Dating the emergence of truffle-like fungi in Australia, by using an augmented meta-analysis
Elizabeth M. Sheedy A F , Martin Ryberg B , Teresa Lebel C , Tom W. May C , Neale L. Bougher D and P. Brandon Matheny EA Department of Botany, National Museum of Nature and Science, 4-1-1 Amakubo, Tsukuba, Ibaraki 305-0005, Japan.
B Department of Organismal Biology, Uppsala University, Norbyvägen 18D, SE-75236 Uppsala, Sweden.
C Royal Botanic Gardens Victoria, Birdwood Avenue, South Yarra, Vic. 3141, Australia.
D Department of Parks and Wildlife, Science and Conservation Division, Western Australian Herbarium, Bentley Delivery Centre, Kensington, WA 6151, Australia.
E Ecology and Evolutionary Biology, 332 Hesler, University of Tennessee, Knoxville, TN 37996-1610, USA.
F Corresponding author. Email: biz.sheedy@gmail.com
Australian Systematic Botany 29(5) 284-302 https://doi.org/10.1071/SB16025
Submitted: 10 June 2016 Accepted: 14 October 2016 Published: 22 December 2016
Abstract
Australia supports a high diversity of sequestrate (truffle-like) macrofungi. This has long been thought to be related to the predominantly or seasonally dry climate. The present study posits that if aridity were a key factor in the evolution of sequestrate fruit-bodies, most sequestrate species would have emerged in Australia only after it began to aridify, which occurred post-separation with Antarctica (c. 32 million years ago). Focusing on the high phylogenetic diversity of sequestrate taxa in the Agaricomycetes in Australia, dates of sequestrate nodes were compiled directly from published phylogenies (four lineages) or created using sequences available on GenBank that were processed in BEAST using a secondary calibration method (nine lineages). Although the morphologically diverse Hysterangiales was found to be the first group to become sequestrate, c. 83 million years ago, overall sequestration in Australia occurred more recently. Models were created and compared and support was found for an increased rate of sequestration in Australia at some point between 34 and 13 million years ago (during the Oligocene and Miocene). Although the rate of sequestration is shown to have increased in Australia after separation from Antarctica, the timing also overlaps with the radiation of potential mycorrhizal plant associates, and the emergence of specialised mycophagous marsupials. Although aridification is evidently not the sole driver of sequestration, it is still likely to have had a major influence on the diversity of sequestrate fungi in Australia. Comparisons with other regions of high sequestrate diversity will be informative.
Additional keywords: aridification, Agaricomycetes, sequestrate, Basidiomycota, Cortinariaceae, Russulaceae.
Introduction
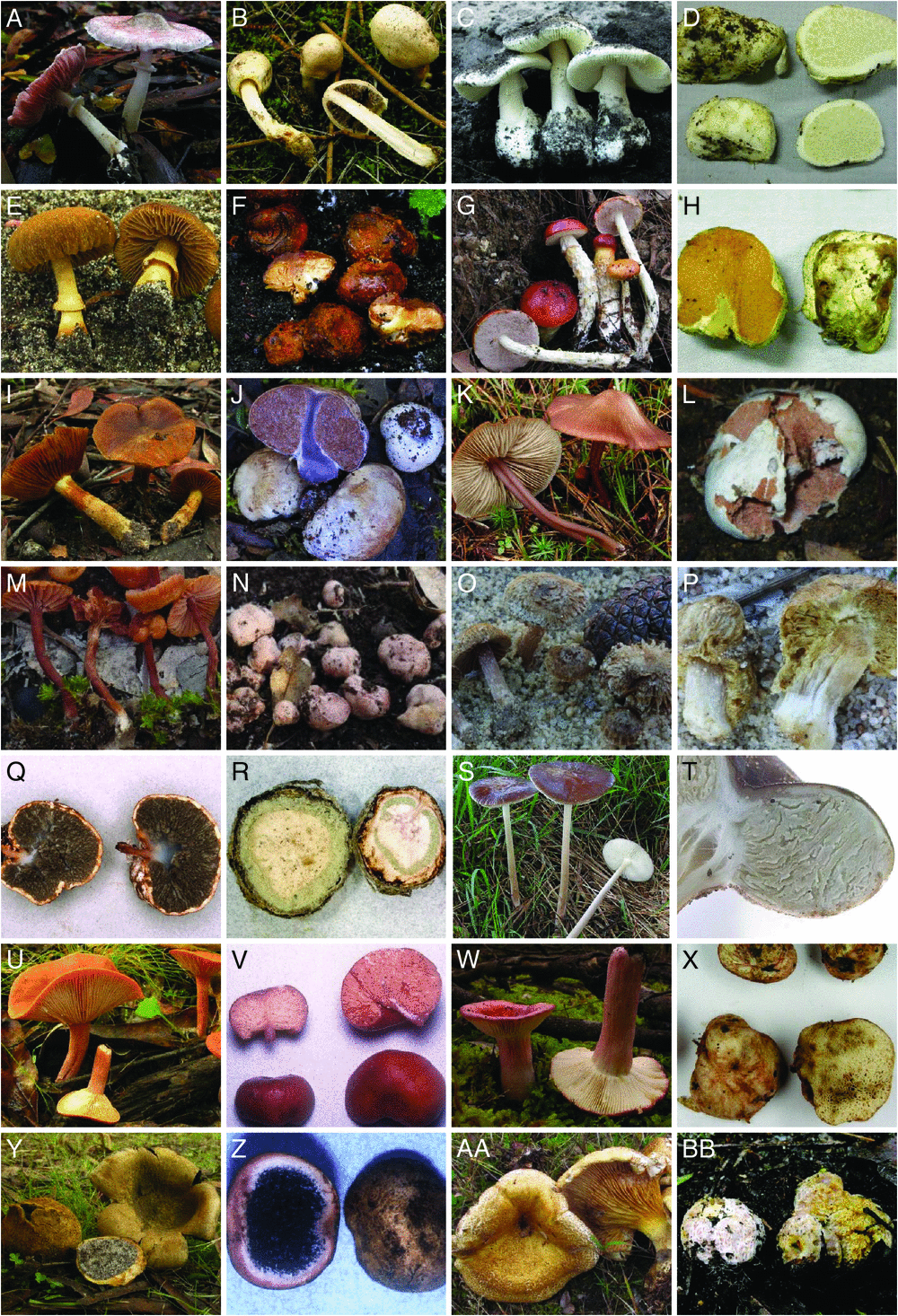
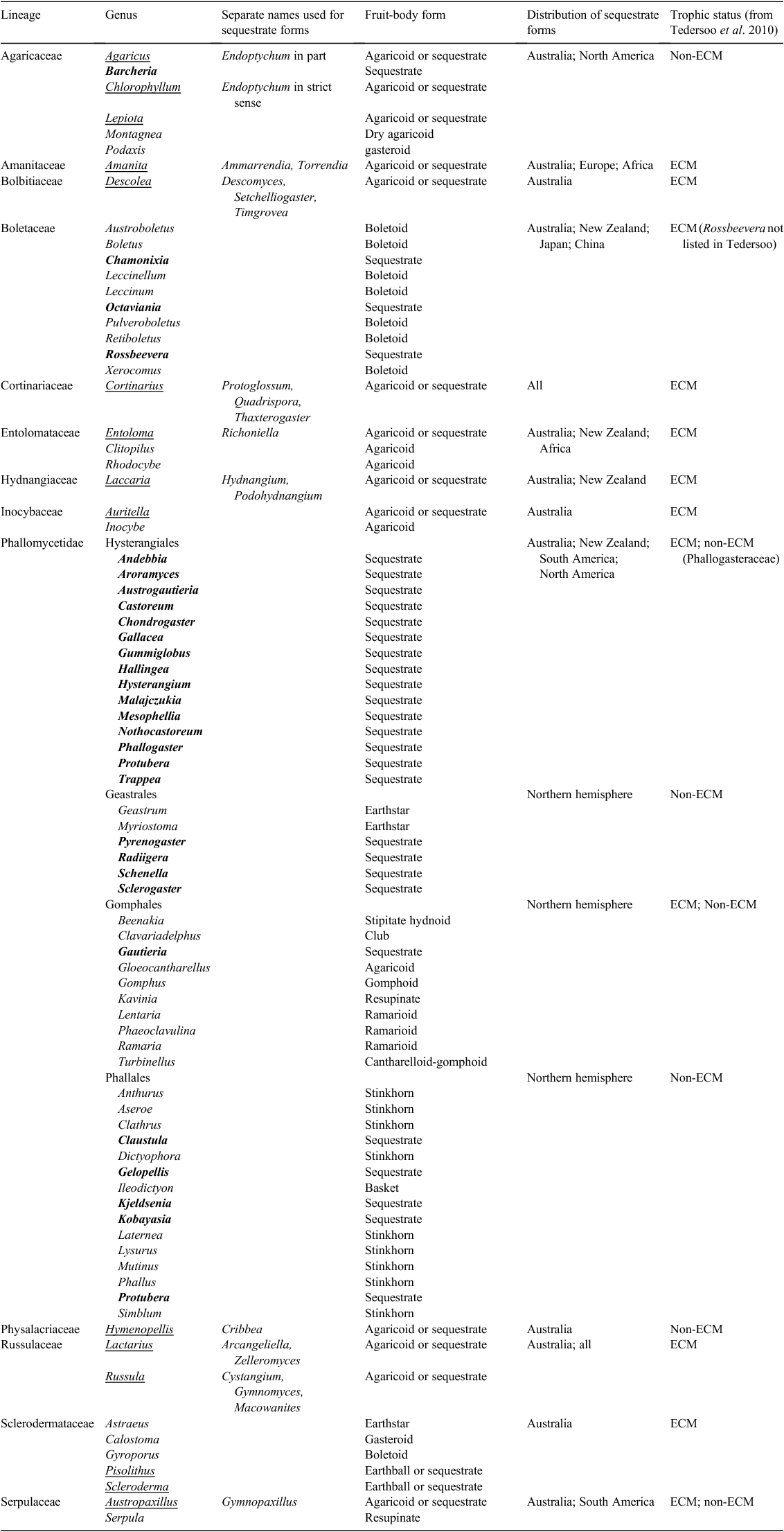
Many evolutionary lineages of fungi that mainly consist of species that produce a certain type of fruit-body morphology (such as a mushroom) also contain some species that produce sequestrate fruit-bodies. The term ‘sequestrate’ encompasses a highly polyphyletic assemblage of macroscopic morphologies where the fruit-body may be highly reduced, ‘truffle-like’ (usually fruiting below ground, termed hypogeous, or occasionally emergent), or intermediate in appearance, ‘secotioid’ (typically emergent), but nearly always with an enclosed hymenium (fertile spore producing layer) at maturity, and spores that are not actively discharged from spore-producing structures such as asci or basidia (Kendrick 1992). This definition of sequestrate is broad and includes taxa from the following three phyla: Ascomycota, Basidiomycota and Glomeromycota and the subphylum Mucoromycotina. Australia contains a high diversity of sequestrate fungi (Bougher and Lebel 2001) with representatives of all three phyla including numerous endemic species, some endemic genera and even an endemic family (Mesophelliaceae). The greatest diversity of sequestrate fungi in Australia is within the Agaricomycetes (Basidiomycota), with ~250 described species distributed in the Phallomycetidae, Russulales, Boletales and Agaricales (see Fig. 1 for examples). The timing of emergence of these sequestrate fruit-body forms in Australia is the focus of the present study (Table 1).
Historically, sequestrate fungi have been grouped together artificially into unique genera and even higher-level taxa based on gross morphology, even though phylogenetic links to non-sequestrate fungi such as mushrooms (with agaricoid fruit-bodies) have long been recognised (e.g. Singer 1951; Singer and Smith 1959; Thiers 1984; Albee-Scott 2007). It is now clear from well supported molecular phylogenies that many genera established for sequestrate fungi are nested within otherwise non-sequestrate (agaricoid, boletoid) genera; indeed, sequestrate taxa are often polyphyletic within these genera (e.g. Peintner et al. 2001; Lebel and Syme 2012). In contrast, there are also examples of sequestrate genera that are monophyletic in lineages such as the Hysterangiales and the Boletaceae (Hosaka et al. 2006; Lebel et al. 2012). Some families have both independent sequestrate genera and genera that contain mixtures of non-sequestrate and sequestrate taxa, such as in the Agaricaceae, where the sequestrate Endoptychum has been subsumed within Chlorophyllum, Agaricus and Macrolepiota, but the monotypic Barcheria is an independent sequestrate genus (Lebel and Syme 2012). Similarly, within the Sclerodermataceae, there are sequestrate taxa within the puffball genera Pisolithus and Scleroderma, but also a wholly sequestrate genus (with two species) Horakiella (Trappe et al. 2010).
For most sequestrate species, this morphology is observed to be stable across collections; however, a few species show considerable morphological plasticity. For example, the sequestrate Hydnangium sublamellatum can produce highly variable fruit-body morphologies, including agaricoid-like forms, although all forms lack active spore discharge (Bougher et al. 1993). Conversely, agaricoid species may, rarely, form sequestrate fruit-bodies under certain conditions (Watling and Martín 2003; Braaten et al. 2014) or in culture (Watling 1971). The stimulus that creates an adaptive benefit for agaricoid ‘mushrooms’ and other forms to evolve the sequestrate form is still unknown, but was originally thought to be related to a dry environment, including mountainous regions where rain may be seasonally absent (Thiers 1984). In these environments, spores that are enclosed or stay beneath the soil surface would be better protected from desiccation and so survive or remain competitive. This hypothesis is supported by the high diversity of sequestrate fungi in the dry continent of Australia (Bougher and Lebel 2001; Danks et al. 2010; Trappe et al. 2010; Castellano et al. 2011; Lebel and Syme 2012; Lebel et al. 2015) as well as seasonally dry western North America (Fogel and States 2001, 2002; Trappe et al. 2009). Alternatively, the stimulus may be related to an association with plant hosts because most sequestrate taxa are ectomycorrhizal (Tedersoo et al. 2010), and distributions and diversity of sequestrate fungi are thought to be driven more generally by plant host availability and diversity (Jumpponen et al. 2004; Bonito et al. 2013). In addition to the dependence of ectomycorrhizal fungi on their plant hosts, sequestrate fungi also rely on animals (insects, reptiles, birds and mammals) to disperse their spores (Claridge and May 1994; Vernes 2010).
Following the separation of Australia from Antarctica (c. 32 million years ago; Lawver and Gahagan 2003), there was an increase in selective pressures that may have favoured sequestration. The process of aridification began during this time, although the first major step towards aridification in Australia occurred in the mid-Miocene (c. 15 million years ago; Martin 2006; Byrne et al. 2008; Crisp and Cook 2013). Prior to this process, the early to mid-Paleogene climate in Australia was relatively wet and the continent supported tropical to temperate rainforest assemblages (Fujioka and Chappell 2010). Radiation of iconic sclerophyllous flora of Australia, such as eucalypts and casuarinas, occurred between 25 and 10 million years ago (Crisp et al. 2004) as the country became increasingly arid. This floristic diversification potentially promoted sequestration through provision of ectomycorrhizal hosts in the dry or seasonally dry habitats thought to benefit sequestrate morphologies. The floristic changes that occurred during the mid-Miocene, including the expansion of sclerophyll forest and grasslands, enabled the radiation of terrestrial marsupials such as kangaroos and wallabies and, thus, improved the spore-dispersal potential for sequestrate fungi (Meredith et al. 2009). Additionally, marsupials highly specialised for mycophagy, such as potoroids, and which currently consume sequestrate fungi in particular, emerged c. 16 million years ago (Claridge and May 1994; Meredith et al. 2009; Vernes 2010).
Here, we test the hypothesis that the emergence of 13 diverse lineages of sequestrate Agaricomycetes in Australia corresponded with Miocene aridification following separation of Australia from Antarctica. We apply molecular dating techniques in a meta-analysis of available phylogenies, to determine when sequestrate fungi emerged in Australia. Dates of sequestration are analysed across phylogenetic groups. The timing of emergence is discussed with respect to different fruit-body morphologies, dates of continental aridification and diversification dates of plant associates and potential spore dispersers. We also use model-based ancestral-state reconstruction to test whether there has been a change in the rate of sequestration in Austral lineages corresponding to the aridification of Australia.
Materials and methods
Fruit-body form
Sequestrate fungi are defined as having fruit-bodies that are usually hypogeal (below ground), occasionally emergent, with a hymenium that remains enclosed and spores that are passively discharged. This definition excludes gasteroid fungi that are often epigeous (fruit above ground) and have a powdery gleba (spore mass) and some form of dehiscence of the peridium (outer covering), such as where a stoma (opening) exists, that facilitates passive spore dispersal (e.g. Calostoma, Lycoperdon, Bovista and Geastrum) or where the peridium falls away at maturity (Battarrea, Podaxis) or largely erodes (Calvatia). Gasteroid fungi occupy an ecological niche different from that of sequestrate fungi and so were beyond the scope of the present study. In Pisolithus and Scleroderma, most species are gasteroid with epigeal (above ground) fruit-bodies with powdery gleba and eroding peridia; however, there are species in each genus that have fully hypogeal fruit-bodies regarded here as sequestrate because erosion of the peridium does not occur. Also not considered as sequestrate were genera producing epigeal fruit-bodies with a dry lamellate hymenium (Montagnea, and those species of Agaricus formerly in Gyrophragmium). Lineages with sequestrate taxa that have been introduced to Australia with non-native plants were also excluded from the analysis (e.g. Hymenogaster sensu stricto, Castellano and Bougher 1994).
Taxon sampling
Thirteen lineages were selected for meta-analysis on the basis that they contained Australian taxa with sequestrate fruit-bodies and that molecular phylogenies and constituent sequences, alignments or dated phylogenies were available (Table 2). The present study concentrates on events occurring within lineages. Relationships among lineages have been described in Hibbett et al. (2014). A complete list of all sequences used, including GenBank numbers, is provided in Table S1, available as Supplementary material for this paper. Each lineage is referred to by the name of the family (or in the case of the Phallomycetidae, the subclass), although, in some cases, only a subset containing the sequestrate taxa of the particular family were sampled, such as for Rossbeevera and Octaviania within the Boletaceae. The 13 lineages contain much of the sequestrate taxa from Australia for which sequences are available. Sequestrate genera known from Australia whose phylogenetic positions are yet to be established on molecular data (at least for the Australian species) include Gymnoglossum (Cortinarius), Horakiella, Mycoamaranthus, Hysterogaster (Descolea, Bolbitiaceae), Leratiomyces and Wakefieldia (Cortinariaceae). Some updates to phylogenetic position for sequestrate taxa occurred subsequent to the analyses and, hence, the following taxa are not included: Agaricus (Lebel 2013), Stephanospora (Lebel et al. 2015), Lepiota (Lebel and Vellinga 2013) and Gymnogaster and Royoungia (Halling et al. 2012). The effect of taxon sampling on overall conclusions is considered further in the discussion.
|
Note that taxonomy of sequestrate fungi is still in a state of transition. Many new combinations have been made to ensure that genera are monophyletic irrespective of fruit-body morphology, such as transferral of members of the sequestrate Thaxterogaster to the previously agaricoid Cortinarius, resulting in genera of mixed morphology. However, some genera composed of sequestrate taxa remain separate, even though they are nested within agaricoid genera (and sometimes polyphyletic), as for Hydnangium and Podohydnangium that still need to be formally synonymised within Laccaria.
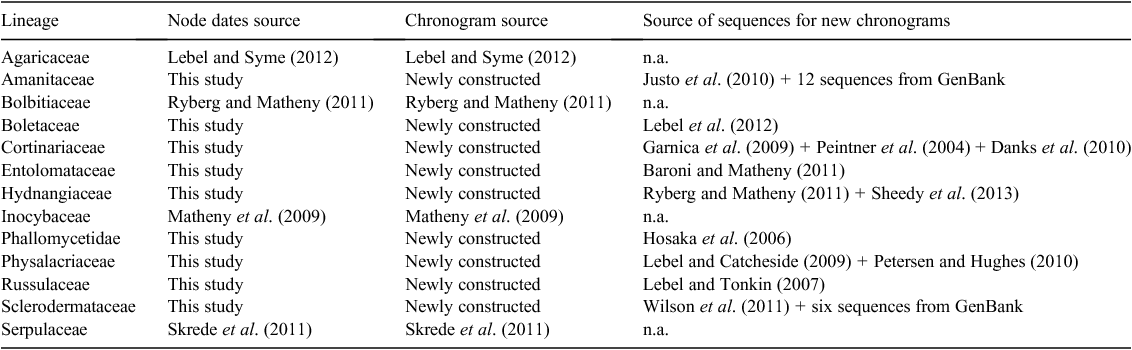
Node dates for sequestrate taxa were used directly from dated phylogenies already published when possible; otherwise, node dates were established by constructing new dated phylogenies from available sequence data. For four lineages, suitable dated phylogenies were already present from other studies (Matheny et al. 2009; Ryberg and Matheny 2011; Skrede et al. 2011; Lebel and Syme 2012). For the other nine lineages, datasets were compiled from sets of GenBank sequences as utilised in published studies, and, in two cases, these were augmented with further sequences from GenBank. The published Sclerodermataceae phylogeny (Wilson et al. 2011) was augmented to include ‘Pisolithus sp. MURU’ (Pisolithus hypogaeus) and its closest five BLAST matches on GenBank. The published Amanitaceae phylogeny (Justo et al. 2010) did not include any agaricoid taxa from Australia, so the Amanitaceae alignment was augmented with sequences from 12 agaricoid species from Australia. Outgroup taxa and taxa that would provide calibration points (see below) were also included in each of the nine lineages for which dated phylogenies were newly constructed.
Ingroup taxa were scored as Australian or non-Australian and as sequestrate or non-sequestrate by using meta-data on GenBank, the original sequence publication or literature on the species (Table S1). Ancestral state reconstruction for the character states ‘Australian’ or ‘non-Australian’ was performed using unordered parsimony in Mesquite, ver. 3.03 (W. P. Maddison and D. R. Maddison, see http://mesquiteproject.org, accessed 1 November 2016).
Alignment construction
For the nine dated phylogenies that were newly created, DNA sequences (including one or more of ITS, LSU, rpb2 and tef-1α; Table 3) were aligned with Multiple Alignment using Fast Fourier Transform (MAFFT), ver. 6.85 (Katoh and Toh 2008). The variability of the sequence regions across large phylogenetic distances prevented the lineages from being combined into a single Agaricomycetes lineage. Maximum likelihood (ML) trees were constructed using RAxML, ver. 7.2.8 (Stamatakis 2006). All duplicate sequences (within a branch-length distance of 0.01) were trimmed from the alignments (Ryberg and Matheny 2012). Ambiguous regions of the alignments were excluded from further analysis. In large alignments, some taxa outside clades of interest were removed to decrease computational burden. Further three taxa (Phlebogaster laurisylvicola, Phallobata alba and Trappea darkeri) were removed from the Phallomycetidae alignment after preliminary analyses, and subsequent BLAST searches suggested identification or labelling errors.
Dating analyses
Nine dated phylogenies were newly created using BEAST, ver. 1.6.2, and the ancillary programs BEAUTi, LogCombiner and TreeAnnotator (Drummond and Rambaut 2007). Five runs for 100 million generations, sampling every 5000 steps, were performed for each lineage on a Linux cluster. Starting trees (shared across runs) were reconstructed for each lineage using RAxML (Stamatakis 2006). The substitution model used was the general time-reversible model with parameters that estimated the proportion of invariable sites and that accommodated rate heterogeneity (GTR+I+G), which followed previous studies (Hosaka et al. 2006; Matheny et al. 2009; Baroni and Matheny 2011; Lebel et al. 2012; Skrede et al. 2011; Ryberg and Matheny 2011, 2012; Lebel and Syme 2012). Where alignments from multiple loci were available, each locus was unlinked for the substitution model and clock rate and then linked for the tree topology and node ages. Protein-coding genes were partitioned further by codon positions (1 + 2) and 3. The clock rate was modelled by the relaxed uncorrelated log-normal distribution (UCLD) model with an ucld.mean prior of 0–1 units. The tree was modelled by the Yule process with a yule.birthrate prior also of 0–1 units. The starting tree for the Phallomycetidae was constrained to maintain larger inclusive clades (orders) recovered by Hosaka et al. (2006).
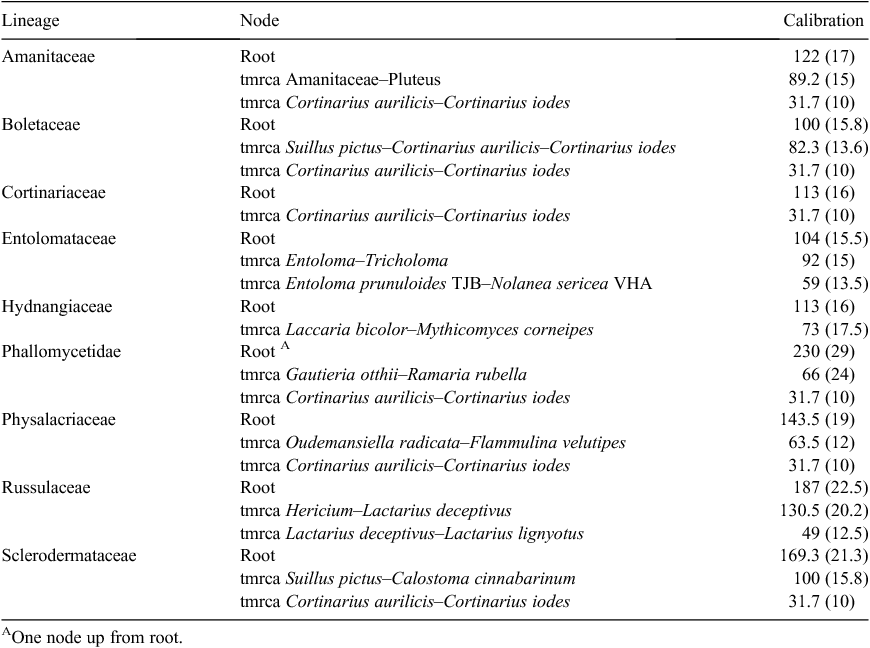
A secondary calibration method (Renner 2005; Matheny et al. 2009) was used to date the nine newly created phylogenies. All phylogenies were individually calibrated to a timescale using dates (and associated error) derived from a reference tree, a five-gene phylogeny of the Basidiomycota in Ryberg and Matheny (2011), wherein the calibration node was the split between Agaricomycotina and Ustilaginomycotina at 430 million years ago, not the root of the Basidiomycota as was erroneously written in the text. Calibration points for each lineage were at the root of the tree and also within the tree where possible and were specified to follow a normal distribution (Ho 2007; Table 4). The incorporation of error was to reduce creating artificial accuracy in the results.
|
All other priors were left at default, with one exception. The Cortinarius data were analysed in BEAST multiple times under different modelling conditions and with adjustment of priors before adequate mixing and sufficient effective sample sizes for all parameters were achieved. The priors for the GTR substitution parameters for this lineage were altered from the default gamma distributions to uniform distributions of 0–10 units.
Log files from each run were examined in Tracer, ver. 1.4 (A. Rambaut and A. J. Drummond, see http://beast.bio.ed.ac.uk/tracer, accessed 1 November 2016), to confirm appropriate mixing whereby the data (not just the priors) were informing the posterior, and that each run within the lineage had converged to a similar likelihood and that the combined effective sample size (ESS) was sufficient (>200) for each of the parameters. Tree files were examined in AWTY (Nylander et al. 2008), and convergence was assessed using the slide function with a 10% burn-in and five windows. The tree topologies were also assessed for pair-wise congruency across the five runs using the cumulative function in AWTY. When these conditions were met, trees from the five runs were combined using LogCombiner with the burn-in (10%) removed, and then processed in TreeAnnotator to produce a maximum clade-credibility tree, with dated node ages and associated 95% confidence intervals. In two cases (Phallomycetidae and Cortinarius), the number and size of the trees processed by TreeAnnotator exceeded the amount of memory (2 GB) allocated to function. In these cases, the tree files were pruned to 10 000 trees (from 90 000 trees) in LogCombiner by sampling at a lower frequency.
Hypothesis testing
Custom-produced R scripts (Boxes S1, S2, available as Supplementary material for this paper) were used to compare four models of sequestration evolution, calculating the likelihoods using the package ‘APE’, ver. 3.2 (Paradis et al. 2004), with the assistance of the package ‘laser’, ver. 2.4-1 (D. Rabosky and K. Schliep, see http://CRAN.R-project.org/package=laser, accessed 1 November 2016). The input data for each lineage were the maximum clade-credibility tree files produced by BEAST, and taxon lists with columns for the binary ‘sequestrate’ and ‘Australian’ scores. All models assume that sequestration is irreversible (see ‘Assumptions’ in the discussion). The first model implemented a uniform rate of evolution, and the second, a uniform rate that ignored phylogeny (i.e. as star trees; Mooers et al. 1999; Vanderpoorten and Goffinet 2006). To test whether the rate of sequestration was higher after the aridification of Australia, a two-rate model increased the rate of evolution along branches scored as Australian by multiplying the branch lengths by a rate multiplier. Branches where parsimony reconstructions (see ‘Taxon sampling’ for method) of the ‘Australian’ scores were uncertain were treated in one round as ‘Australian’ and then in a second round as ‘not Australian’. The two-rate model was initially set to change rate at 32 million years ago (the third model), and in the fourth model, the date was allowed to vary to find an optimal date. Both rate multiplier (elongating branch lengths) and the date of rate change were estimated simultaneously, with time being the variable of prime interest. The likelihood surface for rate shifts from 100 million years ago until the present was also investigated, given the optimal rate multiplier at each date. For comparison with the four models of interest, the variable two-rate model was additionally used to look at sequestrate taxa globally. However, given the non-targeted global sampling, whereby many other regions are under-represented, this test does not represent a true global view. The rate to become sequestrate was estimated separately for each lineage (using the ace function of APE in R) for different parameter values of the time of shift and the rate increase (multiple of base rate). The latter two were optimised for all lineages combined (using the optim function in R), to increase the power. Akaike information criterion (AIC) scores were calculated for the models by using free parameter values of one for the uniform-rate models, two for the fixed two-rate model and three for the variable two-rate models. The models were then compared using ΔAIC. For the likelihood surface, a difference of two in the log-likelihood was considered to indicate a significant difference when comparing parameter values for a model and comparing a model with the same number of free parameters (Vanderpoorten and Goffinet 2006).
Results
Sources of dates and dated phylogeny construction
Four of the thirteen lineages that were included in the present study had previously been published with dates for the emergence of sequestrate taxa available, whereas the remaining nine lineages required dated phylogenies to be constructed from available sequences to date the nodes (Agaricaceae, Lebel and Syme 2012; Bolbitiaceae, Ryberg and Matheny 2011; Inocybaceae, Matheny et al. 2009; Serpulaceae, Skrede et al. 2011; Table 2). Summary statistics, including the loci, number of base pairs and number of Australian sequestrate and non-sequestrate taxa that were used to create the dated phylogenies, are given in Table 3. Data matrices and trees have been uploaded to TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S18448, accessed 3 December 2016). Appropriate ESS for all parameters and convergence of tree topologies were achieved for all lineages, unless specified below.
Dates of sequestration within Australia by lineage
Node ages, given in the accounts below, are medians with 95% highest posterior density (HPD) written here as lower–median–upper when support for the node was >50% in the maximum clade-credibility tree. Crown nodes are the nodes that define the first split of a clade that contains only sequestrate taxa, and stem nodes are one node deeper, where sequestrate taxa split from their non-sequestrate sister taxa. Sequestrate taxa that are not members of a sequestrate clade (singletons) have only a stem age. The actual transition to sequestrate form could have occurred at any point along the branch between the stem and crown nodes (where present), or the present day for singletons. A summary of median node ages is provided at the end of the Results section.
Fifty-nine origins of the sequestrate morphology in Australian taxa were estimated considering all datasets. These origins included either single species (singletons) or clades (24, which occurred in 9 of the lineages). No obvious relationship exists between the number of sequestrate origins and the number of clades. For example, some lineages rich in sequestrate forms have many clades compared with singletons, such as in Bolbitiaceae (four clades of six origins) and Russulaceae (six clades of eleven origins), whereas others have relatively few clades, such as in Agaricaceae (one clade of ten origins). In the Physalacriaceae, both of the two origins of sequestrate taxa resulted in sequestrate clades. In the Hysterangiales, there is a single origin of sequestration leading to a clade of 108 species.
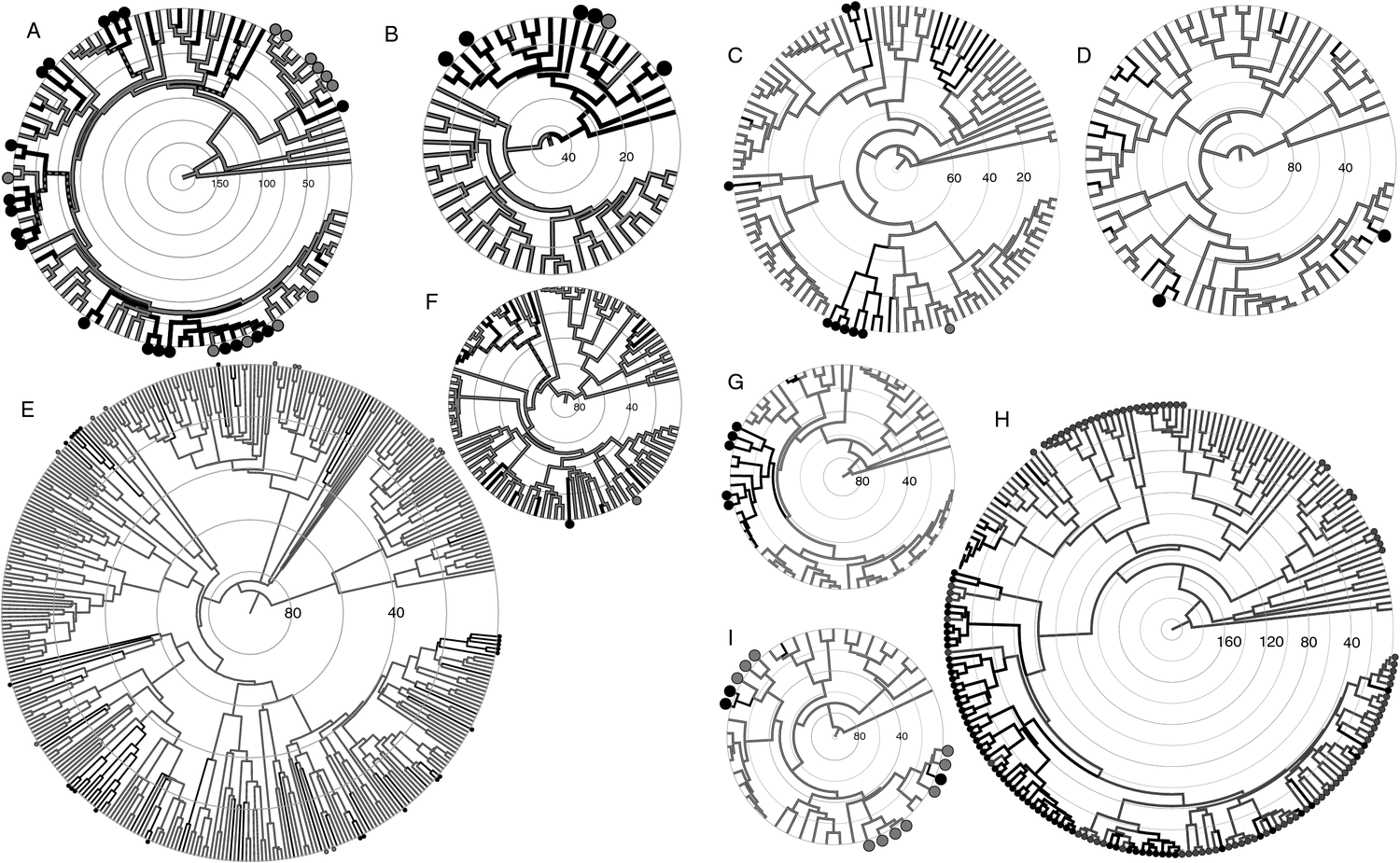
An overview of the newly constructed trees to highlight the positions of sequestrate taxa in Australia is provided in Fig. 2. Phylogenies that include taxon names, posterior probabilities and error bars associated with node ages are available for individual lineages in Fig. S1, available as Supplementary material for this paper.
Agaricaceae
The ages used for the Agaricaceae analysis (Lebel and Syme 2012) were mean node ages (as opposed to the median node ages used in the present study). Sequestrate taxa do not form a monophyletic clade. The Agaricaceae includes 10 transitions to sequestrate morphology, nine of which are single branches. Barcheria is on an extremely long branch, the stem node of which dates back to 57–65–72 million years ago and is the clear outlier in the family. Barcheria has fruit-bodies that are loculate and empty chambered, in contrast to those in the remaining sequestrate taxa of this family that begin with this form but mature to have a single large chamber and powdery spores (with various development of stipe, central core or columnella in Agaricus p.p.). With one further exception (Agaricus melanosporus; 26–34–43 million years ago, stem age), the remainder of the node ages all occurred <15 million years ago.
Amanitaceae
All quality-control checks were passed for the log files of this lineage, although the tree files in AWTY showed splits that were non-uniform in some of the runs. To determine whether combining these runs was appropriate, the node ages for transition nodes (agaricoid to sequestrate) were compared among the maximum clade-credibility trees from each run. All median node ages of interest were within 1.01 million years of each other across the five runs, which is a reasonably small margin of error for this study, so data from the runs were combined.
The transition to sequestrate morphology occurred five times in the Amanitaceae, four of which were in Australia. Sequestrate morphology in this lineage is loculate or sublamellate with empty or filled chambers. The majority of the Australian sequestrate taxa were collected from Western Australia, with ‘Amarrendia sp.’ being the only recorded collection of a sequestrate Amanita from eastern Australia. Mean age of emergence for the Australian sequestrate clades was 21 million years ago (from four stem nodes) and 9 million years ago (two crown nodes).
The inclusion of an additional 12 Australian agaricoid taxa from GenBank to the alignment of Justo et al. (2010) suggested that some of the sequestrate clades in the present study have new sister taxa. The new agaricoid sequences also divided into two the sequestrate ‘Amarrendia clade’ of Justo et al. (2010).
Bolbitiaceae
The Descolea clade of the Bolbitiaceae contains sequestrate taxa that are loculate or sublamellate, with empty chambers and with both stipitate and astipitate forms. Sequestrate taxa in the Descolea clade are predominately from Australia and New Zealand, although the agaricoid Descolea is itself more widespread. In this dated phylogeny, a greater abundance of sequestrate than agaricoid taxa was observed. Six transitions to a sequestrate form were inferred to have evolved, including four sequestrate clades and two singletons from Australia. The averages of the node ages were 12 million years ago (four crown nodes) and 23 million years ago (six stem nodes). Whereas most of the clades contained either stipitate or astipitate sequestrate taxa, one of the clades, listed as Descolea4, had both Descomyces (astipitate) and Setchelliogaster (stipitate). This is one of few observations where a transition from agaricoid through an intermediate secotioid to sequestrate form may be supported.
Boletaceae
The transition to sequestrate occurred once in Rossbeevera and once in Octaviania (the Chamonixia is not Australian but also appears to be a separate transition). The sister taxa for the Australian sequestrate taxa in these genera were other non-Australian species of Rossbeevera and Octaviania respectively. The Rossbeevera clade was well supported, but with low internal branch support (Fig. S1), and the two Australian sequences formed a grade at the base. The transition to a sequestrate form in Rossbeevera occurred between 13 and 25 million years ago (crown-stem), and in Octaviania between 25 and 43 million years ago (crown-stem). For Octaviania, this transition occurred before dispersal to Australia, and the node ages of the Australian clade were 0.5–3–7 million years for the crown and 7–16–28 million years for the stem.
Cortinariaceae
The Cortinariaceae is rich in Australian sequestrate species. Sequestrate forms in the Cortinariaceae include stipitate secotioid forms such as those formerly placed in Thaxterogaster, as well as completely astipitate forms such as formerly placed in Protoglossum and Quadrispora. There were 12 sequestration events, of which five were clades (one clade of five taxa, one clade of four taxa and three taxon pairs). This phylogeny was well sampled with over 370 collections, 60 of which were Australian. However, given the large size of the dataset, we were not assured that convergence was met, and it produced a maximum clade-credibility tree with wide HPD intervals. The oldest transitional node, with correspondingly high variation, was for a singleton sequence of Protoglossum sp. (16–45–51 million years ago stem node). The mean crown node age was 9 million years (five clades) and stem node age was 19 million years (12 stem nodes).
Entolomataceae
Two sequestrate taxa were included in the present analysis, one of which was from Australia (E. gasteromycetoides; stem node 32 million years). The support for the placement of the sequestrate taxon was poor. The sister taxon for E. gasteromycetoides was the agaricoid E. sericellum collected in Belgium. The other sequestrate species was Entoloma asterosporum (as Richoniella asterospora in the original analysis) from Tennessee, USA, which had a node age of 4–8–14 million years and was sister to an Entoloma sp. (as Inocephalus) from Argentina, an agaricoid species.
Hydnangiaceae
Within the Hydnangiaceae, northern hemisphere species occurred in a clade separate from species from Australia and New Zealand. All of the sequestrate taxa were in the Australia–New Zealand group, with representatives from both countries.
Five transitions to sequestrate forms were determined, with an average stem node age of 10 million years ago. However, in only one instance did two sequestrate taxa form sister taxa. This was between Podohydnangium australe from Australia and a Hydnangium sp. from New Zealand. This clade had a crown age 3–10–19 million years and a stem age of 16 million years. Within the sequestrate species H. carneum, different collections identified as this species were separated throughout the tree, with sometimes different node ages. The age for one Hydnangium collection (H. carneum TL2359) was young, 0.1–3–9 million years, whereas another collection (H. carneum TL2361) had an older node age of 21 million years.
Inocybaceae
Dates for this lineage were obtained from Matheny et al. (2009), and it included two singleton sequestrate species from Australia, namely, Inocybe ‘cazaresii’ and Auritella geoaustralis. The dates given for these species were 12–27–41 million years and 8–18–30 million years respectively. Both of these taxa are secotioid in morphology. An additional sequestrate (secotioid) form, I. bicornis f. secotioides, has been observed in the family in Australia and represents a variant of the agaricoid form, I. bicornis f. bicornis (Braaten et al. 2014).
Phallomycetidae
Within the subclass Phallomycetidae, the Australian sequestrate taxa were found only in the order Hysterangiales, of which they constitute a considerable proportion, particularly in the families Gallaceaceae and Mesophelliaceae (Fig. 2H). The Hysterangiales is composed entirely of sequestrate fungi, and its sister clade (the grouping of Phallales and Gomphales) contains a mixture of fruit-body morphologies, including stinkhorns, cages and clubs (all of which are epigeous), as well as other sequestrate fruit-bodies in non-Australian species.
It is not known whether the ancestral state of the clade containing the Hysterangiales, Phallales and Gomphales was sequestrate or non-sequestrate. Given that the ancestor of the Gomphales was epigeous (Hosaka et al. 2006), the Hysterangiales is considered here as likely to represent a single transition to the sequestrate form early during its diversification. The median age of the Hysterangiales crown node was 58–83–114 million years, but the transition could have occurred as long ago as the stem node at 96–134–172 million years ago. The majority of the Hysterangiales are ectomycorrhizal (ECM), with only the Phallogastraceae as possibly saprotrophic (represented by the clade containing Trappea phillipsii, T. pinyonensis, Phallogaster saccatus, Protubera hautensis, P. nothofagi and Protubera sp. T20068; Hosaka et al. 2008). The node ages of the ECM clade (the group containing Hysterangiaceae, Mesophelliaceae, Gallaceaceae) were crown 46–65–88 million years and stem 58–83–114 million years.
Within the Hysterangiales, the Mesophelliaceae has a specialised morphology, consisting of a sterile central core covered by a layer of powdery spores and protected by a hard outer layer that is entwined with roots of its ECM plant associate. The family is endemic to Australasia. The node ages of Mesophelliaceae were crown 29–43–58 million years, stem 37–54–71 million years and, for the Mesophellia–Malajczukia–Nothocastoreum group, which includes some of the more commonly ingested (by small mammals) sequestrate taxa in Australia, 9–14–20 million years and 14–21–29 million years.
Physalacriaceae
The morphology of sequestrate forms is loculate or sublamellate, with empty chambers. The sequestrate taxa are non-monophyletic within this lineage, having evolved at least twice. The ages of each of these transition nodes are quite divergent, one having occurred between 23 and 33 million years ago (crown to stem) and the other 5–12 million years ago (crown to stem). Sequestrate taxa in this lineage are restricted to Australia.
Russulaceae
The Russulaceae, and in particular the genus Russula, is one of the more sequestrate-rich lineages in Australia, with some 55 species having been described (Lebel and Castellano 1999, 2002). For this phylogeny, sequences were derived from Lebel and Tonkin (2007), which focused on russuloid taxa, not lactarioid taxa, and therefore only a single Zelleromyces sequence was included to represent the Australian sequestrate taxa within Lactarius here. However, 18 sequences of sequestrate Russula (historically classified as Macowanites, Cystangium and Gymnomyces) were included. These represent 11 transitions to a sequestrate form. The morphology of these taxa are loculate or sublamellate with empty chambers and include stipitate and astipitate forms. The stipitate form was more abundant and more highly distributed across several different subclades compared to astipitate.
The average crown node age of sequestrate Russulaceae was 18 million years, with the oldest node belonging to Zelleromyces daucinus, in the underrepresented Lactarius clade, at 21–39–64 million years, and the youngest node belonging to Cystangium theodouri at 3–9–19 million years. This lineage did not include many recent (<10 million years ago) or very recent (<5 million years ago) sequestrate transitions, which were observed in other lineages.
Sclerodermataceae
Currently, only three sequestrate species are known in Pisolithus (P. hypogaeus) and Scleroderma (S. mcalpinei, S. paradoxicum) in Australia. In the present study, only one species from each genus was sampled. Pisolithus and Scleroderma typically have a gasteroid morphology that differs from a sequestrate morphology primarily because of the spore mass becoming exposed on maturity as the fruit-body becomes emergent, cracks and erodes, whereas the sequestrate forms remain hypogeous and the peridium does not split. The transition to a sequestrate fruit-body form in this lineage has occurred at least twice from the puffball (non-sequestrate) morphology. The median node age for the sequestrate Pisolithus sp. MURU was 2–7–15 million years and for the sequestrate Scleroderma mcalpinei 3–9–16 million years.
Serpulaceae
The Serpulaceae contains a divergent mix of resupinate, agaricoid and sequestrate fruit-body morphologies. The transition to the sequestrate form was from an agaricoid ancestor, Austropaxillus. Although no exact dates were given for the transitional node from Austropaxillus to Gymnopaxillus (a sequestrate genus), the dates between A. macnabbii, which was the sister species to a clade formed by G. vestitus and G. nudus (in Skrede et al. 2011, additional file 7), and A. muelleri are considered to be a conservative approximation. The following dates were rounded from Node 7 in table 1 of Skrede et al. (2011) for the transition to the sequestrate Gymnopaxillus: 7–15–24 million years ago.
Summary of emergence dates of sequestration within Australia
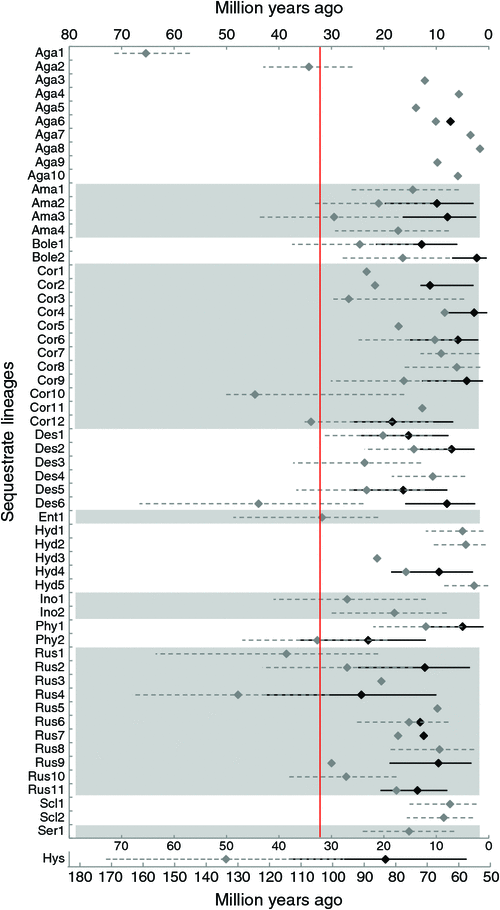
The node ages of transitions to sequestrate fruit-bodies in Australia that were described above for 13 lineages are summarised in Fig. 3. In total, 24 crown and 59 stem node ages were tallied. The oldest sequestrate node belonged to the Hysterangiales at 83–133 million years ago (crown–stem). The other four nodes that were older than 40 million years were stem nodes for singleton genus Barcheria of the Agaricaceae (65 million years ago), a clade in the Russulales (Macowanites rostracystidia–M. variispora; 48 million years ago), a singleton Protoglossum sp. in Cortinariaceae (45 million years ago) and a clade of Descomyces angustisporum and Setchelliogaster (44 million years ago). The mean crown and stem node ages for all transitions were 14 and 21 million years respectively. The 23 crown ages, not including Hysterangiales, were distributed in the following time periods (number of nodes in parentheses): 21–30 million years ago (2), 11–20 million years ago (9) and <10 million years ago (12). Similarly, the stem ages were distributed as follows: >41 million years ago (5), 31–40 million years ago (5), 21–30 million years ago (13), 11–20 million years ago (18) and <10 million years ago (17).
|
The upper and lower bounds of the HPDs averaged within 10 million years of the crown node median (interval average was 17 million years) and within 12 million years of the stem node median (interval average was 23 million years). The median dates for the Hysterangiales had the most variation associated with them; HPD intervals were 56 million years for the crown and 76 million years for the stem nodes.
In relation to the age of the split between Australia and Antarctica 32 million years ago, only one crown node of 24 was older than 32 million years. A further two crown nodes had 95% HPD intervals that were older than 32 million years. Of the 59 stem node ages, only nine were older than 32 million years, although again, the 95% HPD interval of a further 18 nodes crossed this mark. In relation to the emergence of mycophagous animals 16 million years ago, five crown nodes were older than this date.
Although the majority of sequestrate transitions in Australia occurred from agaricoid ancestors within the past 32 million years, the sequestrate Sclerodermataceae had puffball ancestors, Gymnopaxillus diverged from a agaricoid ancestor that itself emerged in Australia only during this time (from a resupinate ancestor) and Octaviania was probably already sequestrate before arriving in Australia. However, only the Hysterangiales would reject a hypothesis of aridification being a key stimulus for sequestration because it has been present in Australia since the Cretaceous.
Hypothesis testing
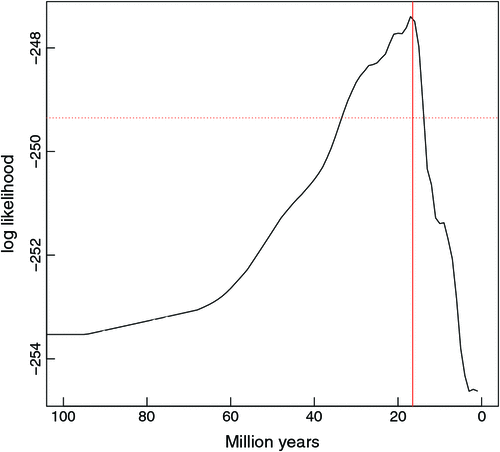
The rate at which Agaricomycetes became sequestrate in Australia was likely to have increased at a point between 13 and 34 million years ago (two log-likelihoods from the maximum, Fig. 4). This increase in sequestration is most likely to have begun c. 16 million years ago. Although median node ages were used to calculate these dates, the 95% credibility intervals around nodes between 34 and 13 million years ago spanned on average 22 million years (Fig. 3); a similar range of error as calculated from the two log-likelihoods. The model that fit the data with the highest likelihood was the two-rate model where the rate change in Australian clades was not fixed in time. When the sequestration rate was set to increase at 32 million years ago (a two-rate model with the rate change fixed in time), the likelihood was reduced. For this model, the AIC score was 3 or 10 higher than for the best fitting model (depending on whether clades with uncertain ancestral origin were scored as Australian or not). The likelihood was even more reduced for the two-rate variable model that included global sequestrate taxa (AIC of 57 worse than for the best fitting model). However, it should again be stressed that because sequestrate taxa were not targeted globally for inclusion, this result is likely to be biased. The rate multipliers were estimated at ~10–11 (depending on uncertain clades as Australian or not) for the fixed-time model and 16 (negligible effect of uncertain clades) for the variable model. Uniform rates of evolution both had much higher (worse) AIC scores than did the best fitting model, being 78 and 234 higher for the uniform rates that regarded and disregarded phylogeny respectively.
|
Discussion
The lineages in the present study were chosen to encompass a diverse assemblage of sequestrate Agaricomycetes, with the aim of detecting patterns unrestricted by phylogenetic lineage, sequestrate morphology type and mode of nutrition. The hypothesis testing enabled us to combine lineages into a single, broad dataset, with enough power to detect the increase in rate of sequestration in Australia during the mid-Miocene, an effect that was unable to be determined from individual lineages alone (data not shown).
Historical climatic effects on sequestration of Agaricomycetes in Australia
Aridification has been an important climatic process that has shaped both the flora and fauna of Australia (Byrne et al. 2008). The results of the present study suggest that aridification has also created an environment that favours the evolution of taxa with sequestrate fruit-bodies. Sequestration provides fruit-bodies with protection from extremes in moisture, such as desiccation or saturation from rain. As agaricoid fruit-bodies desiccate, spores cease to be discharged (Haard and Kramer 1970). Such protection from desiccation would be beneficial in not only annually dry regions, but also in regions of climate variability or unpredictability. The effect of climate on Australia’s vegetation is notable; for example, in the expansion and dominance of iconic arid-adapted flora such as Eucalyptus, a genus with over 600 species that began to rapidly radiate c. 25 million years ago (Ladiges et al. 2003; Crisp et al. 2004). Drying climates are also known to affect vegetation structure in other systems in the world, such as, for example, in western North America (Donoghue and Smith 2004), which is another region with a diverse assemblage of sequestrate fungi.
The sequestrate Pisolithus hypogaeus (as P. sp. MURU) and Scleroderma mcalpinei included in this analysis represent two of the three currently known Australian sequestrate species within the Sclerodermataceae. The ancestral fruit-body morphology for these sequestrate taxa is the powdery-spored and emergent puffball fruit-body type, not an agaricoid fruit-body. Although the sequestrate forms evolved recently, the puffball morphology of Pisolithus and Scleroderma evolved much earlier (c. 60 million years ago) and arguably represents an arid-adapted morphology in its own right. However, if the puffball morphology was the optimal adaptation to Australia’s climate, there would be no selective pressure from aridity for the sequestrate morphology to evolve. The puffball fruit-body may not represent the optimum arid-adapted morphology or there are other contributing evolutionary drivers for sequestrate fruit-bodies.
An alternative morphology hypothesised as an adaptation to prevent fruit-body desiccation is deliquescence or auto-digestion, which is associated with accelerated formation of the fruit-body (Nagy et al. 2011). As with sequestration, deliquescence has evolved more than once in four notable lineages in the Psathyrellaceae. Given very disjunct dates for when deliquescence evolved, Nagy et al. (2011) concluded that deliquescence, and fruit-body morphologies more broadly, are likely to be adaptations to independent events, rather than to large-scale environmental change. In contrast, the results of the current study on sequestration (distinct from gasteromycetation in Nagy et al. 2011) showed that the majority of sequestration events within Australia occurred within the same geological envelope and likely under similar environmental pressures.
Although the sequestration events reported here represent a (predominately) temporally localised expansion into a new niche, few of the individual transitions produced a radiation of sequestrate species. Within Cortinarius, Peintner et al. (2001) suggested that, once established, sequestrate taxa were not only morphologically stable but had a tendency to radiate. This is logically appealing first because sequestrate species will have a more limited dispersal capacity than do wind-dispersed species, so speciation due to isolation may occur more frequently. Second, newly evolved sequestrate species will be exploiting a different ecological niche, with a chance for adaptive radiation to occur. However, within the Cortinarius clade of Peintner et al. (2001), there were three sequestrate clades that contained just two species and only one sequestrate clade that contained more than two species. Compared with the 17 sequestration events they found, only a small proportion appears to have undergone further diversification. Analysis of the Cortinariaceae lineage in the present study showed similar results (12 Australian transitions, three clades of n = 2, two clades of n > 2). Across all lineages, there was a high proportion of singletons; of 59 sequestrate transitions, only 24 resulted in clades and only 12 of these clades had more than two species. This is in contrast to the successful radiation of the Hysterangiales into more than 100 species after evolving the sequestrate habit during the Cretaceous. Prior to the separation of Australia from Antarctica, the Hysterangiales had already diversified such that not only were the six family-level groups identified in Hosaka et al. (2008) present, but 19 branches could be bisected by the 32 million years line.
After the Hysterangiales, the most speciose sequestrate clades belong to the Descolea group and Russula, which had clades containing seven and six species respectively. Clades of two species were produced for all other Australian transitions in Russula, so in this lineage, radiations, although small as yet, do appear to be more common. Approximately 55 species of sequestrate Russula have been described from Australia (of which only 18 were included here), with many more ‘known, undescribed’ (Bougher and Lebel 2001). It remains to be seen where these species will be positioned phylogenetically and whether they represent further individual sequestration events or speciation within already detected transitions. The same can be said for the estimated 1278–2450 species of sequestrate fungi in Australia yet to be described and sequenced (Bougher and Lebel 2001).
Despite the emergence of sequestrate forms in Australia clearly coinciding with a major shift towards an arid climate, when sequestration occurred in the Hysterangiales (late Cretaceous), the climate was temperate with high humidity (Dettmann et al. 1992). The aridity or seasonal periods without rain in Australia were, therefore, not necessary for sequestrate fruit-bodies to evolve or diversify; however, extreme wet could rot the fruit-bodies, so maintaining an even humidity may be just as important (i.e. in New Zealand, the diverse truffle flora is mostly epigeal). Further, although the majority of sequestrate fungi in Australia appears to have transitioned locally, Octaviania and Rossbeevera, two sequestrate genera of Boletaceae, were already sequestrate when they arrived in Australia from Asia (Orihara et al. 2016). Therefore, conditions in Australia did not influence their sequestration, but must have been conducive to the establishment of these species following their dispersal.
Although aridification may still be an important factor that favours sequestration, it cannot be the only process involved. In Australia, sequestrate species are not currently limited to the arid interior and are often collected in wetter areas, including cool- or warm-temperate to tropical rainforest. Some sequestrate species, for example, Podohydnangium australe (Hydnangiaceae), are known to occur only with Nothofagus in cool-temperate rainforests (Beaton et al. 1984). Other sequestrate taxa have been described from tropical forests, such as Guyanagaster from South America (Henkel et al. 2010), and species from Java, Indonesia (Smith and Schmull 2011). This, combined with the early emergence of the Hysterangiales in Australia, is compelling evidence that evolution of the sequestrate morphology is not solely selected for by an arid climate.
Plant associates
Most sequestrate fungi are known to have an ECM mode of nutrition (Trappe and Claridge 2005). Of the 13 lineages investigated in the present study, only the following sequestrate fungi were considered non-ECM (after Tedersoo et al. 2010): all sequestrate fungi in the Agaricaceae (representing 10 transitions); Trappea and Phallogaster (at the base of the Hysterangiales, not included here as a separate sequestrate transition and suggesting that the transition was non-ECM in this lineage); and Cribbea in the Physalacriaceae (representing two transitions). The difference was minor in average ages of sequestrate transitions between ECM (19 million years ago, stem age) and non-ECM (17 million years ago, stem age) species.
Plant associates are generally understood to play an important role in determining the diversity and distribution of the ECM fungi that depend on them. In Australia, important ECM plants include the Myrtaceae, Fabaceae, Nothofagaceae and Casuarinaceae. The ancestral host for the ECM Hysterangiales was reconstructed by Hosaka et al. (2008) as Myrtaceae. They hypothesised that the age of ECM Hysterangiales should, therefore, be as old or younger than the Myrtaceae, which was estimated at ~86 million years old (Sytsma et al. 2004). A more recent study of the Myrtaceae places the crown node between 84 and 88 million years ago (Thornhill et al. 2012), and the crown node for the ECM Hysterangiales is estimated here at c. 65 million years ago, thus supporting the prediction of Hosaka et al. (2008). Although the Hysterangiales is the oldest lineage in the present analysis, the true-truffle ascomycete genus, Tuber, not known to occur in Australia, is also ECM and diverged even earlier, at about the same time as did the Eudicots in the late Cretaceous (c. 142 million years ago; Bonito et al. 2013).
Of common ECM hosts in Australia, only the Nothofagaceae is not adapted to dry seasonal climates. In Australia, if the high diversity of sequestrate species is related to the expansion of arid-adapted and sclerophyll forests, we would expect to find a greater diversity of sequestrates in sclerophyll communities than in rainforest communities. This notion seemingly contradicts Danks et al. (2013) who found that species richness from sequestrate fruit-bodies in northern New South Wales was higher in cooler and wetter forests (grassy woodland and wet sclerophyll) than in warmer and drier forests (dry sclerophyll). It should be noted that this region has experienced numerous localised shifts of habitat boundaries in response to fire, and the rainforest habitat was not included. In North America, Lehmkuhl et al. (2004) also found a higher diversity of sequestrate taxa in mixed conifer and mesic forests than in open and dry ponderosa pine forests.
Mycophagy
Whereas larger animals such as deer or wallabies may occasionally eat fungi to supplement their diet, smaller animals such as potoroids can depend almost entirely on fungi for their nutrition (Luoma et al. 2003; Nguyen et al. 2005). Sequestrate fungi make up to 90% of the diet in some marsupials, such as Potorus longipes (the long-nosed potoroo). The potoroid digestive tract has evolved a foregut where pre-digestive fermentation occurs, enabling it to survive almost exclusively on fungi (Claridge and Cork 1994). Some of the most commonly eaten fungi in Australia belong to the Mesophelliaceae (Hysterangiales) in the Mesophellia–Malajczukia–Nothocastoreum group (Claridge and May 1994).
The Mesophelliaceae exhibits a ‘broom and handle’ shaped pattern of evolution, having diverged c. 54 million years ago with a long stem, or ‘handle’, before the crown age at 43 million years ago when the group rapidly diversified, creating the ‘bristles’. Accurately estimating the crown age of broom- and handle-type radiations is difficult and may require a more targeted analysis than what was performed in the present study. For example, Crisp et al. (2014) tested a random local clocks (RLC) model of molecular evolution against the more commonly used uncorrelated log-normal (UCLN) relaxed clock model (used here) for a broom-shaped clade with a substantial rate change. In this case, the Xanthorrhoea (grasstree) clade was known to evolve much slower than its sister group; however, the UCLN produced crown node ages that were much too young, whereas the RLC model recovered accurate ages.
The Mesophelliaceae radiated from 43 million years ago, whereas the clade responsible for the Mesophellia–Malajczukia–Nothocastoreum group emerged between 21 and 14 million years ago. This coincides with when the mycophagy specialist Potoroidae diverged from the Macropodidae, a group that includes kangaroos and wallabies (16 million years ago; Meredith et al. 2009). The presence of specialist spore dispersers may have supported an increased diversity of sequestrate fungi; however, that cannot be determined from these data. Prior to the evolution of mycophagy specialists, sequestrate fungi would have been dispersed by more generalist feeders known to ingest these types of fungi, including other mammals, such as possums and rodents, and also insects (Claridge and May 1994). In the absence of any animals, limited dispersal by water as fruit-bodies break down in the soil may also occur (Claridge and May 1994).
Morphology
Most of the genera can be grouped into the following two main morphology types: (I) loculate or sublamellate with empty chambers (with various development of a stipe-columella), shown by the majority of taxa included in our analyses; and (II) a single large chamber with powdery spores (with various development of stipe, central core or columella). Morphology type I contains the most phylogenetic diversity, whereas Morphology type II consists predominately of species from the Hysterangiales plus some Agaricus. Other morphologies found within the Phallomycetidae include a chamber or chambers filled or lined with a gelatinous or liquefying spore mass (Gelopellis, Austrogautieria, Hysterangium, Claustula). Sequestrate members of Scleroderma and Pisolithus as well as Amanita (former Amarrendia) are characterised by fleshy or filled chambers, with no stipe-columella.
The genetic basis for sequestration in the lineages we explored is not known. In at least one other case, namely that of the secotioid form of Lentinus tigrinus, a mutation at a single locus has been suggested as responsible (Hibbett et al. 1994). Genome sequencing of pairs of closely related agaricoid and sequestrate taxa will be instructive. Our analysis has provided a framework for selecting such pairs from across a range of lineages.
Habitat variables
The Australian landscape has a long history with fire, both naturally occurring and through human intervention. Many groups of plants exhibit fire-adapted characteristics, such as the production of highly flammable oils and sprouting from epicormics buds in eucalypts. Sequestrate fungi, in particular members of the Mesophelliaceae, are also considered fire-adapted because of their anatomy and also as many fruit deeply enough in the soil to survive burns and are available in abundance immediately post-fire (Claridge et al. 2009). Given the short time frame in which these species dominate the sequestrate niche after fire (less than 3 months; Vernes et al. 2004) and the assumed time that would be required to produce such biomass, it is unlikely that the fruiting is stimulated by fire (Trappe et al. 2005). Rather, the surviving fruit-bodies produce a strong aroma and are easier to locate at burnt sites (Trappe et al. 2005). Similarly, burnt sites were not found to support an increased diversity of sequestrate species in the years following fire, compared with unburnt sites (Trappe et al. 2006). It has generally been found that fire does not play a strong role in governing sequestrate diversity, rather other environmental factors such as litter cover, moisture levels, temperature, elevation, soil properties and fallen wood can have a greater impact (Claridge et al. 2009; Danks et al. 2013). Further, nutrient-deficient soils, such as are common in Australia, are correlated with high plant diversity (Huston 2012). Fine-scale patterning of soils has been suggested as a mechanism to explain this relationship (Crisp and Cook 2013), which may, in turn, be extended to include sequestrate fungi. Ectomycorrhizal species of Hysterangiales often produce dense hyphal mats (Hosaka et al. 2006) that can alter the surrounding soil properties (Griffiths et al. 1994).
Assumptions
Results in the present study are interpreted under the assumption that morphological transitions switch from ‘agaricoid’ fruit-bodies towards sequestrate fruit-bodies. Transitions in the reverse, from sequestrate to agaricoid forms, are considered improbable because of the need, in that case, to re-evolve the mechanism for active spore discharge (Hibbett 2007). Although the assumption made here is generally accepted, Binder and Hibbett (2006) surprisingly found that gasteromycetation (a broader, more inclusive group of organisms than defined by sequestration in the present study) was reversed once in Suillineae. Given the improbability of such occurrence, also noted by the authors despite support in the analysis, the evidence is considered insufficient to warrant altering the assumptions of the present study.
Dating a molecular tree by using a secondary calibration is not ideal, but still useful in cases of organisms with a poor fossil record, providing the error associated with the date is incorporated (Ho 2007). In the present study, all calibration dates were taken from the same calibration source, namely Ryberg and Matheny (2011). Even with the large error bars in the results, the pattern of sequestrate evolution in Australia is apparent.
The transition from species with agaricoid fruit-bodies to those with wholly sequestrate fruit-bodies has been suggested to occur by an ‘intermediate’ fruit-body form (Thiers 1984). Some lineages analysed here contained representatives of all three fruit-body types; these included Laccaria (agaricoid), Podohydnangium (sequestrate, stipe present) and Hydnangium (sequestrate, no stipe); Descolea (agaricoid), Setchelliogaster (sequestrate, stipe present) and Descomyces (sequestrate, no stipe); Russula (agaricoid), Macowanites (sequestrate, stipe present) and Gymnomyces (sequestrate, no stipe); and Cortinarius (agaricoid) and the species of this genus formerly placed in Thaxterogaster (sequestrate, stipe present) and Protoglossum (sequestrate, no stipe). The phylogenetic positions of the ‘intermediate’ sequestrate species that have stipes, in relation to the agaricoid and sequestrate species lacking stipes, suggest that they were not part of a stepwise pattern of evolution; although the number of data points are few. Bruns et al. (1989) suggested that intermediate forms are likely to be ill-adapted and would be rapidly eliminated. Although it is not clear what length of time should be considered ‘rapid’, our observations support the contention of Peintner et al. (2001) for Cortinarius that extant intermediate forms should be considered as stable morphologies.
The present study has not addressed extinction rates. A scenario of differential extinction rates between sequestrate and agaricoid forms could lead to lack of persistence of older sequestrate lineages. However, there is no evidence for such differential extinction rates. Wilson et al. (2011) did examine extinction in gasteroid fungi (again a broader definition than the sequestrate fungi defined here). Although noting that the low occurrence of gasteroid forms was consistent with the idea that these fungi are at a heightened risk of extinction, their results suggested that gasteroid fungi may be diversifying more rapidly (or at least no less rapidly) than their non-gasteroid counterparts (Wilson et al. 2011). Persistence for millions of years to the current day by sequestrate fungi across several lineages suggests some degree of stability. It will be of interest to apply emerging techniques of extinction analysis (Pyron and Burbrink 2013) to sequestrate lineages.
Effect of taxon sampling
Available molecular data on Australian sequestrate Agaricomycetes were comprehensively sampled in the present study. Given the ranges of morphology types and phylogenetic diversity that were tested, inclusion of additional sequestrate taxa is not expected to alter the overall pattern of the results, which showed that the majority of sequestration occurred after 32 million years ago. In contrast, Australian agaricoid taxa are quite under-represented and including more of these could potentially make the agaricoid form older in Australia or, more importantly, discover a sister taxon for sequestrate taxa (or within sequestrate clades), and thus make the emergence of the sequestrate form more recent than is currently shown.
For the Agaricaceae, two additional sequestrate species have been discovered (Lebel 2013) subsequent to publication of the dated phylogeny (Lebel and Syme 2012). On the basis of Bayesian analysis of ITS, one of the new species, Agaricus lamelliperditus, was positioned within a well supported clade consisting of the sequestrate A. wariatodes and A. chartaceus. Hence, the addition of A. lamelliperditus to a dated phylogeny would not represent a new transition to a sequestrate morphology, but merely contribute to a radiation. The second new species, A. colpeteorum, is more difficult to position, because its sister species A. aridicola (also sequestrate), from the northern hemisphere, was not included in the dated phylogeny. However, because A. colpeteorum and sister occur within section Minores, the stem age will be more recent than the 35 million years ago of A. melanosporus, which was positioned at the base of the genus (Lebel 2013).
Conclusions
The mid-Miocene period of 25–10 million years ago was when the first major step in aridification occurred in Australia and included the radiation of sclerophyllous flora and divergence of specialised mycophagists. On the basis of our modelling, the emergence of sequestrate Agaricomycetes in Australia increased at some point between 34 and 13 million years ago, and these dates place sequestration near the mid-Miocene timeframes described. It is not possible to pinpoint a probable reason for the emergence of sequestrate forms on the basis of dates though, because the current competing factors overlap temporally and are probably interrelated. However, it is clear that sequestration in Australia cannot be due to aridification alone because the Hysterangiales emerged and diversified in a humid and wet climate before the Miocene, and there are at least some sequestrate taxa in the Boletales (specifically Boletaceae) that dispersed into Australia. However, it is likely that aridification was important, at least indirectly, in the evolution of most Australian sequestrate Agaricomycetes (other Boletales, Agaricales and Russulales).
Acknowledgements
We thank two anonymous reviewers for their considered and helpful comments. This research began as a visiting graduate scholarship to E. M. Sheedy to attend the Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville. Funding for participant support was provided by National Science Foundation grant DEB-094951 awarded to P. B. Matheny and N. L. Bougher.
References
Albee-Scott S (2007) The phylogenetic placement of the Leucogastrales, including Mycolevis siccigleba (Cribbeaceae), in the Albatrellaceae using morphological and molecular data. Mycological Research 111, 653–662.| The phylogenetic placement of the Leucogastrales, including Mycolevis siccigleba (Cribbeaceae), in the Albatrellaceae using morphological and molecular data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVKrtLbJ&md5=1d36d3323c60434c3ce5b9cb7d6ee7d7CAS |
Baroni TJ, Matheny PB (2011) A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from eastern North America. Harvard Papers in Botany 16, 293–310.
| A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from eastern North America.Crossref | GoogleScholarGoogle Scholar |
Beaton G, Pegler DN, Young TWK (1984) Gasteroid Basidiomycota of Victoria State, Australia I. Hydnangiaceae. Kew Bulletin 39, 499–508.
| Gasteroid Basidiomycota of Victoria State, Australia I. Hydnangiaceae.Crossref | GoogleScholarGoogle Scholar |
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98, 971–981.
| Molecular systematics and biological diversification of Boletales.Crossref | GoogleScholarGoogle Scholar |
Bonito G, Smith ME, Nowak M, Healy RA, Guevara G, Cázares E, Kinoshita A, Nouhra ER, Domínguez LS, Tedersoo L, Murat C, Wang Y, Moreno BA, Pfister DH, Nara K, Zambonelli A, Trappe JM, Vilgalys R (2013) Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PLoS One 8, e52765
| Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptVaisQ%3D%3D&md5=f648e5cc75f7775d8e66a6d074813bc4CAS |
Bougher NL, Lebel T (2001) Sequestrate (truffle-like) fungi of Australia and New Zealand. Australian Systematic Botany 14, 439–484.
| Sequestrate (truffle-like) fungi of Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar |
Bougher NL, Tommerup IC, Malajczuk N (1993) Broad variation in developmental and mature basidiome morphology of the ectomycorrhizal fungus Hydnangium sublamellatum sp. nov. bridges morhologically based generic concepts of Hydnangium, Podohydnangium and Laccaria. Mycological Research 97, 613–619.
| Broad variation in developmental and mature basidiome morphology of the ectomycorrhizal fungus Hydnangium sublamellatum sp. nov. bridges morhologically based generic concepts of Hydnangium, Podohydnangium and Laccaria.Crossref | GoogleScholarGoogle Scholar |
Braaten CC, Matheny PB, Viess DL, Wood MG, Williams JH, Bougher NL (2014) Two new species of Inocybe from Australia and North America that include novel secotioid forms. Botany 92, 9–22.
| Two new species of Inocybe from Australia and North America that include novel secotioid forms.Crossref | GoogleScholarGoogle Scholar |
Bruns TD, Fogel R, White TJ, Palmer JD (1989) Accelerated evolution of a false-truffle from a mushroom ancestor. Letters to Nature 339, 140–142.
| Accelerated evolution of a false-truffle from a mushroom ancestor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXkt1Wjs7w%3D&md5=4d087c0315c2686c7ef4b38b4eb0a9bdCAS |
Byrne M, Yeates DK, Joseph L, Kearney M, Bowler J, Williams MAJ, Cooper S, Donnellan SC, Keogh JS, Leys R, Melville J, Murphy DJ, Porch N, Wyrwoll KH (2008) Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjhvFGruw%3D%3D&md5=2b12a4d7a8eca8300a43b294a15af11dCAS |
Castellano MA, Bougher NL (1994) Consideration of the taxonomy and biodiversity of Australian ectomycorrhizal fungi. Plant and Soil 159, 37–46.
| Consideration of the taxonomy and biodiversity of Australian ectomycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar |
Castellano MA, Trappe JM, Vernes K (2011) Australian species of Elaphomyces (Elaphomycetaceae, Eurotiales, Ascomycota). Australian Systematic Botany 24, 32–57.
| Australian species of Elaphomyces (Elaphomycetaceae, Eurotiales, Ascomycota).Crossref | GoogleScholarGoogle Scholar |
Claridge AW, Cork SJ (1994) Nutritional value of hypogeal fungal sporocarps for the long-nosed potoroo (Potorous tridactylus), a forest-dwelling mycophagous marsupial. Australian Journal of Zoology 42, 701–710.
| Nutritional value of hypogeal fungal sporocarps for the long-nosed potoroo (Potorous tridactylus), a forest-dwelling mycophagous marsupial.Crossref | GoogleScholarGoogle Scholar |
Claridge AW, May TW (1994) Mycophagy among Australian mammals. Australian Journal of Ecology 19, 251–275.
| Mycophagy among Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Claridge AW, Trappe JM, Mills DJ, Claridge DL (2009) Diversity and habitat relationships of hypogeous fungi. III. Factors influencing the occurrence of fire-adapted species. Mycological Research 113, 792–801.
| Diversity and habitat relationships of hypogeous fungi. III. Factors influencing the occurrence of fire-adapted species.Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Cook LG (2013) How was the Australian flora assembled over the last 65 million years? A molecular phylogenetic perspective. Annual Review of Ecology Evolution and Systematics 44, 303–324.
| How was the Australian flora assembled over the last 65 million years? A molecular phylogenetic perspective.Crossref | GoogleScholarGoogle Scholar |
Crisp M, Cook L, Steane D (2004) Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities? Philosophical Transactions of the Royal Society of London – B. Biological Sciences 359, 1551–1571.
| Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities?Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Hardy NB, Cook LG (2014) Clock model makes a large difference to age estimates of long-stemmed clades with no internal calibration: a test using Australian grasstrees. BMC Evolutionary Biology 14, 263
| Clock model makes a large difference to age estimates of long-stemmed clades with no internal calibration: a test using Australian grasstrees.Crossref | GoogleScholarGoogle Scholar |
Danks M, Lebel T, Vernes K (2010) ‘Cort short on a mountaintop’: eight new species of sequestrate Cortinarius from sub-alpine Australia and affinities to sections within the genus. Persoonia 24, 106–126.
| ‘Cort short on a mountaintop’: eight new species of sequestrate Cortinarius from sub-alpine Australia and affinities to sections within the genus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjgs1aluw%3D%3D&md5=2408fb6cb4284971bd775bd62050c194CAS |
Danks M, Lebel T, Vernes K, Andrew N (2013) Truffle-like fungi sporocarps in a eucalypt-dominated landscape: patterns in diversity and community structure. Fungal Diversity 58, 143–157.
| Truffle-like fungi sporocarps in a eucalypt-dominated landscape: patterns in diversity and community structure.Crossref | GoogleScholarGoogle Scholar |
Dettmann ME, Molnar RE, Douglas JG, Burger D, Fielding CR, Clifford HT, Francis J, Jell P, Rich T, Wade M, Rich PV, Pledge N, Kemp A, Rozefelds A (1992) Australian Cretaceous terrestrial faunas and floras: biostratigraphic and biogeographic implications. Cretaceous Research 13, 207–262.
| Australian Cretaceous terrestrial faunas and floras: biostratigraphic and biogeographic implications.Crossref | GoogleScholarGoogle Scholar |
Donoghue MJ, Smith SA (2004) Patterns in the assembly of temperate forests around the northern hemisphere. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 359, 1633–1644.
| Patterns in the assembly of temperate forests around the northern hemisphere.Crossref | GoogleScholarGoogle Scholar |
Drummond A, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214
| BEAST: Bayesian evolutionary analysis by sampling trees.Crossref | GoogleScholarGoogle Scholar |
Fogel R, States J (2001) Materials for a hypogeous mycoflora of the Great Basin and adjacent cordilleras of the western United States. III: Saprogaster gen. et sp. nov. (Basidiomycota, Phallales). Mycotaxon 80, 315–320.
Fogel R, States J (2002) Materials for a hypogeous mycoflora of the Great Basin and adjacent cordilleras of the western United States. VIII: Pachyphloeus lateritius sp. nov. and Cazia quericola sp. nov. (Ascomycota, Pezizales). Mycotaxon 81, 83–89.
Fujioka T, Chappell J (2010) History of Australian aridity: chronology in the evolution of arid landscapes. Geological Society of London, Special Publications 346, 121–139.
| History of Australian aridity: chronology in the evolution of arid landscapes.Crossref | GoogleScholarGoogle Scholar |
Garnica S, Weiss M, Oertel B, Ammirati J, Oberwinkler F (2009) Phylogenetic relationships in Cortinarius, section Calochroi, inferred from nuclear DNA sequences. BMC Evolutionary Biology 9, 1
| Phylogenetic relationships in Cortinarius, section Calochroi, inferred from nuclear DNA sequences.Crossref | GoogleScholarGoogle Scholar |
Griffiths RP, Baham JE, Caldwell BA (1994) Soil solution chemistry of ectomycorrhizal mats in forest soil. Soil Biology & Biochemistry 26, 331–337.
| Soil solution chemistry of ectomycorrhizal mats in forest soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXis12jtrw%3D&md5=c5b90f9c7004a94014a6593f8e500760CAS |
Haard R, Kramer C (1970) Periodicity of spore discharge in the Hymenomycetes. Mycologia 62, 1145–1169.
| Periodicity of spore discharge in the Hymenomycetes.Crossref | GoogleScholarGoogle Scholar |
Halling RE, Nuhn M, Osmundson T, Fechner N, Trappe JM, Soytong K, Arora D, Hibbett DS, Binder M (2012) Affinities of the Boletus chromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Australian Systematic Botany 25, 418–431.
| Affinities of the Boletus chromapes group to Royoungia and the description of two new genera, Harrya and Australopilus.Crossref | GoogleScholarGoogle Scholar |
Henkel TW, Smith ME, Aime MC (2010) Guyanagaster, a new wood-decaying sequestrate fungal genus related to Armillaria (Physalacriaceae, Agaricales, Basidiomycota). American Journal of Botany 97, 1474–1484.
| Guyanagaster, a new wood-decaying sequestrate fungal genus related to Armillaria (Physalacriaceae, Agaricales, Basidiomycota).Crossref | GoogleScholarGoogle Scholar |
Hibbett DS (2007) After the gold rush, or before the flood? Evolutionary morphology of mushroom-forming fungi (Agaricomycetes) in the early 21st century. Mycological Research 111, 1001–1018.
| After the gold rush, or before the flood? Evolutionary morphology of mushroom-forming fungi (Agaricomycetes) in the early 21st century.Crossref | GoogleScholarGoogle Scholar |
Hibbett DS, Tsuneda A, Murakami S (1994) The secotioid form of Lentinus tigrinus: genetics and development of a fungal morphological innovation. American Journal of Botany 81, 466–478.
| The secotioid form of Lentinus tigrinus: genetics and development of a fungal morphological innovation.Crossref | GoogleScholarGoogle Scholar |
Hibbett DS, Bauer R, Binder M, Giachini AJ, Hosaka K, Justo A, Larsson E, Larsson KH, Lawrey JD, Miettinen O, Nagy LG, Nilsson RH, Weiss M, Thorn RG (2014) Agaricomycetes. In ‘The Mycota vol. 7A: Systematics and Evolution’, 2nd edn. (Eds DJ McLaughlin, JW Spatafora) pp. 373–429. (Springer-Verlag: Berlin, Germany)
Ho SYW (2007) Calibrating molecular estimates of substitution rates and divergence times in birds. Journal of Avian Biology 38, 409–414.
| Calibrating molecular estimates of substitution rates and divergence times in birds.Crossref | GoogleScholarGoogle Scholar |
Hosaka K, Bates ST, Beever RE, Castellano MA, Colgan W, Domínguez LS, Nouhra ER, Geml J, Giachini AJ, Kenney SR, Simpson NB, Spatafora JW, Trappe JM (2006) Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders. Mycologia 98, 949–959.
| Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltlOgu70%3D&md5=b8f3f15cda40bfc3fa5b1bb32dc0b9f2CAS | http://www.ncbi.nlm.nih.gov/pubmed/17486971
Hosaka K, Castellano MA, Spatafora JW (2008) Biogeography of Hysterangiales (Phallomycetidae, Basidiomycota). Mycological Research 112, 448–462.
| Biogeography of Hysterangiales (Phallomycetidae, Basidiomycota).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvVeqsb4%3D&md5=32d134ce9b7c167dc9d2ec72effe6592CAS |
Huston MA (2012) Precipitation, soils, NPP, and biodiversity: resurrection of Albrecht’s curve. Ecological Monographs 82, 277–296.
| Precipitation, soils, NPP, and biodiversity: resurrection of Albrecht’s curve.Crossref | GoogleScholarGoogle Scholar |
Jumpponen A, Claridge AW, Trappe JM, Lebel T, Claridge DL (2004) Ecological relationships among hypogeous fungi and trees: inferences from association analysis integrated with habitat modeling. Mycologia 96, 510–525.
| Ecological relationships among hypogeous fungi and trees: inferences from association analysis integrated with habitat modeling.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FktVKnsA%3D%3D&md5=b742970f10954627228d8fce67061d1fCAS |
Justo A, Morgenstern I, Hallen-Adams HE, Hibbett DS (2010) Convergent evolution of sequestrate forms in Amanita under Mediterranean climate conditions. Mycologia 102, 675–688.
| Convergent evolution of sequestrate forms in Amanita under Mediterranean climate conditions.Crossref | GoogleScholarGoogle Scholar |
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9, 286–298.
| Recent developments in the MAFFT multiple sequence alignment program.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpt1artrs%3D&md5=4ae8346d6a347a8ee0542d09bcad47bfCAS |
Kendrick B (1992) ‘The Fifth Kingdom.’ (Focus Information Group, Inc.: Newburyport, MA, USA)
Ladiges PY, Udovicic F, Nelson G (2003) Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae. Journal of Biogeography 30, 989–998.
| Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae.Crossref | GoogleScholarGoogle Scholar |
Lawver LA, Gahagan LM (2003) Evolution of cenozoic seaways in the circum-antarctic region. Palaeogeography, Palaeoclimatology, Palaeoecology 198, 11–37.
| Evolution of cenozoic seaways in the circum-antarctic region.Crossref | GoogleScholarGoogle Scholar |
Lebel T (2013) Two new species of sequestrate Agaricus (section Minores) from Australia. Mycological Progress 12, 699–707.
| Two new species of sequestrate Agaricus (section Minores) from Australia.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Castellano MA (1999) Australasian truffle-like fungi. IX. History and current trends in the study of the taxonomy of sequestrate macrofungi from Australia and New Zealand. Australian Systematic Botany 12, 803–817.
| Australasian truffle-like fungi. IX. History and current trends in the study of the taxonomy of sequestrate macrofungi from Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Castellano MA (2002) Type studies of sequestrate Russulales II. Australian and New Zealand species related to Russula. Mycologia 94, 327–354.
| Type studies of sequestrate Russulales II. Australian and New Zealand species related to Russula.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Catcheside PS (2009) The truffle genus Cribbea (Physalacriaceae, Agaricales) in Australia. Australian Systematic Botany 22, 39–55.
| The truffle genus Cribbea (Physalacriaceae, Agaricales) in Australia.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Syme A (2012) Sequestrate species of Agaricus and Macrolepiota from Australia: new species and combinations and their position in a calibrated phylogeny. Mycologia 104, 496–520.
| Sequestrate species of Agaricus and Macrolepiota from Australia: new species and combinations and their position in a calibrated phylogeny.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Tonkin JE (2007) Australasian species of Macowanites are sequestrate species of Russula (Russulaceae, Basidiomycota). Australian Systematic Botany 20, 355–381.
| Australasian species of Macowanites are sequestrate species of Russula (Russulaceae, Basidiomycota).Crossref | GoogleScholarGoogle Scholar |
Lebel T, Vellinga EC (2013) Description and affinities of a sequestrate Lepiota (Agaricaceae) from Australia. Mycological Progress 12, 525–532.
| Description and affinities of a sequestrate Lepiota (Agaricaceae) from Australia.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Orihara T, Maekawa N (2012) The sequestrate genus Rosbeeva T.Lebel & Orihara gen. nov. (Boletaceae) from Australasia and Japan: new species and new combinations. Fungal Diversity 52, 49–71.
| The sequestrate genus Rosbeeva T.Lebel & Orihara gen. nov. (Boletaceae) from Australasia and Japan: new species and new combinations.Crossref | GoogleScholarGoogle Scholar |
Lebel T, Castellano MA, Beever RE (2015) Cryptic diversity in the sequestrate genus Stephanospora (Stephanosporaceae: Agaricales) in Australasia. Fungal Biology 119, 201–228.
| Cryptic diversity in the sequestrate genus Stephanospora (Stephanosporaceae: Agaricales) in Australasia.Crossref | GoogleScholarGoogle Scholar |
Lehmkuhl JF, Gould LE, Cázares E, Hosford DR (2004) Truffle abundance and mycophagy by northern flying squirrels in eastern Washington forests. Forest Ecology and Management 200, 49–65.
| Truffle abundance and mycophagy by northern flying squirrels in eastern Washington forests.Crossref | GoogleScholarGoogle Scholar |
Luoma DL, Trappe JM, Claridge AW, Jacobs KM, Cazares E (2003) Relationships among fungi and small mammals in forested ecosystems. Mammal community dynamics: management and conservation. In ‘Coniferous Forests of Western North America’. (Eds CJ Zabel, RG Anthony) pp. 343–373. (Cambridge University Press: New York)
Martin H (2006) Cenozoic climatic change and the development of the arid vegetation in Australia. Journal of Arid Environments 66, 533–563.
| Cenozoic climatic change and the development of the arid vegetation in Australia.Crossref | GoogleScholarGoogle Scholar |
Matheny PB, Aime MC, Bougher NL, Buyck B, Desjardin DE, Horak E, Kropp BR, Lodge DJ, Soytong K, Trappe JM, Hibbett DS (2009) Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. Journal of Biogeography 36, 577–592.
| Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae.Crossref | GoogleScholarGoogle Scholar |
Meredith RW, Westerman M, Springer MS (2009) A phylogeny of Diprotodontia (Marsupialia) based on sequences for five nuclear genes. Molecular Phylogenetics and Evolution 51, 554–571.
| A phylogeny of Diprotodontia (Marsupialia) based on sequences for five nuclear genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlslajs7Y%3D&md5=d711a6f8023bbcfa1dc391b094f24cbdCAS |
Mooers AØ, Vamosi SM, Schluter D (1999) Using phylogenies to test macroevolutionary hypotheses of trait evolution in cranes (Gruinae). American Naturalist 154, 249–259.
| Using phylogenies to test macroevolutionary hypotheses of trait evolution in cranes (Gruinae).Crossref | GoogleScholarGoogle Scholar |
Nagy LG, Walther G, Házi J, Vágvölgyi C, Papp T (2011) Understanding the evolutionary processes of fungal fruiting bodies: correlated evolution and divergence times in the Psathyrellaceae. Systematic Biology 60, 303–317.
| Understanding the evolutionary processes of fungal fruiting bodies: correlated evolution and divergence times in the Psathyrellaceae.Crossref | GoogleScholarGoogle Scholar |
Nguyen VP, Needham AD, Friend JA (2005) A quantitative dietary study of the ‘Critically Endangered’ Gilbert’s potoroo Potorous gilbertii. Australian Mammalogy 27, 1–6.
| A quantitative dietary study of the ‘Critically Endangered’ Gilbert’s potoroo Potorous gilbertii.Crossref | GoogleScholarGoogle Scholar |
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24, 581–583.
| AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVKis7g%3D&md5=2e1e924417b8789e0cdc926f04ffd0faCAS |
Orihara T, Lebel TE, Ge Z-W, Smith ME, Maekawa N (2016) Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of the ITS minisatellite-like insertions for molecular identification. Persoonia 37, 173–198.
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290.
| APE: analyses of phylogenetics and evolution in R language.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXms1eitg%3D%3D&md5=6916b7d175804198fb11c011fc70ac01CAS |
Peintner U, Bougher NL, Castellano MA, Moncalvo JM, Moser MM, Trappe JM, Vilgalys R (2001) Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). American Journal of Botany 88, 2168–2179.
| Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mngt1agtg%3D%3D&md5=9ee55ba2751b188cf925eee8ec08c6b5CAS |
Peintner U, Moncalvo J, Vilgalys R (2004) Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota). Mycologia 96, 1042–1058.
| Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpvVyrtb0%3D&md5=ae3acf66ca59d5d9ab2834b01baa6b00CAS |
Petersen RH, Hughes KW (2010) ‘The Xerula/Oudemansiella complex (Agaricales)’, Nova Hedwegia, Beiheft supplemental series 137. (J. Cramer: Stuttgart, Germany)
Pyron RA, Burbrink FT (2013) Phylogenetic estimates of speciation and extinction rates for testing ecological and evolutionary hypotheses. Trends in Ecology & Evolution 28, 729–736.
| Phylogenetic estimates of speciation and extinction rates for testing ecological and evolutionary hypotheses.Crossref | GoogleScholarGoogle Scholar |
Renner SS (2005) Relaxed molecular clocks for dating historical plant dispersal events. Trends in Plant Science 10, 550–558.
| Relaxed molecular clocks for dating historical plant dispersal events.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFOmtL3M&md5=15882251222a1fd4a0195d4e2d71b674CAS |
Ryberg M, Matheny PB (2011) Dealing with incomplete taxon sampling and diversification of a large clade of mushroom-forming fungi. Evolution 65, 1862–1878.
| Dealing with incomplete taxon sampling and diversification of a large clade of mushroom-forming fungi.Crossref | GoogleScholarGoogle Scholar |
Ryberg M, Matheny PB (2012) Asynchronous origins of ectomycorrhizal clades of Agaricales. Proceedings. Biological Sciences 279, 2003–2011.
| Asynchronous origins of ectomycorrhizal clades of Agaricales.Crossref | GoogleScholarGoogle Scholar |
Sheedy EM, Van de Wouw AP, Howlett BJ, May TW (2013) Multigene sequence data reveal morphologically cryptic phylogenetic species within the genus Laccaria in southern Australia. Mycologia 105, 547–563.
| Multigene sequence data reveal morphologically cryptic phylogenetic species within the genus Laccaria in southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpt1Cht7k%3D&md5=08df042e6049b645651f30ec8edbb121CAS |
Singer R (1951) Thaxterogaster: a new link between Gasteromycetes and Agaricales. Mycologia 43, 215–224.
| Thaxterogaster: a new link between Gasteromycetes and Agaricales.Crossref | GoogleScholarGoogle Scholar |
Singer R, Smith AH (1959) Studies on secotiaceous fungi VI. Setchelliogaster Pouzar. Madroño 15, 73–78.
Skrede I, Engh IB, Binder M, Carlsen T, Kauserud H, Bendiksby M (2011) Evolutionary history of Serpulaceae (Basidiomycota): molecular phylogeny, historical biogeography and evidence for a single transition of nutritional mode. BMC Evolutionary Biology 11, 230
| Evolutionary history of Serpulaceae (Basidiomycota): molecular phylogeny, historical biogeography and evidence for a single transition of nutritional mode.Crossref | GoogleScholarGoogle Scholar |
Smith ME, Schmull M (2011) Tropical truffles: English translation and critical review of F. von Höhnel’s truffles from Java. Mycological Progress 10, 249–260.
| Tropical truffles: English translation and critical review of F. von Höhnel’s truffles from Java.Crossref | GoogleScholarGoogle Scholar |
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690.
| RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKlsbfI&md5=2c4e6894fe988f809d27c552e1656574CAS |
Sytsma KJ, Litt A, Zjhra ML, Pires JC, Nepokroeff M, Conti E, Walker J, Wilson PG, Journal I, Walker J, Wilson PG (2004) Clades, clocks, and continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the southern hemisphere. International Journal of Plant Sciences 165, S85–S105.
| Clades, clocks, and continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the southern hemisphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptFOhurk%3D&md5=7672d458cfb04b2ab1be2b0360e95de8CAS |
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20, 217–263.
| Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages.Crossref | GoogleScholarGoogle Scholar |
Thiers HD (1984) The secotioid syndrome. Mycologia 76, 1–8.
| The secotioid syndrome.Crossref | GoogleScholarGoogle Scholar |
Thornhill AH, Popple LW, Carter RJ, Ho SYW, Crisp MD (2012) Are pollen fossils useful for calibrating relaxed molecular clock dating of phylogenies? A comparative study using Myrtaceae. Molecular Phylogenetics and Evolution 63, 15–27.
| Are pollen fossils useful for calibrating relaxed molecular clock dating of phylogenies? A comparative study using Myrtaceae.Crossref | GoogleScholarGoogle Scholar |
Trappe JM, Claridge AW (2005) Hypogeous fungi: evolution of reproductive and dispersal strategies through interactions with anmals and mycorrhizal plants. In ‘The Fungal Community. Its Organisation and Role in the Ecosystem’. (Eds J Dighton, JF White, P Oudemans) pp. 599–611. (CRC Press: Boca Raton, FL, USA)
Trappe JM, Claridge AW, Jumpponen A (2005) Fire, hypogeous fungi and mycophagous marsupials. Mycological Research 109, 516–518.
| Fire, hypogeous fungi and mycophagous marsupials.Crossref | GoogleScholarGoogle Scholar |
Trappe JM, Nicholls AO, Claridge AW, Cork SJ (2006) Prescribed burning in a Eucalyptus woodland suppresses fruiting of hypogeous fungi, an important food source for mammals. Mycological Research 110, 1333–1339.
| Prescribed burning in a Eucalyptus woodland suppresses fruiting of hypogeous fungi, an important food source for mammals.Crossref | GoogleScholarGoogle Scholar |
Trappe JM, Molina R, Luoma DL, Cázares E, Pilz D, Smith JE, Michael A, Miller SL, Trappe MJ (2009) Diversity, ecology, and conservation of truffle fungi in forests of the Pacific Northwest. USDA Forest Service, Pacific Northwest Research Station, General Technical Report PNW-GTR-772. US Department of Agriculture: Portland, OR, USA.
Trappe JM, Kovács GM, Claridge AW (2010) Comparative taxonomy of desert truffles of the Australian outback and the African Kalahari. Mycological Progress 9, 131–143.
| Comparative taxonomy of desert truffles of the Australian outback and the African Kalahari.Crossref | GoogleScholarGoogle Scholar |
Vanderpoorten A, Goffinet B (2006) Mapping uncertainty and phylogenetic uncertainty in ancestral character state reconstruction: an example in the moss genus Brachytheciastrum. Systematic Biology 55, 957–971.
| Mapping uncertainty and phylogenetic uncertainty in ancestral character state reconstruction: an example in the moss genus Brachytheciastrum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s7ks1WgtA%3D%3D&md5=b49fdbbfc7922c0dfcaa04d95898d64eCAS |
Vernes K (2010) Mycophagy in a community of macropodoid species. In ‘Macropods: the biology of Kangaroos, Wallabies and Rat-Kangaroos’. (Eds G Coulson, M Eldridge) pp. 155–169. (CSIRO Publishing: Melbourne, Vic., Australia)
Vernes K, Johnson CN, Castellano MA (2004) Fire-related changes in biomass of hypogeous sporocarps at foraging points used by a tropical mycophagous marsupial. Mycological Research 108, 1438–1446.
| Fire-related changes in biomass of hypogeous sporocarps at foraging points used by a tropical mycophagous marsupial.Crossref | GoogleScholarGoogle Scholar |
Watling R (1971) Polymorphism in Psilocybe merdaria. New Phytologist 70, 307–326.
| Polymorphism in Psilocybe merdaria.Crossref | GoogleScholarGoogle Scholar |
Watling R, Martín MP (2003) A sequestrate Psilocybe from Scotland. Botanical Journal of Scotland 55, 245–257.
| A sequestrate Psilocybe from Scotland.Crossref | GoogleScholarGoogle Scholar |
Wilson AW, Binder M, Hibbett DS (2011) Effects of gasteroid fruiting body morphology on diversification rates in three independent clades of fungi estimated using binary state speciation and extinction analysis. Evolution 65, 1305–1322.
| Effects of gasteroid fruiting body morphology on diversification rates in three independent clades of fungi estimated using binary state speciation and extinction analysis.Crossref | GoogleScholarGoogle Scholar |