotheca Lichenologica’, Vol. 88. pp. 409–42. (The New York Botanical Garden Press: Bronx, NY)
Lücking R (2008) Foliicolous lichenized fungi. Flora Neotropica Monograph 103, 1–867.
Lumbsch HT, Huhndorf SM (2010) Myconet Volume 14. Part one. Outline of Ascomycota – 2009. Fieldiana Life and Earth Sciences 1, 1–42.
Maddison WP, Maddison DR (2010) Mesquite: a modular system for evolutionary analysis Version 2.73. Available at http://mesquiteproject.org [Verified 25 February 2014]
Malcolm WM, Vězda A (1995) New foliicolous lichens from New Zealand 1. Folia Geobotanica et Phytotaxonomica 30, 91–96.
| New foliicolous lichens from New Zealand 1.Crossref | GoogleScholarGoogle Scholar |
Mangold A, Martin MP, Lücking R, Lumbsch HT (2008) Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota: Ostropales). Taxon 57, 476–486.
McCarthy PM (1993) Saxicolous species of Porina Müll Arg. (Trichotheliaceae) in the southern hemisphere. Bibliotheca Lichenologica 52, 1–134.
McCarthy PM (1995) A reappraisal of Clathroporina Müll. Arg. (Trichotheliaceae). Lichenologist 27, 321–350.
McCarthy PM (2001a) Myeloconaceae. In ‘Flora of Australia’, Vol. 58A. pp. 104–105. (CSIRO Publishing and Australian Biological Resources Study: Melbourne)
McCarthy PM (2001b) Appendix: Myeloconaceae. In ‘Flora of Australia’, Vol. 58A. pp. 227. (CSIRO Publishing and Australian Biological Resources Study: Melbourne)
McCarthy PM (2001c) Trichotheliaceae. In ‘Flora of Australia’, Vol. 58A. pp. 105–157. (CSIRO Publishing and Australian Biological Resources Study: Melbourne)
McCarthy PM (2003) Catalogue of the lichen family Porinaceae. Bibliotheca Lichenologica 87, 1–164.
McCarthy PM (2013) Catalogue of Porinaceae. Australian biological resources study, Canberra. Version 4 December 2013. Available http://www.anbg.gov.au/abrs/lichenlist/PORINACEAE.html [Verified 25 February 2014]
McCarthy PM, Elix JA (1996) Myeloconis, a new genus of pyrenocarpous lichens from the tropics. Lichenologist 28, 401–414.
McCarthy PM, Kantvilas G (2000) A new bryophilous Porina from Tasmania, and notes on the diversity, ecological groups and biogeographical affinities of the Tasmanian Trichotheliaceae. Lichenologist 32, 247–256.
| A new bryophilous Porina from Tasmania, and notes on the diversity, ecological groups and biogeographical affinities of the Tasmanian Trichotheliaceae.Crossref | GoogleScholarGoogle Scholar |
McCarthy PM, Malcolm WM (1997) The genera of Trichotheliaceae. Lichenologist 29, 1–8.
McCarthy PM, Kantvilas G, Elix JA (2001) Amphorothecium, a new pyrenocarpous lichen genus from New South Wales, Australia. Lichenologist 33, 291–296.
| Amphorothecium, a new pyrenocarpous lichen genus from New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In ‘Proceedings of the Gateway Computing Environments Workshop (GCE)’, 14 November 2010, New Orleans, LA. pp. 1–8. (Institute of Electrical and Electronics Engineers)
Nelsen MP, Rivas Plata E, Andrew CJ, Lücking R, Lumbsch HT (2011a) Phylogenetic diversity of trentepohlialean algae associated with lichen-forming fungi. Journal of Phycology 47, 282–290.
| Phylogenetic diversity of trentepohlialean algae associated with lichen-forming fungi.Crossref | GoogleScholarGoogle Scholar |
Nelsen MP, Lücking R, Mbatchou JS, Andrew CJ, Spielmann AA, Lumbsch HT (2011b) New insights into relationships of lichen-forming Dothideomycetes. Fungal Diversity 51, 155–162.
| New insights into relationships of lichen-forming Dothideomycetes.Crossref | GoogleScholarGoogle Scholar |
Nelsen MP, Lücking R, Andrew CJ, Rivas Plata E, Chaves JL, Cáceres MES, Ventura N (2012) Dismantling Herpothallon: Herpothallon antillarum (Arthoniomycetes: Arthoniaceae) is a member of the genus Diorygma (Lecanoromycetes: Graphidaceae). The Bryologist 115, 313–321.
| Dismantling Herpothallon: Herpothallon antillarum (Arthoniomycetes: Arthoniaceae) is a member of the genus Diorygma (Lecanoromycetes: Graphidaceae).Crossref | GoogleScholarGoogle Scholar |
Nylander JA, Wilgenbusch WC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24, 581–583.
| AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVKis7g%3D&md5=bc5ce04ccc0e11394dccf9614316c76dCAS | 17766271PubMed |
Orange A, Purvis OW, James PW (2009) Porina. In ‘The lichens of Great Britain and Ireland’. (Eds CW Smith, A Aptroot, BJ Coppins, A Fletcher, OL Gilbert, PW James, PA Wolseley) pp. 729–737. (British Lichen Society: London)
Rambaut A, Grassly NC (1997) Seq-Gen: an application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic trees. Bioinformatics 13, 235–238.
| Seq-Gen: an application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic trees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksVGntL4%3D&md5=51c6bf83949435b1399ae13634148f8bCAS |
Rivas Plata E, Lumbsch HT, Lücking R (2012) A new classification for the lichen family Graphidaceae s.lat. (Ascomycota: Lecanoromycetes: Ostropales). Fungal Diversity 52, 107–121.
| A new classification for the lichen family Graphidaceae s.lat. (Ascomycota: Lecanoromycetes: Ostropales).Crossref | GoogleScholarGoogle Scholar |
Rivas Plata E, Parnmen S, Staiger B, Mangold A, Frisch A, Weerakoon G, Hernández MJE, Cáceres ME, Kalb K, Sipman HJM, Common RS, Nelsen MP, Lücking R, Lumbsch HT (2013) A molecular phylogeny of Graphidaceae (Ascomycota, Lecanoromycetes, Ostropales) including 428 species. MycoKeys 6, 55–94.
| A molecular phylogeny of Graphidaceae (Ascomycota, Lecanoromycetes, Ostropales) including 428 species.Crossref | GoogleScholarGoogle Scholar |
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539–542.
| MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space.Crossref | GoogleScholarGoogle Scholar | 22357727PubMed |
Santesson R (1952) Foliicolous lichens I. A revision of the taxonomy of the obligately foliicolous, lichenized fungi. Symbolae Botanicae Upsalienses 12, 1–590.
Santesson R, Moberg R, Nordin A, Tønsberg T, Vitikainen O (2004) ‘Lichen-forming and lichenicolous fungi of Fennoscandia.’ (Museum of Evolution, Uppsala University: Uppsala, Sweden)
Shimodaira H, Hasegawa M (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution 16, 1114–1116.
| Multiple comparisons of log-likelihoods with applications to phylogenetic inference.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltVyksrg%3D&md5=badc5249ae6db1adb40ad89917174533CAS |
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690.
| RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKlsbfI&md5=99af42d78c661258cd286bb3f9d2e18dCAS | 16928733PubMed |
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57, 758–771.
| A rapid bootstrap algorithm for the RAxML web servers.Crossref | GoogleScholarGoogle Scholar | 18853362PubMed |
Swinscow TDV (1962) Pyrenocarpous lichens. 3. The genus Porina in the British Isles. Lichenologist 2, 6–56.
| Pyrenocarpous lichens. 3. The genus Porina in the British Isles.Crossref | GoogleScholarGoogle Scholar |
Swofford DL, Olsen GJ, Waddell PJ, Hillis DM (1996) Phylogenetic inference. In ‘Molecular systematics’. (Eds DM Hillis, C Moritz, BK Mable) pp. 407–514. (Sinauer: Sunderland, MA)
Vainio EA (1896) Lichenes Antillarum a W. R. Elliott collecti. Journal of Botany British and Foreign 34, 292–297.
Vainio EA (1915) Additamenta ad lichenographiam Antillarum illustrandum. Annales Academiae Scientiarum Fennicae, Series A 6, 1–226.
Vainio EA (1923) Lichens in insula Trinidad a professore R. Thaxter collecti. Proceedings of the American Academy of Arts and Sciences 58, 131–147.
| Lichens in insula Trinidad a professore R. Thaxter collecti.Crossref | GoogleScholarGoogle Scholar |
Vězda A (1968) Taxonomische revision der Gattung Thelopsis Nyl. (lichenisierte Fungi). Folia Geobotanica et Phytotaxonomica 3, 363–406.
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172, 4238–4246.
Wilgenbusch JC, Warren DL, Swofford DL (2004) AWTY: a system for graphical exploration of MCMC convergence in Bayesian phylogenetic inference. Available at http://ceb.csit.fsu.edu/awty [Verified 25 February 2014]
Zoller S, Scheidegger C, Sperisen C (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31, 511–516.



