A revision of the Triodia basedowii species complex and close relatives (Poaceae: Chloridoideae)
Benjamin M. Anderson A B C , Kevin R. Thiele A and Matthew D. Barrett A BA School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B Kings Park and Botanic Garden, Botanic Gardens and Parks Authority, Fraser Avenue, Kings Park, WA 6005, Australia.
C Corresponding author. Email: banderson2914@gmail.com
Australian Systematic Botany 30(3) 197-229 https://doi.org/10.1071/SB17011
Submitted: 20 February 2017 Accepted: 7 July 2017 Published: 20 October 2017
Abstract
Perennial grasses commonly known as ‘spinifex’ (Triodia R.Br.) are iconic Australian plants, predominantly found in the arid interior of the continent. In some areas, such as the economically important Pilbara region of Western Australia, current species taxonomy does not account for observed diversity. Previous morphological and molecular studies of Triodia basedowii E.Pritz. and related species have revealed multiple unnamed lineages requiring taxonomic recognition. Here, we describe and name eight new species of Triodia, including T. birriliburu B.M.Anderson, T. chichesterensis B.M.Anderson, T. glabra B.M.Anderson & M.D.Barrett, T. infesta B.M.Anderson & M.D.Barrett, T. mallota B.M.Anderson & M.D.Barrett, T. nana B.M.Anderson, T. scintillans B.M.Anderson & M.D.Barrett, and T. vanleeuwenii B.M.Anderson & M.D.Barrett. We also provide recircumscriptions and revised descriptions for T. basedowii, T. lanigera Domin, T. concinna N.T.Burb. and T. plurinervata N.T.Burb. A key to species and photographs are included.
Additional keywords: new species, Pilbara, spinifex, Western Australia.
Introduction
The genus Triodia R.Br. comprises perennial grasses that form a dominant component of hummock grasslands covering more than 18% of Australia (Department of the Environment 2006). Triodia occurs primarily in the arid central region of Australia, but is also found in the northern tropical savannas and southern semi-arid temperate regions, and is ecologically important as a food source and habitat for animals (Bolton and Latz 1978; Kitchener et al. 1983; Morton and James 1988; Westoby et al. 1988; Lundie-Jenkins et al. 1993; Dickman et al. 1999; Daly et al. 2008). Triodia (common name ‘spinifex’) is known for its needle-like leaf blades and distinctive growth form, many species forming mounded hummocks from repeated branching and stoloniferous expansion, with older plants sometimes forming rings as older material in the centre dies, while newer expansion continues around the periphery (Lazarides 1997). The genus currently comprises 73 described species (Lazarides 1997; Barrett et al. 2005; Armstrong 2008; Hurry et al. 2012; Barrett and Barrett 2015; Crisp et al. 2015).
This revision focuses on a group of Triodia species that has been taxonomically challenging, especially in the Pilbara region of Western Australia, where extensive vegetation surveys are conducted in the course of mining developments. Two names, Triodia basedowii E.Pritz. and T. lanigera Domin, have been applied to an apparent complex of species in the Pilbara, and have been recognised as being difficult to distinguish from each other by Lazarides et al. (2005). The need for taxonomic revision has become more evident with the recent erection of multiple informal names to account for putative new taxa, such as, for example, T. sp. Shovelanna Hill (S. van Leeuwen 3835) and T. sp. Peedamulla (A.A. Mitchell PRP 1636). (Names of informal taxa follow the Australian convention for undescribed taxa, see Barker 2005, and are on the Australian Plant Census, see https://biodiversity.org.au/nsl/services/APC, accessed August 2017.) This group of species is referred to here as the T. basedowii species complex (not equivalent to either iteration of the ‘Basedowii Group’ in Lazarides (1997) or in Lazarides et al. (2005)). In addition to the complex, this revision includes treatments of three close relatives (T. concinna N.T.Burb., T. plurinervata N.T.Burb. and the new species T. infesta B.M.Anderson & M.D.Barrett) that were included with the complex in previous analyses of morphological and molecular data (Anderson et al. 2016, 2017).
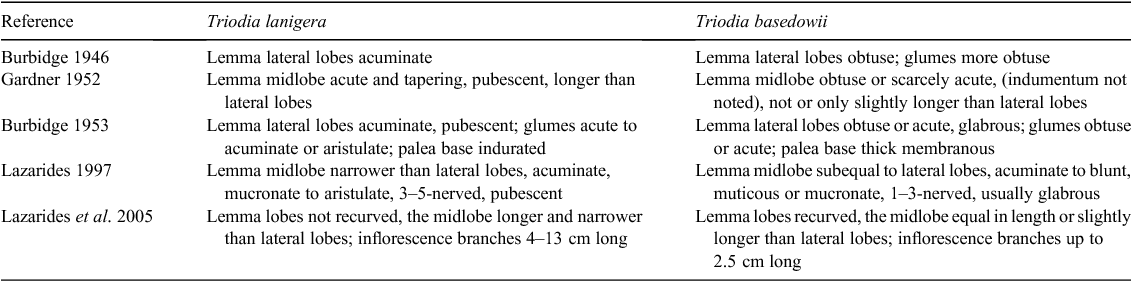
Triodia lanigera was erected by Domin (1912), who noted its leaf sheaths with dense white wool that could be wiped off, making the older sheaths partly or totally glabrous, and spikelet structure that allowed for easy differentiation from known Triodia species. Triodia basedowii was later erected by Pritzel (1918), who noted affinities with T. pungens R.Br. but distinguished T. basedowii by its woolly branches, more deeply trilobed lemmas with more obtuse lobes, and larger spikelets. Revisionary work and flora treatments since then have distinguished between T. lanigera and T. basedowii primarily by using characters of the lemma lobes (Table 1). Two close relatives of the T. basedowii complex included in this revision were described and named by Burbidge (1960). Triodia plurinervata was distinguished from T. spicata N.T.Burb. by its racemose inflorescence and many-nerved, tridentate lemmas, and T. concinna was distinguished from T. inutilis N.T.Burb. by its broadly ovate, purplish spikelets and shortly lobed lemmas.
|
Since the most recent revision of the genus (Lazarides 1997), several informal names have been raised for putative new taxa, as botanists conducting environmental surveys noted regional variation in T. basedowii and T. lanigera. Triodia sp. Shovelanna Hill (S. van Leeuwen 3835) from the Pilbara and T. sp. Little Sandy Desert (S. van Leeuwen 4935) from the Little Sandy Desert were recognised at the Western Australian Herbarium (PERTH) in 2003 and 2005 respectively. Three additional phrase names were raised during the course of the current study. Triodia sp. Peedamulla (A.A. Mitchell PRP 1636) was recognised as distinct from T. lanigera in having glabrous leaf sheaths and orifices and lemma midlobes, with preliminary ITS sequence data supporting the distinction. Triodia sp. Warrawagine (A.L. Payne PRP 1859) was recognised as distinct from T. basedowii and T. lanigera in having shorter leaves and sparkly droplets on young leaf sheaths and inflorescence axes, and from T. sp. Shovelanna Hill (with which it shares short leaves and droplets) in having more branched inflorescences with more spikelets. Finally, T. sp. Pannawonica (B.M. Anderson & M.D. Barrett BMA 89) was recognised as distinct from T. lanigera in having a racemose inflorescence with short pedicels, and from T. plurinervata (with similar inflorescence structure) in having densely woolly leaf sheaths and orifices.
Current species taxonomy in Triodia is based almost entirely on morphological characters. Morphological species delimitation is usually sufficient when species differ strongly, but in taxonomically difficult species complexes where morphological characters intergrade and there are subtle differences between potentially distinct species, other sources of evidence are needed to accurately delimit species, i.e. using integrative taxonomy (Dayrat 2005; Padial et al. 2010; Yeates et al. 2011). Species in this revision, therefore, are delimited using genetic and genomic evidence from two previous studies (Anderson et al. 2016, 2017) in addition to morphology. As outlined in the delimitation work of Anderson et al. (2016), we adopt de Queiroz’s (1998, 2007) general lineage concept of species, under which species are defined as separately evolving segments of metapopulation lineages. In short, criteria that are used to define species under other concepts (e.g. morphological discontinuities, monophyly) are used as lines of evidence for detecting evolutionarily independent lineages (de Queiroz 1998, 2007). The species in this revision are described and diagnosed using morphological characters, following traditional practice, but underlying taxonomic delimitations are informed by differences in chloroplast and nuclear ribosomal sequences as well as genomic divergence.
Materials and methods
Morphological descriptions in the present revision are based on measurements and observations made in the field and on herbarium specimens. Collections made during the course of studies on the T. basedowii complex include ~140 specimens and cover a wide geographical area in Western Australia and the Northern Territory, being concentrated particularly on Western Australia’s Pilbara region where most of the morphological and genetic diversity in the complex is found. In most cases, specimens were collected to voucher populations used for genetic and genomic analyses. Field material was also used to produce the included photographs. Herbarium material (abbreviations from Index Herbariorum, see http://sweetgum.nybg.org/ih/, accessed August 2017) included the collection at PERTH and loans from AD, BRI, CANB, DNA, E, MEL, NSW, and NT. In total, ~1050 herbarium specimens were examined.
In cases where morphological characters between two or more species overlap, ITS sequences from Anderson et al. (2016) were used to make diagnoses clearer. Genomic divergences estimated from distance-based clustering and phylogenetic branch lengths (Anderson et al. 2017) were evaluated in the context of previous morphological and genetic work on the complex (Anderson et al. 2016). Taxa that showed strong evidence for distinction (e.g. co-occurrence, with fixed character differences and without evident mixing; e.g. T. basedowii and T. vanleeuwenii) provided a rough guide to typical genomic divergence and discontinuities between species in the complex, with taxa on much longer branches considered genomically distinct.
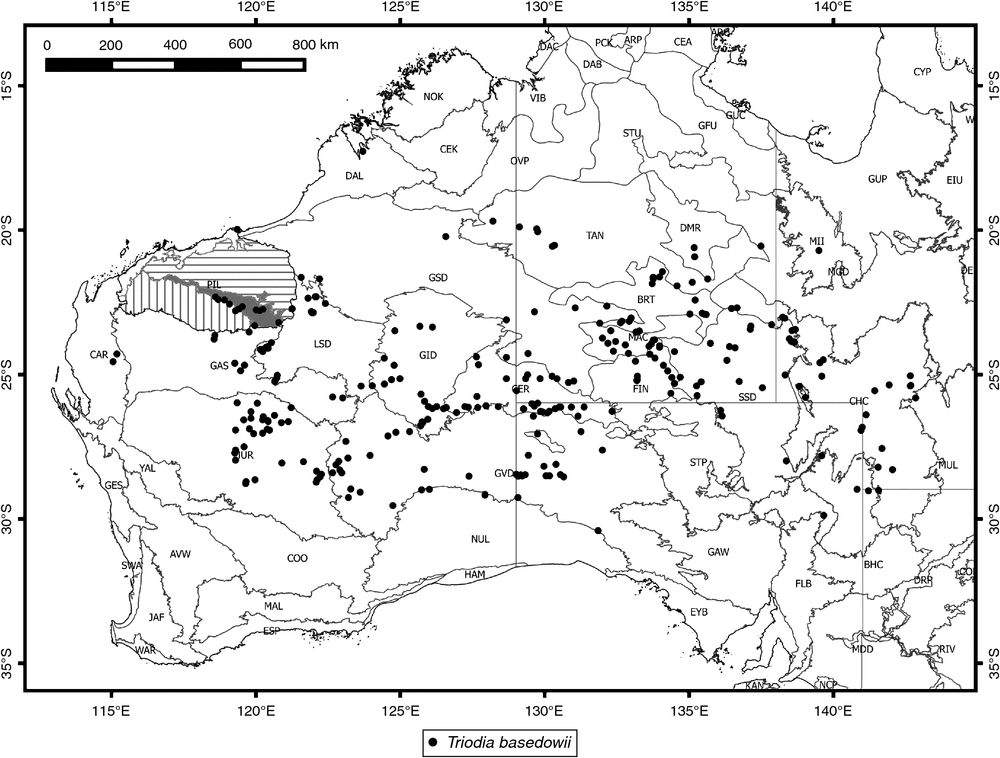
Distribution maps are based on specimens that could be confidently assigned to the species. Due to uncertainty in locality or determination, and limited collecting in some areas, the full extent of each distribution is likely to be slightly larger than shown on the provided maps. Flowering months are recorded on the basis of specimens with fresh or recently dried florets (florets in Triodia may be retained on the plant for multiple seasons following actual flowering, but then appear degraded and aged).
Morphological descriptions were partly compiled with the aid of the package ‘monographaR’ (Reginato 2016) in R, ver. 3.2.5 (R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/, accessed August 2017).
Conservation assessments follow Department of Parks and Wildlife (2015) for P codes (Priority species) or IUCN (2012) for the Least Concern (LC) category.
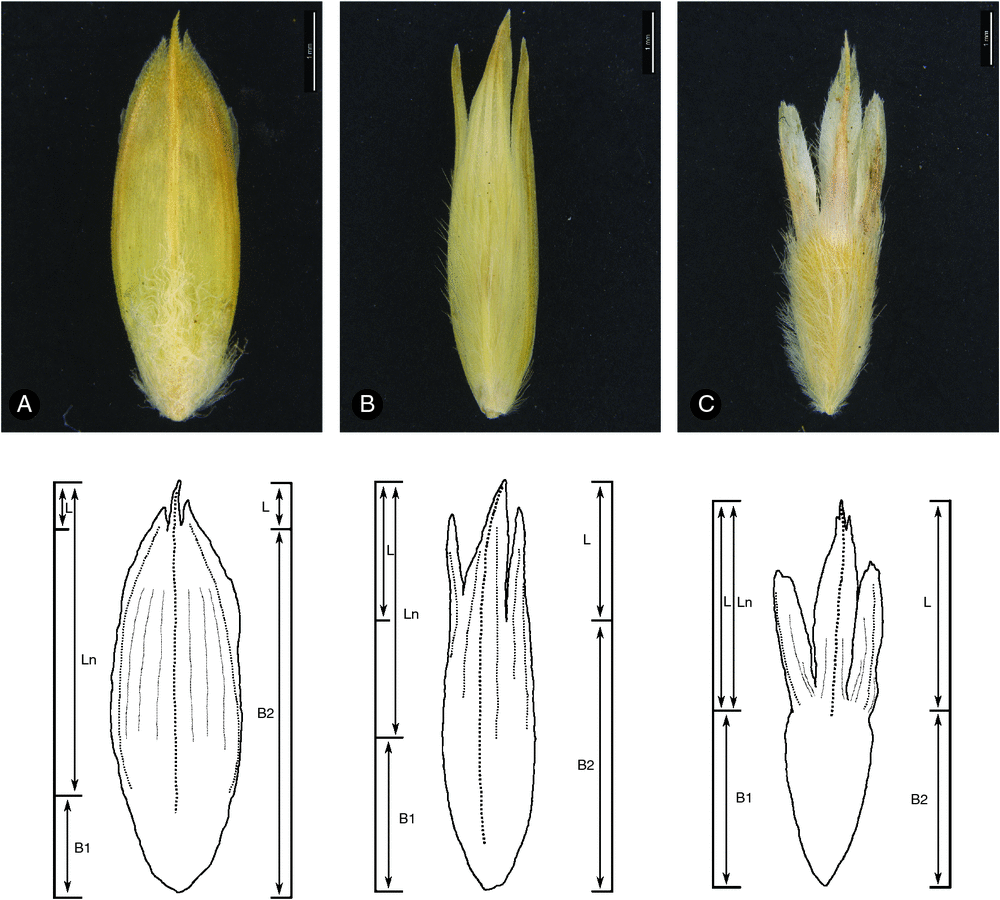
Character measurement and terminology
Morphological characters were measured by hand with a ruler and dissecting microscope. Character measurements were based on a variable number of specimens per taxon, depending on the availability and condition of material. Florets were rehydrated in boiling water with a small amount of detergent. Floret measurements are based on the lowest floret in each of three spikelets per specimen.
‘Orifice hairs’ refer to hairs at the apex of the leaf sheath, between the blade and culm, and including those on auricular extensions. The ligule in the T. basedowii complex comprises a fringe of short, straight hairs; these are not included when reporting orifice indumentum.
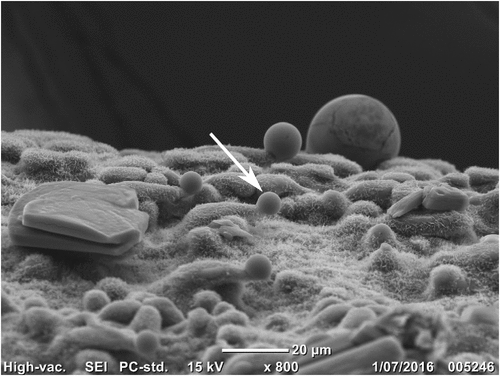
On young material (often leaf sheaths) of T. vanleeuwenii and T. scintillans, there are noticeable droplets that we observed to sparkle in sunlight in the field. These droplets can remain a viscous liquid or become crystalline following specimen drying, and they can even be observed on older herbarium material, depending on the quality of the material at the time of collection. The droplets are soluble in water and may be washed off, and are usually missing from older leaf sheaths near the base of the plant. The droplets were observed to form at the ends of microhairs (Fig. 1), and are possibly homologous to microhair exudates described from chloridoid and panicoid grasses, comprising polysaccharides or proteins (Amarasinghe 1990). Note that these droplets are a distinct phenomenon from the well-known resin-covered leaves of Triodia species not included in this revision.
|
Glume restriction was taken as the proportion of spikelet length held within the glumes, i.e. the furthest extent of the glumes along the spikelet length divided by the spikelet length. Upper glumes and lower glumes are morphologically similar in all members of the group; so, only measurements of the lower glumes are reported.
Lemma morphology is important in differentiating some species. Measurements made for morphometric analyses (Anderson et al. 2016) treated lemma lobing differently from previous taxonomic treatments (e.g. Lazarides 1997), in an attempt to ensure homology among taxa. Species in the T. basedowii complex and close relatives have lemma lobes fused to various extents, and a varying texture differentiation between the lobed portion and the lemma body (Fig. 2). Whereas previous treatments have focused on delineating the lemma into free portions (called lobes) and body, measurements for the present revision use nerve development and texture change to demarcate a ‘lobed’ nerved portion from the more or less nerveless body (Fig. 2). Nerved portion length is, therefore, measured here regardless of fusion, from the tips of the lobes to the point where the nerves, visible in the less-indurated lobes, disappear at the junction of lobe and body. There is, thus, a midlobe ‘nerved portion’ and a lateral lobe ‘nerved portion’ for each species. For consistency with previous treatments, the length of the free portion of the lobes is reported in species with fused lobes, and the length of the midlobe in species with limited lobe fusion is provided for comparison. Thus, the midlobe nerved portion length for T. concinna is reported as 2.5–5 mm, but the actual free portion is only 0.25–1 mm long. The width of the lemma body was measured on flattened lemmas (the measurement is thus the length of the arc around the curve of the lemma body rather than the apparent width when looking at an unflattened lemma).
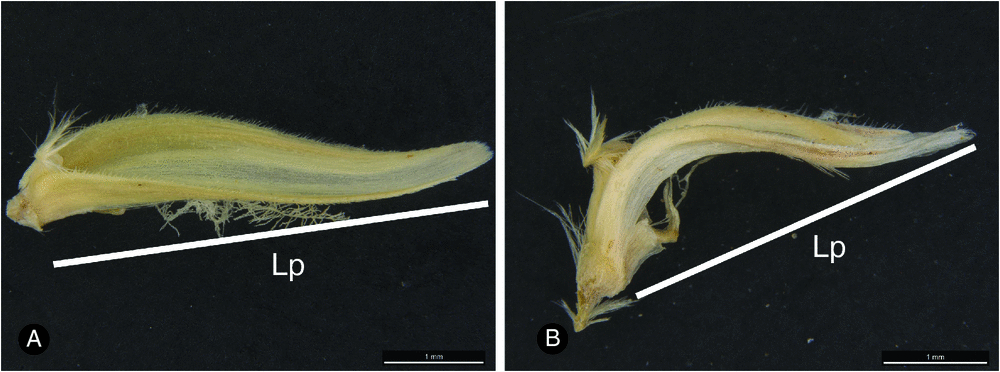
Palea measurements included length, which comprised a straight-line distance from base to tip but without flattening the palea completely (Fig. 3). In strongly curved paleas (most species), this is akin to a hypotenuse and is not equivalent to the actual bent length of the palea.
Outer surfaces of the lemma and palea relative to the floret axis (i.e. the exterior of a floret) are referred to as abaxial surfaces.
Results and discussion
Largely on the basis of previous investigations of the Triodia basedowii complex and close relatives (Anderson et al. 2016, 2017), and in conjunction with additional morphological measurements for the current study, we found evidence to support the recognition of eight new species, four of which are largely restricted to the Pilbara region of Western Australia. A taxonomic account of the group is provided in the following section.
Fire responses of species in the complex, which are likely to be ecologically important, are not reported here, owing to insufficient data for confident scoring of this character. Fire responses in Triodia have been shown to vary both among and within species (Burbidge 1943; Rice and Westoby 1999). Most species in the present revision have no data on fire response, and fire studies may be challenging to interpret because of a previous lack of taxonomic resolution. For example, the response of T. basedowii is reported in Casson and Fox (1987) but the species studied is T. vanleeuwenii. A report that the widespread T. basedowii is fire-killed (Rice and Westoby 1999) is based on a handful of burnt sites that may not encompass a wide-enough range of fire intensities. In different fire conditions, T. basedowii may be able to resprout (B. M. Anderson, pers. obs.).
Taxonomy
Species described here include all known members of the T. basedowii species complex and three closely related species. Together, this group of species shares the following three key character states that, in combination, set them apart from other Triodia species: evenly distributed leaf abaxial stomatal grooves, many-nerved glumes and awnless lemmas. As ‘hard’ Triodia species (see Gardner 1952; Burbidge 1953; Mant et al. 2000), members of the study group have stomatal grooves distributed evenly around the abaxial and adaxial surfaces of their leaf blades (‘soft’ Triodia species have grooves on the adaxial surface and only in a narrow central strip on the abaxial surface). All the species in the present revision have glumes with ≥5 nerves, whereas all but two other ‘hard’ Triodia species have <5 nerves. The two other ‘hard’ species with many-nerved glumes, T. rigidissima (Pilg.) Lazarides and T. desertorum (C.E.Hubb.) Lazarides, differ from the T. basedowii complex and close relatives in having long-awned cf. awnless lemmas.
The group comprises 12 species, with all but one being restricted to Western Australia. The widespread species (T. basedowii) is found in Western Australia, the Northern Territory, South Australia, Queensland and New South Wales. For ease of use, the dichotomous key includes the names of species that follow each couplet choice.
Key to species|
1. Lemmas deeply 3-lobed, the free portions typically at least 1/2 the length of the lemma |
|
2 Inflorescence a panicle, the spikelets with pedicels 2–19 mm long (some always >5 mm long) |
|
3 Leaf sheaths and orifices densely woolly with crimped hairs 1–2 mm long; leaf blades 8–17 cm long; ligule 1–2 mm long |
|
4 Spikelets 12.5–17 mm long; glumes 5.2–6.2 mm long; paleas 5.2–6 mm long |
|
5 Paleas not or only slightly winged, the wings not visible when looking at the broad side of the intact spikelet; leaf blades 7–25 cm long; inflorescence (6.5–)8.2–13.3 cm long; glumes 10–17-nerved |
|
6 Leaf blades 2.5–11(–13.7) cm long, the longest on any specimen rarely >10 cm long; inflorescences 2.8–9.8 cm long with 5–22 spikelets |
|
7 Orifice glabrous to puberulent or rarely pubescent but hairs ≤1(–1.5) mm long, the hairs (if present) not extending along the pseudopetiole onto the blade |
|
8 Callus 0.8–1.5(–1.8) mm long; lemma midlobe nerved portion 4.2–11 mm long, 1.1–2.2× the length of the lateral lobe nerved portion; glumes 6–13.5(–15.8) mm long |
|
9 Body of palea strongly flattened so that the rhachilla diverges approximately perpendicularly away from the palea; inflorescence with (8–)13–29 spikelets on pedicels 2–19 mm long |
|
10 Lemma midlobe nerved portion 2.2–5.2 mm long, abaxially glabrous; lemma lateral lobe nerved portion 2.2–4.2 mm long; spikelets 7–13 mm long. [Gibson Desert or south of the Fortescue River in the Pilbara or, if north of the Fortescue River, in the eastern Pilbara] |
|
11 Minute droplets (sparkly in sunlight) often evident on leaf sheaths; palea 2.8–4.2 × 1–1.5 mm. [Pilbara and adjacent regions] |
|
12 Inflorescences with 7–19 spikelets, (1–)2–7 branches bearing more than one spikelet. [North of the Fortescue River] |
1. Triodia basedowii E.Pritz., Repert. Spec. Nov. Regni Veg. Beih. 15: 356 (1918). N.T.Burbidge, J. Roy. Soc. W. Australia 30: 19, fig. 1 (1946); J.M.Black, Fl. S. Australia, 2nd edn 2: 112 (1948); C.A.Gardner, Fl. W. Australia 67, pl. 19a (1952); N.T.Burbidge, Austral. J. Bot. 1: 153, fig. 10a (1953); M.Lazarides, Grasses Central Australia 207, pls. 59, 60a (1970); M.Lazarides in J.P.Jessop (ed.), Fl. Central Australia 444, fig. 672a,c (1981); J.P.Jessop, Fl. S. Australia, 4th edn 4: 1869, fig. 952a (1986); S.W.L.Jacobs & K.L.McClay in G.J.Harden (ed.), Fl. New South Wales 4: 545 (1993); M.Lazarides, Austral. Syst. Bot. 10: 408 (1997) p.p.; M.Lazarides, C.M.Weiller & A.McCusker in K.Mallett (ed.), Fl. Australia 44B: 221 (2005) p.p.
(Fig. 4.)
Type: central Australia, H. Basedow 315, 1903 (lecto: NSW 298653, designated here).
Diagnostic features
Triodia basedowii is the most widespread and morphologically variable species in the complex, but can usually be distinguished by a combination of deeply three-lobed lemmas, long leaf blades that have orifice hairs extending onto them, and a lemma midlobe less than 1.5 times as long as the lateral lobes. Whereas plants outside the Pilbara may have woolly leaf sheaths, Pilbara populations have glabrous leaf sheaths, which may help distinguish them from the Pilbara species T. lanigera, which usually has woolly leaf sheaths. Internal transcribed spacer (ITS) sequences (Anderson et al. 2016) may be used to distinguish T. basedowii from T. lanigera, even though they have a fixed difference at only one position: (T. basedowii: T. lanigera) Locus ITS:ITS1 (starting from 18S end) at Position 112 (C:T).
Description
Hummocks 0.15–1 m tall, often forming rings, with flowering culms 0.65–1.5 m above the ground. Leaf sheaths glabrous to densely tangled-villous or woolly with hairs 1–4 mm long; margins sometimes tangled-villous or woolly even when outer surface glabrous; orifice pubescent to densely tangled-villous or woolly with hairs 1–4 mm long; ligule 0.2–1(–2) mm long. Blades 72–280 mm long, with orifice hairs extending 3–11(–20) mm along the pseudopetiole onto adaxial surface; pseudopetiole 1–7 mm long. Inflorescence a narrow to broad panicle, 46–195 mm long, with (10–)13–54 spikelets and (2–)4–10 branches bearing more than 1 spikelet; pedicels 1–12(–17) mm long. Spikelets 6.8–17.2 × 2.5–8 mm with (4–)6–12 florets, restricted by the glumes for 0.32–0.96× the length. Lower glume (4.5–)6–9(–11) × (2.2–)3–4(–5) mm, the widest point at 0.3–0.62× the length, (5–)7–12(–15)-nerved, glabrous to scaberulent, sometimes with hairs on the margins to 2 mm long; apex acute to acuminate or rounded. Lowest lemma (5.2–)6–10.5 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to (3.2–)3.5–5.5(–7) mm long; body (1.5–)2–3.5(–4.5) × (2.2–)2.5–3.5(–4) mm, sparsely to densely appressed-pubescent with hairs 0.5–1.2 mm long in the centre and 1.5–3.5 mm long on the margins; midlobe narrowly elliptic to ovate or narrowly triangular, nerved portion (3.2–)3.5–5.5(–7) × (0.5–)0.8–1.2(–1.8) mm, the widest point at (0.05–)0.2–0.5(–0.67)× the length, 0.8–1.4× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous to pubescent similar to the body past 2/3, the margins with hairs 0.2–1.2 mm long, the apex acute to acuminate or rounded; lateral lobes oblong or broadly ovate to obovate, nerved portion 3–5(–6) × (0.8–)1–1.8(–2.2) mm, the widest point at (0.16–)0.2–0.6(–0.77)× the length, (3–)4–6(–8)-nerved, abaxially glabrous to pubescent similar to the body in the lower half, the inner margins puberulent, the apex acute to rounded. Palea (2.2–)3–4.5(–5) × (0.8–)1–1.5 mm, abaxially glabrous to sparsely pubescent with hairs to 0.5 mm long, the keels puberulent; keels and body indurated, the induration becoming weaker towards the apex; apex truncate and ciliate; under-flaps ~0.2 mm wide, often with straight hairs 0.2–1 mm long. Rhachilla segment 0.5–1(–1.2) mm long. Callus (0.2–)0.5–1 mm long, sparsely to densely pubescent with hairs 0.2–2.2 mm long. Lodicules 0.2–1 mm long, sometimes ciliate. Anthers (2–)2.5–3.5(–4.5) mm long. Caryopsis ovoid, 1.8–2.2 × 0.8–1.2 mm.
Distribution
Widespread across arid Australia, from Western Australia through the Northern Territory and South Australia to western Queensland and far north-western New South Wales (Fig. 5).
Habitat and phenology
Occurs on red sandy soils, sometimes with loam, clay or calcrete, often in dunefields in swales and on dune slopes and rarely crests. Florets observed February–November, presumably flowering after heavy rainfall, which is unpredictable in central Australia.
Proposed conservation assessment
Least Concern (LC). This species is widespread and common.
Notes
Triodia basedowii is morphologically variable across its wide geographic range. The name has previously been applied to many specimens now assigned to new species described in the present revision. There is morphological overlap with some specimens of T. lanigera north of the Fortescue River valley in the Pilbara, possibly indicating past hybridisation between the two species, despite genomic evidence (Anderson et al. 2017) that there is substantial divergence between them. Triodia basedowii is the only member of the complex that occurs outside Western Australia. In the Pilbara, it occurs only on sandy soils along the Fortescue River valley. Triodia basedowii has been found growing with T. vanleeuwenii and T. scintillans, and adjacent to both T. birriliburu and T. nana, but without evident hybrids in each of these cases. Although T. basedowii is morphologically challenging to distinguish from some specimens of T. lanigera, genomic data (Anderson et al. 2017) indicate a great deal of differentiation between the two species. The close ITS-sequence similarity (Anderson et al. 2016) may represent past introgression.
Genomic data (Anderson et al. 2017) show population structure within T. basedowii, with central Australian populations grouping separately from most Western Australian populations. ITS sequences (Anderson et al. 2016) suggest differentiation of a group of populations in southern Western Australia, with some correspondence to the character of woolly leaf sheaths, although leaf-sheath woolliness is variable in T. basedowii. These examples of population differentiation might be expected in a widespread species, and we do not consider them distinct enough to merit recognition at species rank.
In revisions of Triodia (Burbidge 1953; Lazarides 1997), two H. Basedow collections (315 and 425) from 1903 were noted as possibly representing type material. Pritzel’s (1918) protologue states the following:
Hab. in Australia centrali (districtu C sec. cl. Tate) leg. H. Basedow, 1903. Ab expeditione Elderiana ad ‘Arkaringa Creek’ in Australia australi collecta et a cl. F. v. Mueller et R. Tate T. pungens nominata specimina in herbario Kewense adsunt.
This has been interpreted to mean that the type, collected by Basedow in 1903, is from the Elder Expedition, collected at Arkaringa Creek in South Australia. Notes by C. Herscovitch on the two Basedow specimens, however, indicate that Basedow did not participate in this expedition and there is no indication that the specimens are from Arkaringa Creek. Lazarides (1997) indicated that possible syntypes were located at Kew; however, a recent inquiry there did not support this. We interpret the wording of the protologue to mean that T. pungens from Arkaringa Creek collected on the Elder Expedition and held in Kew should be referred to the new species, not that Arkaringa Creek is the type locality. We have chosen the H. Basedow 315 specimen at NSW as the lectotype, given it has more vegetative material than does the syntype specimen H. Basedow 425 (both collected in 1903). It is likely that any duplicates that Pritzel may have worked on at the Berlin Herbarium (B) were destroyed during World War II (R. Vogt, pers. comm.).
Representative specimens examined
2. Triodia birriliburu B.M.Anderson, sp. nov.
(Fig. 6.)
Type: Western Australia, Carnarvon Range, Katjarra, B.M. Anderson 61, 13-May-2014 (holo: PERTH; iso: CANB).
Diagnostic features
A long-leaved species distinguished by the combination of usually glabrous leaf orifices and blades, and partly fused lemma lobes, with the free portions typically less than a third of the lemma length. Distinguished from T. infesta, T. mallota and T. plurinervata (with which it shares fused lemma lobes) by a paniculate cf. racemose or spicate inflorescence. Distinguished from T. concinna (with which it shares fused lemma lobes and paniculate inflorescence) by the absence of palea wings, or, if wings are present, then they are small and not visible when looking at the intact spikelet.
Description
Hummocks 0.4–1 m tall with flowering culms to 1–2 m above the ground. Leaf sheaths glabrous; orifice shortly pubescent with hairs <1(–1.5) mm long; ligule 0.2–0.5 mm long. Blades 70–250 mm long, glabrous; pseudopetiole 1.5–4 mm long. Inflorescence a narrow panicle, (65–)82–133 mm long, with (8–)13–29 spikelets and (2–)4–9 branches bearing more than 1 spikelet; pedicels 2–19 mm long. Spikelets 8.4–14.9 × 4.5–7 mm with 7–13 florets, restricted by the glumes for 0.3–0.72× the length. Lower glume 5–7.2 × 3–4.5 mm, the widest point at 0.48–0.69× the length, 10–17-nerved, scaberulent; apex acute to rounded. Lowest lemma 5.8–7.2 mm long, 3-lobed, indurated for most of its length and the body not clearly differentiated from the lobes, with the free portion of the lobes to 1.5–3.8 mm long; body 3–5 × 3.8–5.2 mm, sparsely pubescent with hairs concentrated in the centre and 0.5–1.5 mm long; midlobe consisting of an ovate to triangular free portion above but partly fused to lateral lobes below, nerved portion 2–4 × 0.8–1.5 mm, 0.6–1.1× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous or rarely with a few body hairs in the lower third, the margins puberulent, the apex acute to acuminate; lateral lobes consisting of an ovate to triangular free portion above but partly fused to midlobe below, nerved portion 3–4 × 1.2–1.8 mm, 4–6-nerved, abaxially glabrous, the inner margins puberulent, the apex acute to rounded. Palea 4.2–5 × 1–1.8 mm, flattened so that it is close to perpendicular to the rhachilla, abaxially glabrous, the keels puberulent; keels indurated and the body less so; apex truncate to rounded, sometimes ciliate; under-flaps 0.2–0.5 mm wide, occasionally sparsely hairy. Rhachilla segment 0.5–1 mm long. Callus 0.2–0.5 mm long, glabrous to densely pubescent with hairs 0.5–1.5(–2) mm long. Lodicules 0.2–0.8 mm long. Anthers 3–4 mm long. Caryopsis ~2 × 0.8 mm.
Distribution
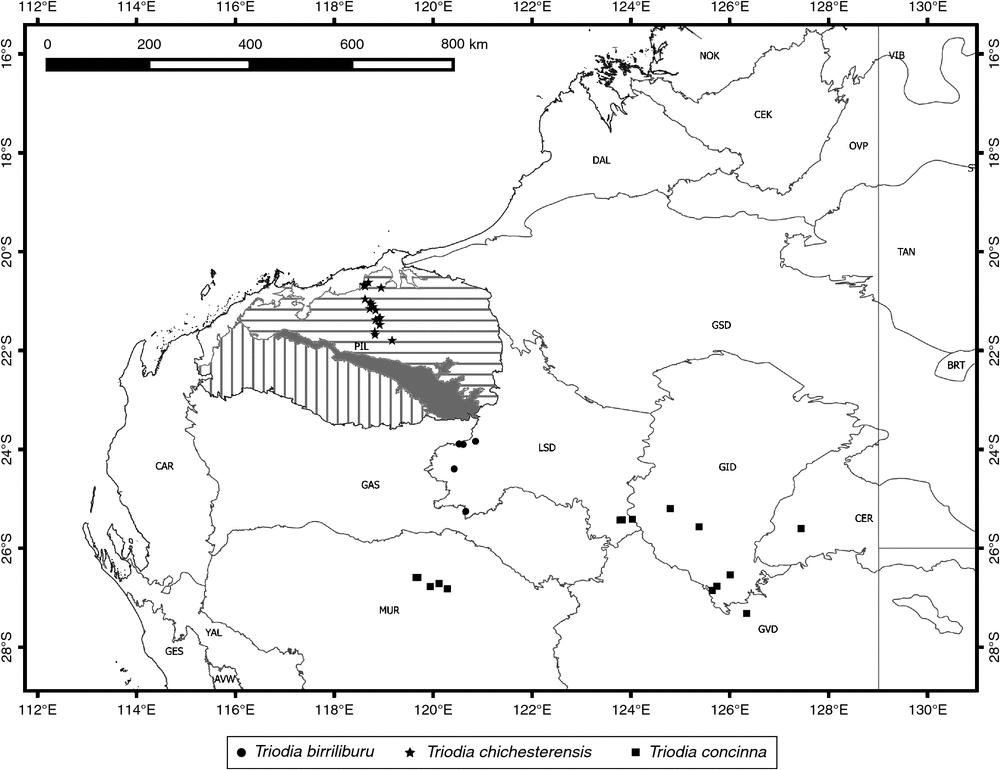
Found only in a narrow portion of the Little Sandy Desert bioregion south of the Pilbara in Western Australia (Fig. 7).
Habitat and phenology
Occurs on red sandy substrates, typically on or adjacent to dunes. Florets observed April–June and August.
Proposed conservation assessment
Priority 3 (P3). This species is known from only a few locations in a narrow area, and partly on protected land. Additional surveys of the Little Sandy Desert and nearby regions are needed to assess the extent of its distribution.
Notes
Triodia birriliburu often grows adjacent to T. basedowii and, although closely related, is distinguished from that species most obviously by its glabrous leaf sheaths and orifices. Where they co-occur, T. birriliburu is preferentially found on or near the dunes, whereas T. basedowii occupies lower parts of the landscape and swales. The partial fusion of lemma lobes and indistinct texture differentiation set T. birriliburu apart from most species in the present study, but those features are similar in T. mallota, T. plurinervata, T. infesta, and T. concinna (see differences above).
ITS and chloroplast sequences (Anderson et al. 2016) do not help distinguish T. birriliburu from T. basedowii, despite the strong morphological differences, whereas genomic data (Anderson et al. 2017) show substantial divergence between the two, although they are consistently recovered as sister taxa. The congruence between morphological and genomic data strongly supports recognition of this species.
Etymology
The specific epithet was chosen by traditional owners of the land where this species is found and is the name for the area: Birriliburu. It is used as a noun in apposition.
Representative specimens examined
3. Triodia chichesterensis B.M.Anderson, sp. nov.
(Fig. 8.)
Type: Western Australia, Pippingarra Road, ~70 km south of the Great Northern Highway, M.D. Barrett 4106, 19-Mar-2013 (holo: PERTH; iso: CANB, K).
Diagnostic features
A short-leaved species, distinguished by the combination of diminutive stature, glabrous leaf sheaths, relatively unbranched inflorescence, often short pedicels, and pubescent lemma midlobe. The short pedicels and pubescent lemma midlobe contrast with the typically longer pedicels and glabrous lemma midlobes of other short-leaved species in the complex (T. nana, T. scintillans, T. vanleeuwenii). Distinguished from the closely related Triodia lanigera by its shorter and less hairy leaves and less branched inflorescence. ITS sequences (Anderson et al. 2016) distinguish it from co-occurring T. lanigera, although hybrids have been observed (see Notes): (T. lanigera: T. chichesterensis) Locus ITS:ITS1 (starting from 18S end) at Position 71 (A:T), 105 (G:A), 112 (T:C); ITS2 (starting from 5.8S end) at Position 24 (C:T), 55–62 (C--AGTGC:CRCAGGCC), 122 (G:A), 143 (T:C), 191 (A:T).
Description
Hummocks diminutive, 0.2–0.4 m tall, with flowering culms to 0.3–0.5 m above the ground. Leaf sheaths glabrous; orifice woolly to straight-pubescent with hairs 2–3 mm long (these sometimes worn off older leaves); ligule 0.2–1 mm long. Blades 30–110 mm long, glabrous or with a few orifice hairs extending onto the pseudopetiole; pseudopetiole 1–4 mm long. Inflorescence a racemose panicle, 30–62 mm long, with 4–11 spikelets and 0–3 branches bearing more than 1 spikelet; pedicels 1–8 mm long. Spikelets 10–16.5 × 5–10 mm with 5–11 florets, restricted by the glumes for 0.42–0.78× the length. Lower glume 6.5–10 × 2.8–3.8 mm, the widest point at 0.3–0.53× the length, 9–14-nerved, glabrous to scaberulent; apex acute to acuminate or rounded. Lowest lemma 6.2–10.5 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to 4.2–8 mm long; body 2–3.8 × 2.5–3.8 mm, pubescent with hairs 0.5–1 mm long in the centre and 1–2 mm long on the margins; midlobe narrowly ovate to elliptic, nerved portion 4.2–8 × 1–1.5 mm, the widest point at 0.23–0.43× the length, 1.1–1.6× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially pubescent to sparsely so typically in the lower half, the margins puberulent to shortly pubescent, the apex acuminate; lateral lobes ovate to elliptic, nerved portion 3.5–5.5 × 1.2–2 mm, the widest point at 0.2–0.5× the length, 5–8-nerved, abaxially glabrous to sparsely pubescent in the lower third, the inner margins puberulent, the apex acute to acuminate or rounded. Palea 3–4.2 × 1–1.2 mm, abaxially glabrous, the keels puberulent; keels and body indurated, the induration becoming weaker towards the apex or sometimes becoming stronger towards the apex; apex truncate to rounded, sometimes ciliate; under-flaps ~0.2 mm wide, occasionally sparsely pubescent. Rhachilla segment 0.5–1 mm long. Callus 0.2–1 mm long, glabrous to sparsely pubescent with hairs 0.2–1 mm long. Lodicules 0.2–0.5 mm long. Anthers 2.8–3.5 mm long. Caryopsis not seen.
Distribution
This species has been found only in a narrow area in the central Chichester region of the Pilbara of Western Australia (Fig. 7). The areas immediately to the west and east of its known distribution are poorly explored, but it is likely to be restricted to an area <100 km beyond current collections, given intensive collecting efforts in the Pilbara.
Habitat and phenology
Occurs in rocky to gravelly substrates of loam or sand, often with quartzite pieces evident on the surface. Florets observed February–April and August.
Proposed conservation assessment
Priority 3 (P3). This species is only known from a narrow area in the northern Pilbara, but is not under immediate threat.
Notes
Triodia chichesterensis is closely related to T. lanigera, and its florets resemble those of that species. It co-occurs with T. lanigera, with little evidence of genomic mixing in some northern populations, but hybridises with T. lanigera in the southern end of its range. It can usually be distinguished from T. lanigera by its shorter and less hairy leaves and less branched inflorescences; however, these differences break down in the hybrid populations in the south. Where the two co-occur in the north, there is a subtle but consistent substrate change that marks the shift in species, with T. lanigera occurring on sandier soils and T. chichesterensis on rockier soils with quartzite pieces. Within a hybrid population in the south with less distinct substrate changes, there is an apparent cline in plant morphology and genomic evidence of variable admixture (see Anderson et al. 2017).
Internal transcribed spacer sequences (Anderson et al. 2016) indicate that this species forms a distinct clade, although hybrid individuals have copies from both putative parents. Despite evidence of hybridisation, genomic data (Anderson et al. 2017) show that this species forms a distinct cluster and remains distinct while co-occurring with T. lanigera in the north, consistent with its recognition here.
Etymology
The specific epithet refers to the Chichester region of the Pilbara where this species is found.
Representative specimens examined
4. Triodia concinna N.T.Burb., Austral. J. Bot. 8: 387, fig. 3a (1960). M.Lazarides, Austral. Syst. Bot. 10: 425 (1997); M.Lazarides, C.M.Weiller & A.McCusker in K.Mallett (ed.), Fl. Australia 44B: 210 (2005)
(Fig. 9.)
Type: Western Australia, 30 miles (~48.3 km) west of Wiluna, N. Speck 1482, 18-Sep-1958 (holo: CANB n.v.; iso: AD n.v., BRI, L n.v., MEL, NSW, PERTH, US n.v., but photograph seen).
Diagnostic features
Distinguished by a combination of paniculate inflorescence, partly fused lemma lobes with the free portion less than a third of the length of the lemma, and noticeable palea wings that are visible when the spikelet is viewed on the broad side.
Description
Hummocks ~0.5–0.7 m tall, sometimes forming rings. Leaf sheaths glabrous, except for occasionally pubescent margins; orifice glabrous to tangled-villous with hairs 0.25–0.75 mm long; ligule 0.2–0.5 mm long. Blades 25–88 mm long, glabrous; pseudopetiole 1–2 mm long. Inflorescence a narrow panicle, 50–88 mm long, with 10–22 spikelets and 3–7 branches bearing more than 1 spikelet; pedicels 2–13 mm long. Spikelets 8–11 × 4–5 mm with 5–10 florets, restricted by the glumes for 0.28–0.56× the length. Lower glume 3.8–5 × 2.2–3.2 mm, the widest point at 0.33–0.59× the length, 5–11-nerved, scaberulent on nerves; apex acute to acuminate. Lowest lemma 4.5–6 mm long, shortly 3-lobed, the lobes not always clearly differentiated from the body, free portion of the lobes to 0.25–1 mm long; body 1–2 × 2.8–3.8 mm, with curly or wavy hairs 0.5–1.5 mm long concentrated in the lower central portion; midlobe comprising a triangular free portion above but fused to lateral lobes below, nerved portion 2.5–5 mm long, 0.8–1.1× the length of the lateral lobe nerved portion, 1- or 2-nerved, abaxially glabrous, the margins puberulent, the apex acute; lateral lobes comprising a triangular free portion above but fused to the midlobe below, nerved portion 3.2–5 mm long, 2–4-nerved, abaxially glabrous, the inner margins puberulent, the apex acute. Palea 4.5–5.2 × 1.2–1.8 mm, abaxially mostly glabrous except for a few curly hairs near curved junction with the rhachilla; keels and body indurated; keels obviously winged above, the wings visible when looking at the intact spikelet; apex narrowing to truncate; under-flaps 0.2–1 mm wide, glabrous. Rhachilla segment 0.2–1 mm long. Callus 0.2–0.5 mm long, glabrous to densely pubescent with hairs to 1.2 mm long. Lodicules 0.2–0.5 mm long. Anthers 2.5–2.8 mm long. Caryopsis round, ~1.2 × 1 mm.
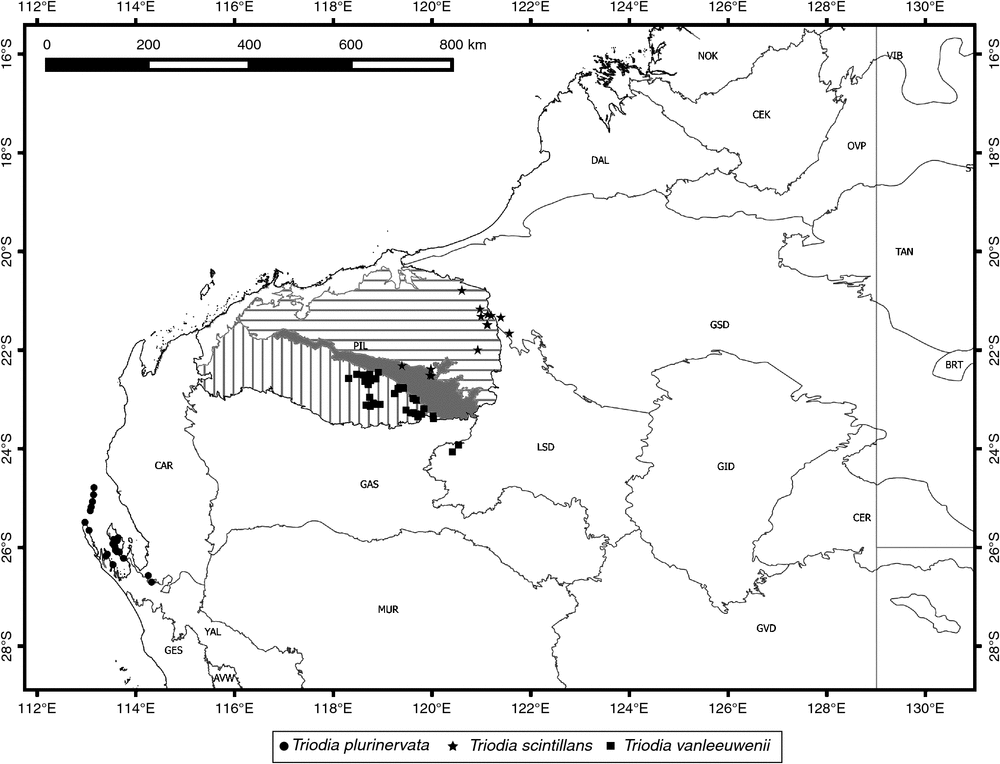
Distribution
Found in two clusters of more or less contiguous populations, these more than ~300 km apart, in central Western Australia (Fig. 7).
Habitat and phenology
Occurs on red or lateritic loam to red sandy silt. Florets observed in April, July and September.
Proposed conservation assessment
Least Concern (LC). This species is widespread and lacks obvious threats.
Notes
Triodia concinna is easily recognised by its distinctive spikelets, with the winged paleas clearly visible when looking at the spikelet’s broad side, a feature not found in the T. basedowii complex. Triodia concinna shares the character of fused lemma lobes with T. mallota, T. plurinervata and T. infesta, but differs from those species in its inflorescence structure (paniculate v. racemose or spicate).
The two disjunct groups of populations are genetically differentiated both in ITS sequences (Anderson et al. 2016) and genomic data (Anderson et al. 2017), but are morphologically very similar and do not show chloroplast differentiation in the rps16–trnK spacer. The two groups consistently form a single clade in genomic analyses (Anderson et al. 2017). Further sampling may reveal more consistent morphological differences or genomic intermediates, but given current sampling, we are hesitant to split this species. The ITS and genomic variation are here treated as phylogeographic structure within a single species.
Representative specimens examined
5. Triodia glabra B.M.Anderson & M.D.Barrett, sp. nov.
(Fig. 10.)
Type: Western Australia, 219 km west of Paraburdoo on the Tom Price–Paraburdoo road, then continuing on the Nanutarra–Wittenoom road, M.D. Barrett 3978B, 13-Mar-2012 (holo: PERTH; iso: CANB).
Diagnostic features
A long-leaved species distinguished by a combination of glabrous leaf sheaths and usually glabrous orifices, and deeply 3-lobed lemmas with mostly glabrous midlobes. Distinguished from T. birriliburu, with which it shares the glabrous leaf sheaths and orifices, by its deeply 3-lobed lemmas (cf. fused lemma lobes) and geographic distribution in the far west of the Pilbara and onto the Carnarvon sandplains (cf. Little Sandy Desert).
Description
Hummocks 0.25–0.8 m tall, with flowering culms to ~0.5–1.2 m above the ground. Leaf sheaths glabrous except for occasionally puberulent margins; orifice glabrous to pubescent with hairs 1–2.5 mm long; ligule 0.2–1 mm long. Blades 50–230 mm long, glabrous; pseudopetiole 1–6 mm long. Inflorescence a narrow panicle, 45–105 mm long, with 9–15 spikelets and 2–5 branches bearing more than 1 spikelet; pedicels 1–11 mm long. Spikelets 6.8–20 × 3–8 mm with 5–10 florets, restricted by the glumes for 0.36–0.69× the length. Lower glume 4.5–10 × 2–5 mm, the widest point at 0.28–0.56× the length, 8–15-nerved, glabrous to scaberulent; apex acuminate to acute or rounded. Lowest lemma 4–11 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to 2.2–7.5 mm long; body 1.8–3.5 × 2–4 mm, appressed-pubescent with hairs 0.8–1.5 mm long in the centre and 1.5–3 mm long on the margins; midlobe narrowly ovate to elliptic, nerved portion 2.2–7.5 × 0.8–2 mm, the widest point at 0.11–0.52× the length, 1.1–1.6× as long as lateral lobe nerved portion, 3–5-nerved, abaxially glabrous or occasionally with body hairs in the lower third, the margins puberulent to shortly pubescent with hairs 0.5 mm long, the apex acuminate to acute; lateral lobes ovate to elliptic or linear, nerved portion 2–6 × 1–2.2 mm, the widest point at 0.18–0.6× the length, 3–8-nerved, abaxially glabrous, the inner margins puberulent, the apex acute to acuminate or rounded. Palea 2.2–4.8 × 0.8–1.8 mm, abaxially glabrous, the keels puberulent; keels and body indurated, the induration becoming weaker towards the apex; apex truncate to rounded, sometimes ciliate and jagged; under-flaps 0.2–0.5 mm wide, glabrous to pubescent with hairs 0.2–0.5 mm long. Rhachilla segment 0.5–1.5 mm long. Callus 0.2–1 mm long, pubescent (sometimes glabrous above) with hairs 0.2–2(–2.5) mm long. Lodicules 0.2–0.5 mm long. Anthers 2.5–3 mm long. Caryopsis ellipsoid, ~1.2 × 0.5 mm.
Distribution
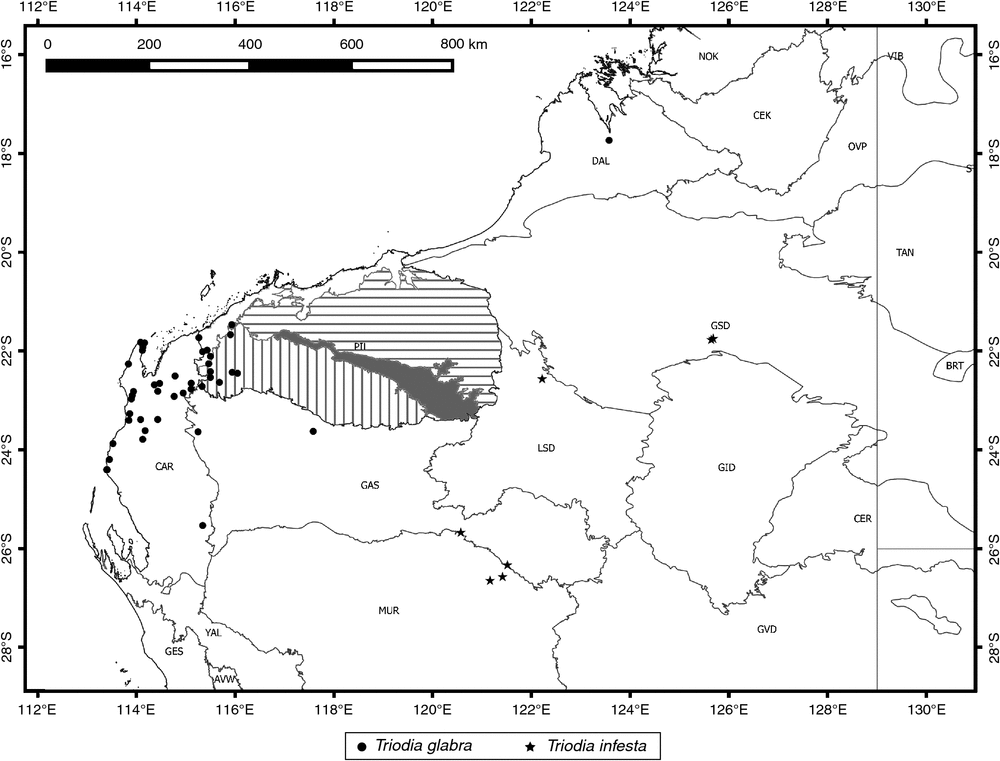
Found in the far western Pilbara and onto the Carnarvon sand plains, including a partially disjunct set of coastal populations occurring in and south of the Cape Range (Fig. 11).
Habitat and phenology
Occurs on sandy to clayey soil in sand plains, dunes or coastal sandhills. Florets observed February–October (presumably following heavy rain).
Proposed conservation assessment
Least Concern (LC). This species is widespread with no obvious threats.
Notes
Some collections of Triodia glabra include annotations identifying the plants as a glabrous-sheathed form of T. lanigera (note: some T. lanigera individuals, especially in southern populations, may have glabrous sheaths). The difference in the leaf orifices (mostly glabrous in this species, but woolly in T. lanigera) is a better character to distinguish the two than is sheath indumentum, besides their geographically disjunct distributions.
Triodia glabra is strongly distinguished from other species in the T. basedowii complex in both ribosomal and chloroplast sequences (Anderson et al. 2016), as well as in genomic data (Anderson et al. 2017).
The western coastal populations of T. glabra have some morphological differences (e.g. smaller spikelets and smaller length difference between lemma midlobes and lateral lobes), but there is overlap. ITS sequences (Anderson et al. 2016) do not distinguish between the two sets of populations, but genomic data (Anderson et al. 2017) suggest that there is differentiation, with some analyses failing to recover the two groups in a single clade. Our limited sampling of the coastal populations makes it difficult to ascertain whether they are distinct enough to be recognised as a separate species, and we are hesitant to split the species on the basis of these differences. The morphological similarities and ITS sequence sharing suggest that the variation may represent phylogeographic structure in the species or the early stages of speciation. The western populations were referred to informally as T. ‘wcoast’ in Anderson et al. (2016, 2017), but are here recognised as part of T. glabra.
Etymology
The specific epithet is from the Latin glaber (hairless, bald), in reference to the most common state of this species with glabrous leaf sheaths and orifices (rarely there are some straight hairs at the orifice and apex of the sheath).
Representative specimens examined
6. Triodia infesta B.M.Anderson & M.D.Barrett, sp. nov.
(Fig. 12.)
Type: Western Australia, 97 km east of Wiluna on Wongawol Road, B.M. Anderson 42, 6-Apr-2014 (holo: PERTH; iso: CANB).
Diagnostic features
Distinguished from species in this revision (apart from T. plurinervata) by the combination of glabrous leaf sheaths, racemose inflorescence, and lemma lobes fused, with the free portion less than one-third of the length. These character states are shared with T. plurinervata, but T. infesta differs primarily in its larger size, having larger hummocks, inflorescences and spikelets. ITS sequences (Anderson et al. 2016) readily distinguish T. infesta from T. plurinervata: (T. infesta: T. plurinervata) Locus ITS:ITS1 (starting from 18S end) at Position 79 (G:C), 188 (G:T), 193 (C:A); ITS2 (starting from 5.8S end) at Position 24 (C:A), 151 (A:T), 196 (C:T).
Description
Hummocks ~0.5–0.8 m tall, often forming rings or half-circles, with flowering culms to 1.2–1.5 m above the ground. Leaf sheaths glabrous except for puberulent margins; orifice puberulent; ligule 0.2 mm long. Blades 47–98 mm long, glabrous; pseudopetiole 1–2 mm long. Inflorescence racemose, 45–75 mm long, with 6–8 spikelets and no branches bearing more than 1 spikelet; pedicels 1–3 mm long. Spikelets 12.5–17 × 3 mm with 6–8 florets, restricted by the glumes for 0.3–0.5× the length. Lower glume 5.2–6.2 × 2.2–2.5 mm, the widest point at 0.35–0.5× the length, 6–9-nerved, scaberulent; apex acute to acuminate. Lowest lemma 5.5–7 mm long, shortly 3-lobed, the indurated body scarcely differentiated from the lobes, with the free portion of the lobes to 0.5–1 mm long; body 2–3 × 3–4 mm, densely pubescent with hairs 1–1.5 mm long in the centre and to 2 mm long on the margins; midlobe comprising a triangular free portion above but fused to lateral lobes below, nerved portion 3.5–4 mm long, 0.8–1.1× the length of the lateral lobe nerved portion, 4–6-nerved, abaxially glabrous including the margins, the apex acute to acuminate; lateral lobes comprising a triangular free portion above but fused to midlobe below, nerved portion 3.5–4.8 mm long, 5–8-nerved, abaxially glabrous including the margins, the apex acute. Palea 5.2–6 × 1–1.2 mm, abaxially sparsely hairy near junction with rhachilla, the keels puberulent; keels indurated, with small wings; apex narrowly truncate; under-flaps not measured. Rhachilla segment 1–1.2 mm long. Callus 0.8–1 mm long, patchy to densely pubescent with hairs 1–2 mm long. Lodicules 0.2–0.5 mm long. Anthers 3–3.5 mm long. Caryopsis ~1.8 × 0.8–1 mm.
Distribution
Known from a few scattered collections in central Western Australia, possibly representing vicariant populations (Fig. 11). It is disjunct by ~600 km from its sister species T. plurinervata on the coast.
Habitat and phenology
Occurs on red loamy flats, dunes and rocky hilltops, sometimes in close proximity to saline substrates (e.g. on a rise in a salt lake). Florets observed April, May and September.
Proposed conservation assessment
Priority 3 (P3). This species is known from only a few widely scattered collections.
Notes
Triodia infesta is similar in most respects to its sister species, T. plurinervata, but differs mostly in its larger size, with substantially larger spikelets. When Burbidge (1960) described T. plurinervata, she noted that a specimen of T. infesta (N.H. Speck 1434) from CANB was distinct from the type of T. plurinervata. She refrained from describing it as a separate species because of concerns that an insect larval infestation had distorted the morphology. Our collections from a nearby locality also showed this larval infestation, which tends to distort the palea so that it is much shorter than the lemma and to lengthen the under-flaps to wrap around the larva. A few florets were found without the infestation, in which the palea is clearly longer (whereas the lemma is largely the same), and on which the length measurements are based.
Triodia infesta is consistently recovered as distinct from T. plurinervata in ITS and chloroplast sequences (Anderson et al. 2016) and genomic data (Anderson et al. 2017). Although the two species share diagnostic characters, the size difference, genetic and genomic divergence and large geographic disjunction suggest that they are distinct.
Etymology
The specific epithet is from the Latin infestus (hostile, dangerous, infested), in reference to the frequent observation of insect larval infestation in the florets, as well as to the first mention of this species by Nancy Burbidge, who commented on the infestation.
Representative specimens examined
7. Triodia lanigera Domin, J. Linn. Soc., Bot. 41: 278 (1912). N.T.Burbidge, J. Roy. Soc. W. Australia 30: 20, fig. 3 (1946) p.p.; C.A.Gardner, Fl. W. Australia 73, pl. 19b (1952) p.p.; N.T.Burbidge, Austral. J. Bot. 1: 155, fig. 10b (1953) p.p.; M.Lazarides, Austral. Syst. Bot. 10: 443 (1997) p.p.; M.Lazarides, C.M.Weiller & A.McCusker in K.Mallett (ed.), Fl. Australia 44B: 222 (2005) p.p
Type: Western Australia, between the Ashburton and Yule Rivers, E. Clement s.n., s. dat. (holo: K n.v., photograph at PERTH 3912205; iso: K n.v., photograph at PERTH 3914348).
Diagnostic features
A long-leaved species distinguished by the combination of woolly leaf orifices, with the indumentum extending onto the blade, and deeply three-lobed lemmas with relatively long midlobes that are 1.1–2.2 times the length of the lateral lobes. Distinguished from T. basedowii in the Pilbara by its usually woolly (cf. glabrous) sheaths, and by an ITS difference (see Diagnostic features under T. basedowii). Distinguished from T. chichesterensis (with which it hybridises) by its usually longer leaves, larger stature and more branched inflorescence, and by ITS differences (see Diagnostic features under T. chichesterensis).
Description
Hummocks 0.2–0.5 m tall, not observed forming rings, with flowering culms to 0.6–1.2 m above the ground. Leaf sheaths glabrous to densely tangled-villous or woolly, the hairs 1–4 mm long; orifice tangled-villous to sometimes straight-pubescent or woolly, the hairs 0.2–4 mm long; ligule 0.2–1.2 mm long. Blades 35–280(–385) mm long, with orifice hairs extending 3–10(–15) mm along the pseudopetiole onto adaxial surface, rarely glabrous; pseudopetiole 1–7 mm long. Inflorescence a narrow panicle, 33–143(–180) mm long, with 5–38 spikelets and (0–)2–9 branches bearing more than 1 spikelet; pedicels 1–19 mm long. Spikelets 10–21(–26.6) × 3–10 mm with 6–11 florets, restricted by the glumes for 0.34–0.84× the length. Lower glume 6–13.5(–15.8) × 3–5.5 mm, the widest point at 0.33–0.58× length, (7–)9–17-nerved, scaberulent to glabrous; apex acute to acuminate or shortly awned. Lowest lemma 7.2–15.5 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to 4.2–11 mm long; body 2.2–4.5 × 2.8–4.5 mm, sparsely to densely appressed-pubescent with hairs 0.2–1 mm long in the centre and 1–2 mm long on the margins; midlobe narrowly ovate to elliptic, nerved portion 4.2–11 × 1–2 mm, the widest point at 0.17–0.47× the length, 1.1–2.2× the length of the lateral lobe nerved portion, 3–7-nerved, abaxially glabrous to pubescent similar to the body past 2/3, the margins puberulent, the apex acute to long-acuminate; lateral lobes elliptic to ovate, nerved portion 3.2–6.5 × 1.2–2 mm, the widest point at 0.25–0.56× length, 5–10-nerved, abaxially glabrous to pubescent similar to the body typically beyond half its length, the inner margins puberulent, the apex acute to acuminate or rounded. Palea 3–5(–6) × 1–1.8 mm, abaxially glabrous to pubescent near the junction with the rhachilla with hairs 0.2–1 mm long, the keels glabrous to puberulent; keels and body indurated, the induration becoming weaker towards the apex; apex truncate, sometimes ciliate; under-flaps 0.2(–0.5) mm wide, glabrous to sparsely pubescent with hairs to 0.5 mm long. Rhachilla segment 0.8–1.5(–1.8) mm long. Callus 0.8–1.5(–1.8) mm long, densely pubescent (sometimes glabrous above) with hairs 0.2–1 mm long. Lodicules 0.2–0.8 mm long, sometimes ciliate. Anthers 1.2–4 mm long. Caryopsis ovoid to broadly ellipsoid, 1.5–2.5 × 0.5–1.2 mm.
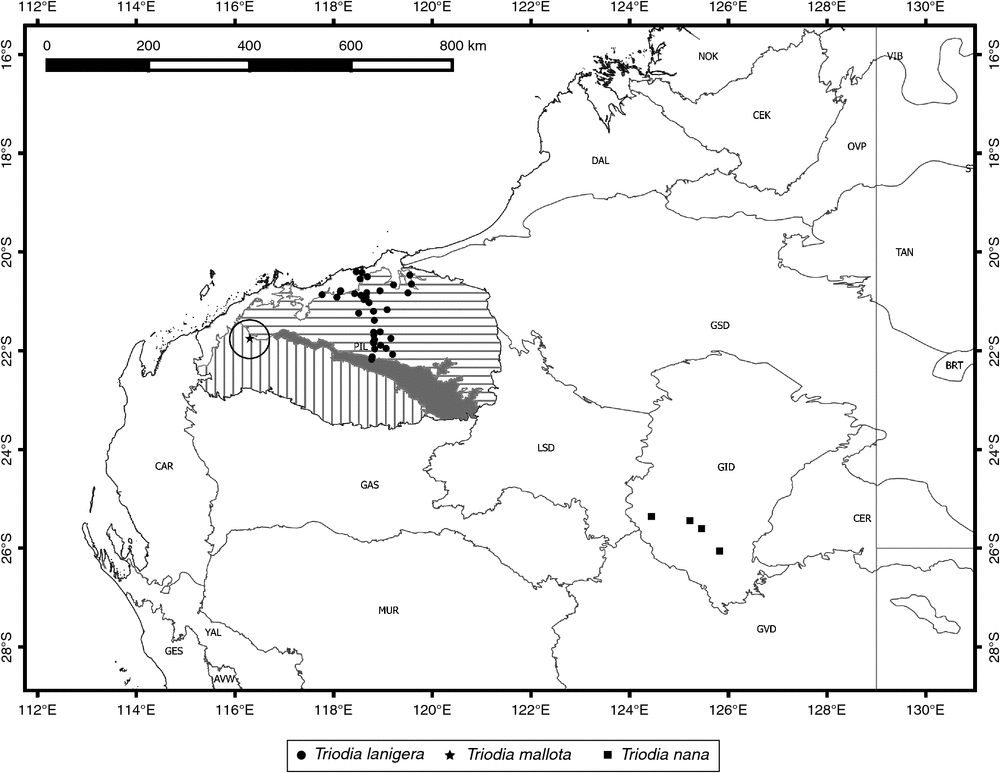
Distribution
Found in the northern Pilbara, particularly in the area south of Port Hedland (Fig. 15). A southern group of populations that is morphologically and genetically divergent occurs just north of the Fortescue River valley.
Habitat and phenology
Occurs primarily on sandy soils, but is also known from rockier soils, sometimes derived from granite. Florets observed February–May.
Proposed conservation assessment
Least Concern (LC). This species is widespread in the northern Pilbara and without obvious threats.
Notes
Triodia lanigera, as recognised here, contains morphological diversity that makes it difficult to distinguish from T. basedowii in the Pilbara. Whereas northern populations are distinct in having hairy leaf sheaths (T. basedowii in the Pilbara has glabrous sheaths), southern populations north of the Fortescue River valley have variable sheath hairiness and may be confused with T. basedowii in the shape of their lemmas as well (midlobes 1.1–1.7× the length of the lateral lobes, v. T. basedowii <1.5).
The shared morphological features near where the ranges of the two species abut is suggestive of possible past hybridisation. ITS sequences (Anderson et al. 2016) are highly similar between T. lanigera and T. basedowii, with the southern populations of T. lanigera being intermediate. Chloroplast sequences (Anderson et al. 2016) and genomic data (Anderson et al. 2017), however, indicate strong divergence between the two species, and the genomic data consistently place the southern populations in the same clade as the northern populations.
The morphological and genomic differences between the northern and southern populations may represent incipient speciation or phylogeographic structure within a single species, and given the morphological overlap and limited genomic divergence, we are uncomfortable splitting the southern populations into a separate species. The southern populations were referred to informally as T. ‘shovb’ in Anderson et al. (2016, 2017), but are here included in a variable T. lanigera.
Representative specimens examined
8. Triodia mallota B.M.Anderson & M.D.Barrett, sp. nov.
(Fig. 16.)
Type: Western Australia, near Pannawonica, S. Reiffer s.n., 19-Mar-2017 (holo: PERTH; iso: BRI, CANB, DNA, K, MEL) [precise locality withheld for conservation reasons].
Diagnostic features
A highly distinctive species in the T. basedowii species complex, distinguished by the combination of a racemose inflorescence with very short pedicels subtending spikelets, and densely woolly leaf sheaths and orifices. The closest relatives outside the complex with a similar inflorescence structure (T. plurinervata and T. infesta) lack the leaf indumentum.
Description
Hummocks to ~0.5 m tall, often forming rings or half-circles. Leaf sheaths densely crimped-woolly with hairs 1–2 mm long; orifice densely crimped-woolly; ligule 1–2 mm long. Blades 70–185 mm long, with orifice hairs extending along the pseudopetiole onto adaxial surface up to 2/3 of the blade length; pseudopetiole 3–7 mm long. Inflorescence a raceme, 55–125 mm long, with 4–7 spikelets and no branches bearing more than 1 spikelet; pedicels 1–2 mm long. Spikelets 11.5–18 × 3.5–4.5 mm with 6–8 florets, restricted by the glumes for 0.33–0.52× the length. Lower glume 6.5–9 × 2–3 mm, 9–14-nerved. Lowest lemma 6.5–8 mm long, shortly 3-lobed, lightly indurated body distinct from upper nerved portion, with the free portion of the lobes to 0.2–1.8 mm long; body 1–3.2 × 3–4 mm, shortly pubescent with hairs 0.5 mm long in the centre and 1 mm long on the margins; midlobe comprising a triangular free portion above but fused to the lateral lobes below, nerved portion 3.5–6.5 mm long, 0.78–1× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous or with body hairs in the lower third, the margins puberulent; lateral lobes comprising a triangular to rounded free portion above but fused to the midlobe below, nerved portion 4–5.5 mm long, 5- or 6-nerved, abaxially glabrous or with body hairs in the lower third, the inner margins puberulent. Palea 4–5 × 1–1.2 mm, abaxially shortly pubescent especially at the junction with the rhachilla, the keels scaberulent; keels indurated and the body less so; apex tapering-truncate, jagged; under-flaps ~0.2 mm wide, pubescent. Rhachilla segment 1–1.2 mm long. Callus 0.5–0.8 mm long, densely pubescent with hairs 0.5 mm long. Lodicules 0.2–0.5 mm long. Anthers 3.2–3.5 mm long. Caryopsis ovoid, 2.2 × 1 mm.
Distribution
Known only from the type population, close to Pannawonica in the Pilbara region of Western Australia (Fig. 15).
Habitat and phenology
Occurs on rocky or gravelly soils. The geology at the type locality is Jeerinah Formation, comprising a mix of pelite, metasandstones and chert. The geological formation runs in a thin band for ~1 km to the west and 35 km to the east, and could be habitat for additional populations of this rare species. Flowering in March, presumably following summer rainfall.
Proposed conservation assessment
Priority 1 (P1). This species is known only from a single small population and has been found to have relatively low heterozygosity (Anderson et al. 2017). The population could represent the last remaining individuals of this species, and is close to active mining.
Notes
Triodia mallota is known only from the type locality. The distinctive combination of its woolly leaf sheaths and inflorescence structure are sufficient to recognise it as a new species, with its many-nerved glumes and unawned lemmas clearly placing it in the T. basedowii complex. Genomic data (Anderson et al. 2017) indicate that it is a highly divergent species in the complex, with low heterozygosity, which is surprising given that it appears to be a tetraploid (Anderson et al. 2017). The low apparent diversity and lack of collections make this species an important target for a future survey in the region.
Etymology
The specific epithet is from the Greek mallotos (fleecy, woolly), in reference to the woolly indumentum on the leaf sheaths and orifices.
Representative specimens examined
9. Triodia nana B.M.Anderson, sp. nov.
(Fig. 17.)
Type: Western Australia, Gibson Desert, 310 km east of Carnegie Homestead on Gunbarrel Highway, B.M. Anderson 52, 7-Apr-2014 (holo: PERTH; iso: CANB).
Diagnostic features
A short-leaved species distinguished by the combination of diminutive stature, typically glabrous leaf blades, small spikelets, and glabrous lemma midlobe. It shares these features with T. scintillans and T. vanleeuwenii, but lacks the minute droplets found on the leaf sheaths of those species and has a slightly narrower palea. It can also be distinguished from T. scintillans and T. vanleeuwenii by its distribution in the Gibson Desert (cf. Pilbara and adjacent regions), and by the following ITS sequences: (T. nana: T. scintillans: T. vanleeuwenii) Locus ITS:ITS1 (starting from 18S end) at Position 71 (A:T:T), 193 (C:T:C), 206 (G:G:A); ITS2 (starting from 5.8S end) at Position 56–62 (CGCAGTG:TGCAGTG:TG----G), 165 (A/T:A:T).
Description
Hummocks diminutive, to ~0.5 m tall, often blue-green in colour. Leaf sheaths glabrous; orifice tangled-villous to straight-pubescent or woolly, the hairs 1.5–3.2 mm long (these sometimes worn off on older leaves); ligule 0.4–0.5 mm long. Blades 25–105 mm long, glabrous or with a few orifice hairs extending along the pseudopetiole onto adaxial surface; pseudopetiole 1–3 mm long. Inflorescence a narrow panicle, 28–68 mm long, with 6–22 spikelets and 0–6 branches bearing more than 1 spikelet; pedicels 0.5–12 mm long. Spikelets 7.5–10.5 × 4–6.8 mm with 5–7 florets, restricted by the glumes for 0.38–0.88× the length. Lower glume 4.8–8 × 2–3.8 mm, the widest point at 0.34–0.6× the length, 5–12-nerved, margins rarely jagged; apex acute to acuminate. Lowest lemma 5.2–6.5 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to 2.2–3.5 mm long; body 2–3 × 2.5–3.5 mm, pubescent with hairs 0.8–1.2 mm long in the centre and 2–2.2 mm long on the margins; midlobe elliptic to ovate, nerved portion 2.2–3.5 × 0.8–1.2 mm, the widest point at 0.23–0.56× the length, 0.8–1.3× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous, the margins puberulent, the apex acuminate to acute or rounded; lateral lobes ovate to obovate, nerved portion 2.5–4 × 1–1.5 mm, the widest point at 0.31–0.7× the length, 4–7-nerved, abaxially glabrous, the inner margins puberulent, the apex rounded. Palea 2.8–3.2 × 0.8–1 mm, abaxially glabrous to rarely sparsely pubescent near the junction with the rhachilla, the keels puberulent; keels and body indurated, the induration becoming weaker towards the apex; apex truncate, ciliate; under-flaps ~0.2 mm wide, puberulent. Rhachilla segment 0.5–1 mm long. Callus 0.2–0.8 mm long, densely pubescent (sometimes glabrous above) with hairs 0.2–1 mm long. Lodicules 0.2–0.5 mm long, rarely ciliate. Anthers 3–3.2 mm long. Caryopsis not seen.
Distribution
Currently known from a small part of the Gibson Desert of Western Australia (Fig. 15). Given that this area is poorly explored, T. nana may be more widespread.
Habitat and phenology
Occurs on rocky to gravelly hills and rises. Florets observed in April (presumably flowering after heavy rainfall).
Proposed conservation assessment
Priority 3 (P3). This species is known from only a few locations, but is not likely to be under immediate threat.
Notes
Triodia nana co-occurs with T. basedowii, but is notably distinct from that species in its lighter blue-green colour and smaller size (leaf length, inflorescences, spikelets). Apart from geographical distribution and the lack of sparkly droplets, it is difficult to distinguish from T. vanleeuwenii and T. scintillans, its possible sister species in the Pilbara, which also tend to occur on rocky areas or substrates over ironstone bedrock. It is marginally distinguished from T. vanleeuwenii by its somewhat more branched inflorescence (0–6 v. 0–3(–5) branches), but there is considerable overlap in this character.
Genomic data (Anderson et al. 2017) indicate that T. nana is differentiated from T. scintillans and T. vanleeuwenii. It comprises a distinct lineage in the phylogenetic analyses, often not recovered as sister to these species. The instability in the phylogenetic analyses suggests significant genomic divergence. The regions between the Gibson Desert (where T. nana occurs) and the Pilbara (where T. vanleeuwenii and T. scintillans occur) are under-collected, and it is possible that there are geographically intermediate populations that would cluster genomically between T. nana and those species, or improve the stability of the phylogenetic analyses and possibly change the current circumscriptions. On the basis of the current sampling, we consider the T. nana samples genomically distinct enough to recognise as a species.
GenBank accession numbers for T. nana sequences from the present study are as follows: MF289175 (B.M. Anderson 49), MF289176 (B.M. Anderson 52) and MF289177 (B.M. Anderson 54).
Etymology
The specific epithet is from the Latin nanus (dwarf), referring to the smaller stature, inflorescences and spikelets than those of co-occurring T. basedowii.
Representative specimens examined
10. Triodia plurinervata N.T.Burb., Austral. J. Bot. 8: 390, fig. 4b (1960). M.Lazarides, Austral. Syst. Bot. 10: 460 (1997) p.p.; M.Lazarides, C.M.Weiller & A.McCusker in K.Mallett (ed.), Fl. Australia 44B: 207, pl. 32 (2005) p.p.
(Fig. 18.)
Type: Western Australia, Shark Bay, Dorre Island, R.D. Royce 5884, 15-Jul-1959 (holo: CANB n.v.; iso: PERTH × 2).
Diagnostic features
Distinguished from other species in this revision (except for T. infesta) by the combination of glabrous leaf sheaths, a spicate or racemose inflorescence, and fused lemma lobes with the free portion less than one-third of the lemma length. These character states are shared with T. infesta, but T. plurinervata differs primarily in its smaller size, having smaller hummocks, inflorescences and spikelets. Triodia plurinervata can be distinguished from T. infesta by using ITS sequences (see Diagnostic features under T. infesta).
Description
Hummocks 0.1–0.6 m tall, sometimes forming rings. Leaf sheaths glabrous to occasionally with puberulent margins; orifice puberulent with hairs <0.2 mm long; ligule 0.2–0.5 mm long. Blades 45–65 mm long, glabrous; pseudopetiole 1–2 mm long. Inflorescence a spike or raceme, 24–55 mm long, with 4–8 spikelets and no branches bearing more than 1 spikelet; pedicels 0–2 mm long. Spikelets 5–9.8 × 1.5–3 mm with 4–8 florets, restricted by the glumes for 0.32–0.69× the length. Lower glume 3–4.2 × 1.2–2 mm, the widest point at 0.21–0.5× the length, 5–8-nerved, glabrous to scaberulent; apex acute to acuminate or rounded. Lowest lemma 3.5–4.8 mm long, shortly 3-lobed to unlobed, the indurated body scarcely differentiated from the lobes, with the free portion of the lobes 0–0.5 mm long; body 1–1.8 × 2–3 mm, appressed-pubescent with hairs 0.5–1 mm long in the centre and 1.25–2 mm long on the margins; midlobe comprising a triangular free portion above but fused to lateral lobes below, nerved portion 2–3 mm long, 1–1.1× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous, the margins occasionally puberulent, the apex acute; lateral lobes comprising a triangular to elliptic free portion above but fused to midlobe below, nerved portion 2–3 mm long, 3–6-nerved, abaxially glabrous, the inner margins occasionally puberulent, the apex acute to rounded. Palea 3–4 × 0.8–1 mm, abaxially glabrous to sparsely pubescent near the junction with the rhachilla, the keels puberulent; keels indurated, with small wings ~0.2 mm wide; apex truncate, sometimes ciliate; under-flaps ~0.2 mm wide. Rhachilla segment (0.2–)0.5–1 mm long. Callus 0.2–0.5 mm long, glabrous to densely pubescent with hairs 0.5–1.8 mm long. Lodicules 0.2–0.5 mm long. Anthers 1.5–2 mm long. Caryopsis not seen.
Distribution
Found in coastal areas around Shark Bay in Western Australia and on near-coastal islands south of 24°S (Fig. 19).
Habitat and phenology
Occurs on red to brown sand on coastal sandplains and low dunes. Florets observed July–October.
Proposed conservation assessment
Priority 4 (P4). This species is locally restricted to the Shark Bay area and nearby islands, and although not immediately threatened, may face danger from continued development in the area.
Notes
Triodia plurinervata is a close relative of the T. basedowii complex; it is distinguished from species in the complex (with the exception of T. mallota) by its inflorescence structure (spicate or racemose with short pedicels subtending spikelets). It can be distinguished from T. mallota by its glabrous cf. densely woolly leaves. Its inflorescence structure distinguishes it from T. concinna (which has a branched inflorescence), with which it shares largely fused lemma lobes. Although morphologically similar to T. infesta, T. plurinervata is smaller, geographically disjunct from T. infesta, and distinct in both ITS and chloroplast data (Anderson et al. 2016) as well as in genomic analyses (Anderson et al. 2017).
Representative specimens examined
11. Triodia scintillans B.M.Anderson & M.D.Barrett, sp. nov.
(Fig. 20.)
Type: Western Australia, Woodie Woodie Road, 19 km south from turn-off to Telfer, M.D. Barrett 4089, 18-Mar-2013 (holo: PERTH; iso: CANB, K).
Diagnostic features
A short-leaved species distinguished by the combination of glabrous leaf blades, sparkly droplets on leaf sheaths and sometimes inflorescences, glabrous lemma midlobes, and a highly branched inflorescence with (1–)2–7 branches bearing more than 1 spikelet. Distinguished from T. vanleeuwenii by its typically more branched inflorescence and disjunct distribution north of the Fortescue River valley (cf. south of the Fortescue River valley). ITS sequence differences (Anderson et al. 2016) also help distinguish T. scintillans from T. vanleeuwenii: (T. scintillans: T. vanleeuwenii) Locus ITS:ITS1 (starting from 18S end) at Position 193 (T:C), 206 (G:A); ITS2 (starting from 5.8S end) at Position 57–62 (GCAGTG:G----G), 74 (G:A), 165 (A:T).
Description
Hummocks 0.2–0.5 m tall, with flowering culms to ~0.7–1 m above the ground. Leaf sheaths glabrous with obvious sparkly droplets (sometimes crystalline on dried material); orifice tangled-villous or woolly, the hairs 1.5–2.5 mm long (these sometimes worn off on older leaves); ligule 0.5–1 mm long. Blades 40–100(–137) mm long, glabrous or rarely with a few hairs spreading onto pseudopetiole; pseudopetiole 1–3 mm long. Inflorescence a racemose panicle, 40–98 mm long, with 7–19 spikelets and (1–)2–7 branches bearing more than 1 spikelet; pedicels 1–18 mm long. Spikelets 7–13 × 3.5–8 mm with 4–10 florets, restricted by the glumes for 0.42–0.93× the length. Lower glume 4–7.8 × 2.5–4 mm, the widest point at 0.3–0.57× the length, 8–14-nerved, glabrous to scaberulent; apex acute to acuminate. Lowest lemma 5–9 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to 2.8–5.2 mm long; body 2–3.2 × 2.5–4 mm, appressed-pubescent with hairs 1–1.5 mm long in the centre and 1.5–3 mm long on the margins; midlobe ovate to elliptic or linear, nerved portion 2.8–5.2 × 0.8–1.2 mm, the widest point at 0.22–0.56× the length, 1–1.4× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous, the margins puberulent, the apex acuminate to acute; lateral lobes ovate to obovate or oblong, nerved portion 2.2–4.2 × 1–2 mm, the widest point at 0.15–0.71× the length, 5–7-nerved, abaxially glabrous, the inner margins puberulent, the apex acute to rounded. Palea 2.8–4.2 × 1–1.2 mm, abaxially glabrous to rarely sparsely pubescent, the keels puberulent; keels indurated and body less so, the induration becoming weaker towards the apex; apex truncate to rounded, sometimes ciliate; under-flaps ~0.2 mm wide. Rhachilla segment 0.5–1 mm long. Callus 0.2–0.8 mm long, pubescent to sparsely so (sometimes glabrous above) with hairs 0.2–0.8(–1.2) mm long. Lodicules 0.2–0.5 mm long. Anthers 2.2–3.5 mm long. Caryopsis not seen.
Distribution
Found north of the Fortescue River valley in the eastern Pilbara region of Western Australia, east to near the margins of the Pilbara in the adjacent Greater Sandy Desert (Fig. 19).
Habitat and phenology
Occurs on sandy to gravelly substrates on plains, knolls and slopes. Florets observed February, March and June–August (typically flowering in response to heavy rain).
Proposed conservation assessment
Least Concern (LC). This species occurs over a large area in the eastern Pilbara; it may be affected by mining in a portion of its range.
Notes
Triodia scintillans is closely related to T. vanleeuwenii, with which it shares sparkly droplets, and possibly hybridises with that species at a population in the Fortescue River valley near Roy Hill. It is distinguished from T. vanleeuwenii by its more branched inflorescence with more spikelets, but most practically by geography (T. scintillans is only found north of, whereas T. vanleeuwenii occurs only south of, the Fortescue River valley). It grows sympatrically with T. basedowii, from which it can be distinguished by its glabrous and shorter leaf blades (v. orifice hairs spreading well onto longer blades in T. basedowii) and by its sparkly droplets on sheaths and often parts of the inflorescence as well.
Internal transcribed spacer sequences (Anderson et al. 2016) for this species form a distinct clade, and external transcribed spacer (ETS) and chloroplast sequences suggest that it is closely related to T. basedowii. Analyses of genomic data (Anderson et al. 2017), however, have consistently placed it in a clade with T. vanleeuwenii, consistent with morphological similarity. The close morphological and genomic relationship between T. scintillans and T. vanleeuwenii makes it a difficult call as to whether to recognise them as distinct. The close occurrence of populations of both species on either side of the Fortescue River valley (less than ~50 km) with the maintenance of significant genomic divergence (no genomic intermediates) despite relative genomic homogeneity within both species across much longer distances, supports their distinction. In addition, ITS and ETS sequences are diagnostically different between the two species, such that hybrid individuals could be identified by detecting ITS copies from both parents (Anderson et al. 2016). Coupled with appearance differences that are difficult to quantify in descriptions, the genetic and genomic differences support our choice to recognise these two entities as separate species.
Etymology
The specific epithet is from the Latin scintillo (to sparkle), in reference to the distinctive sparkly droplets on young material, especially noticeable in sunlight.
Representative specimens examined
12. Triodia vanleeuwenii B.M.Anderson & M.D.Barrett, sp. nov.
(Fig. 21.)
Type: Western Australia, ~13 km south of Mount Robinson rest area on the Great Northern Highway, B.M. Anderson 33, 26-Feb-2014 (holo: PERTH; iso: CANB, K).
Diagnostic features
A short-leaved species distinguished by the combination of glabrous leaf blades, sparkly droplets on leaf sheaths and sometimes inflorescences, glabrous lemma midlobes, and a relatively unbranched inflorescence compared to other species in the complex with 0–3(–5) branches bearing more than one spikelet. Distinguished from T. scintillans by its less branched inflorescence, disjunct distribution, and ITS sequences (see Diagnostic features under T. scintillans).
Description
Hummocks diminutive, 0.15–0.5(–1) m tall, sometimes forming rings, with flowering culms 0.5–1.3 m above the ground. Leaf sheaths glabrous to rarely sparsely villous, with sparkly droplets (sometimes crystalline on dried material); orifice tangled-villous to straight-pubescent or woolly, the hairs (0.5–)1–2(–4) mm long; ligule 0.5–1 mm long. Blades 40–90(–115) mm long, glabrous or rarely with a few orifice hairs spreading onto the pseudopetiole; pseudopetiole 1–4 mm long. Inflorescence a racemose panicle, 34–75 mm long, with 5–9(–14) spikelets and 0–3(–5) branches bearing more than 1 spikelet; pedicels 1–15 mm long. Spikelets 7–12 × 3–5 mm with 5–10 florets, restricted by the glumes for 0.41–0.64× the length. Lower glume 4.2–7 × 2.5–3.8 mm, the widest point at 0.3–0.72× the length, 6–12-nerved, glabrous to scaberulent; apex acuminate to acute or rounded. Lowest lemma 4.8–6.8 mm long, deeply 3-lobed, the indurated body clearly differentiated from the lobes, with the free portion of the lobes to 2.8–4 mm long; body 2–3 × 2.5–4 mm, appressed-pubescent especially towards the base with hairs 0.5–1 mm long in the centre and 1.5–2 mm long on the margins; midlobe ovate to elliptic or linear, nerved portion 2.8–4 × 0.8–1.2 mm, the widest point at 0.17–0.58× the length, 0.9–1.3× the length of the lateral lobe nerved portion, 3–5-nerved, abaxially glabrous, the margins puberulent, the apex acuminate to acute; lateral lobes ovate to elliptic or oblong, nerved portion 2.5–3.5 × 1–1.8 mm, the widest point at 0.17–0.57× the length, 4–6-nerved, abaxially glabrous, the inner margins puberulent, the apex acute to rounded. Palea 2.8–3.5 × 1–1.5 mm, abaxially glabrous, the keels puberulent; keels and body indurated, the induration becoming weaker towards the apex; apex truncate, sometimes ciliate; under-flaps ~0.2 mm wide. Rhachilla segment 0.5–1 mm long. Callus 0.2–0.8 mm long, pubescent (rarely glabrous above) with hairs 0.2–1.2 mm long. Lodicules 0.2–0.5 mm long, sometimes ciliate. Anthers 2.5–3.2 mm long. Caryopsis ellipsoid, 1.8–2 × 0.8–1 mm.
Distribution
Found primarily in the eastern Hamersley Range of the Pilbara region of Western Australia, with a few outlying collections in the adjacent Little Sandy Desert region (Fig. 19).
Habitat and phenology
Occurs in association with banded ironstone, particularly on red to brown skeletal loams and gravelly soils derived from banded ironstone, rarely in sandy areas over sandstone. Florets observed February, March, May, July, August and October (assumed to follow heavy rain, summer or winter).
Proposed conservation assessment
Least Concern (LC). This species is widespread in the eastern Hamersley region of the Pilbara, with some impacts from ongoing mining operations there.
Notes
Triodia vanleeuwenii is often recognised in the field by its small hummocks with short leaves and typically racemose inflorescences. It is the most common member of the complex in the eastern Hamersley region of the Pilbara, and is usually the only member of the complex in that area. It co-occurs with T. basedowii on the edge of its range at the Fortescue River valley. Its leaves are shorter than those of T. basedowii, and it lacks the orifice hairs continuing onto the leaf blade, which is typical of that species. It is closely related to T. scintillans, differing in its fewer spikelets and less-branched inflorescences, and T. nana, which lacks droplets. See the Notes under those species for differences and arguments for distinction.
Etymology
The specific epithet was chosen to honour Stephen van Leeuwen, who has collected and was involved in recognising this species, and has contributed considerably to botanical knowledge of the Pilbara.
Representative specimens examined
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank the Curator and staff at the Western Australian Herbarium (PERTH) for access to their collection and space to work, and the following herbaria for loans of their specimens: AD, BRI, CANB, DNA, E, MEL, NSW, and NT. Field collecting assistance was provided by C. Ellery Mayence, Peter Jobson, Stephen van Leeuwen, Neil Gibson, Margaret Langley, Kate Brown, Nicholas Casson, Rob Davis, Tim Hammer, and Peter Golos. We thank Phil Davidson for alerting us to the location where we found Triodia mallota, and Scott Reiffer for returning to that location and collecting additional material, including the type specimen. We thank Peter Wilson for assistance with interpreting the protologue of T. basedowii and the implications for its typification, as well as assistance translating some of the Latin in the protologues of T. basedowii and T. lanigera. We thank Clare Drinkell at Kew for assistance looking through the collection for possible type material of T. basedowii, and Robert Vogt at the Berlin Herbarium for confirming the absence of duplicates of the Basedow collections. We thank Alex George for advice on correctly forming a specific epithet for T. scintillans. The manuscript was improved following helpful comments from two anonymous reviewers and Brendan Lepschi. This research was partly supported by an Australian Research Council Linkage Project LP120100350 (CIs Grierson, Krauss et al. in collaboration with Rio Tinto Iron Ore, Chevron Australia Pty Ltd, Botanic Gardens and Parks Authority and the Department of Parks and Wildlife). B. M. Anderson was supported by an International Postgraduate Research Scholarship, an Australian Postgraduate Award, and a UWA Top-up Scholarship at the University of Western Australia. Collecting in eastern Western Australia and the NT was supported by an ANZ Trustees Foundation – Holsworth Wildlife Research Endowment to B. M. Anderson.
References
Amarasinghe V (1990) Polysaccharide and protein secretion by grass microhairs. A cytochemical study at light and electron microscopic levels. Protoplasma 156, 45–56.| Polysaccharide and protein secretion by grass microhairs. A cytochemical study at light and electron microscopic levels.Crossref | GoogleScholarGoogle Scholar |
Anderson BM, Barrett MD, Krauss SL, Thiele K (2016) Untangling a species complex of arid zone grasses (Triodia) reveals patterns congruent with co-occurring animals. Molecular Phylogenetics and Evolution 101, 142–162.
| Untangling a species complex of arid zone grasses (Triodia) reveals patterns congruent with co-occurring animals.Crossref | GoogleScholarGoogle Scholar |
Anderson BM, Thiele KR, Krauss SL, Barrett MD (2017) Genotyping-by-sequencing in a species complex of australian hummock grasses (Triodia): methodological insights and phylogenetic resolution. PLoS One 12, e0171053
| Genotyping-by-sequencing in a species complex of australian hummock grasses (Triodia): methodological insights and phylogenetic resolution.Crossref | GoogleScholarGoogle Scholar |
Armstrong G (2008) Triodia caelestialis (Triodieae: Chloridoideae: Poaceae), a new species from the central Kimberley, Western Australia. Journal of the Royal Society of Western Australia 91, 313–317.
Barker B (2005) Standardising informal names in Australian publications. Australian Systematic Botany Society Newsletter 122, 11–12.
Barrett RL, Barrett MD (2015) Twenty-seven new species of vascular plants from Western Australia. Nuytsia 26, 21–87.
Barrett RL, Wells GB, Dixon KW (2005) New taxa and combinations: Subfam. Chloridoideae, Trib. Triodieae, Triodia. In ‘Flora of Australia’. (Ed. K Mallett) pp. 458–459. (CSIRO Publishing: Melbourne, Vic., Australia).
Bolton B, Latz P (1978) The western hare-wallaby Lagorchestes hirsutus (Gould) (Macropodidae), in the Tanami Desert. Australian Wildlife Research 5, 285–293.
| The western hare-wallaby Lagorchestes hirsutus (Gould) (Macropodidae), in the Tanami Desert.Crossref | GoogleScholarGoogle Scholar |
Burbidge N (1943) Ecological succession observed during regeneration of Triodia pungens R.Br. after burning. Journal of the Royal Society of Western Australia 28, 149–156.
Burbidge N (1946) A revision of the Western Australian species of Triodia R.Br. Journal of the Royal Society of Western Australia 30, 15–33.
Burbidge N (1953) The genus Triodia R.Br. (Gramineae). Australian Journal of Botany 1, 121–184.
| The genus Triodia R.Br. (Gramineae).Crossref | GoogleScholarGoogle Scholar |
Burbidge N (1960) Further notes on Triodia R.Br. (Gramineae) with description of five new species and one variety. Australian Journal of Botany 8, 381–395.
| Further notes on Triodia R.Br. (Gramineae) with description of five new species and one variety.Crossref | GoogleScholarGoogle Scholar |
Casson N, Fox J (1987) The post-fire regeneration responses of Triodia wiseana and T. basedowii. Australian Rangeland Journal 9, 53–55.
| The post-fire regeneration responses of Triodia wiseana and T. basedowii.Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Mant J, Toon A, Cook LG (2015) Australian spinifex grasses: new names in Triodia for Monodia and Symplectrodia. Phytotaxa 230, 293–296.
| Australian spinifex grasses: new names in Triodia for Monodia and Symplectrodia.Crossref | GoogleScholarGoogle Scholar |
Daly BG, Dickman CR, Crowther MS (2008) Causes of habitat divergence in two species of agamid lizards in arid central Australia. Ecology 89, 65–76.
| Causes of habitat divergence in two species of agamid lizards in arid central Australia.Crossref | GoogleScholarGoogle Scholar |
Dayrat B (2005) Towards integrative taxonomy. Biological Journal of the Linnean Society. Linnean Society of London 85, 407–415.
| Towards integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
de Queiroz K (1998) The general lineage concept of species, species criteria, and the process of speciation. In ‘Endless Forms Species and Speciation’. (Eds D Howard, S Berlocher) pp. 57–75. (Oxford University Press: Oxford, UK).
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879–886.
| Species concepts and species delimitation.Crossref | GoogleScholarGoogle Scholar |
Department of Parks and Wildlife (2015) Conservation codes for Western Australian flora and fauna. Available at https://www.dpaw.wa.gov.au/plants-and-animals/threatened-species-and-communities [Verified August 2017]
Department of the Environment (2006) MVG 20: hummock grasslands. (Australian Government: Canberra, ACT, Australia) Available at http://www.environment.gov.au/land/publications/factsheets-vegetation-profiles-major-vegetation-groups [Verified August 2017]
Dickman CR, Letnic M, Mahon PS (1999) Population dynamics of two species of dragon lizards in arid Australia: the effects of rainfall. Oecologia 119, 357–366.
| Population dynamics of two species of dragon lizards in arid Australia: the effects of rainfall.Crossref | GoogleScholarGoogle Scholar |
Domin K (1912) Additions to the flora of western and north-western Australia. Journal of the Linnean Society Botany 41, 245–283.
| Additions to the flora of western and north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Gardner C (1952) ‘Flora of Western Australia.’ (William H. Wyatt, Government Printer: Perth, WA, Australia).
Hurry CR, Walsh NG, Murphy DJ (2012) A taxonomic review of Triodia bunicola and T. scariosa (Poaceae: Chloridoideae), based on morphological and molecular data. Australian Systematic Botany 25, 304–312.
| A taxonomic review of Triodia bunicola and T. scariosa (Poaceae: Chloridoideae), based on morphological and molecular data.Crossref | GoogleScholarGoogle Scholar |
IUCN (2012) ‘IUCN Red List Categories and Criteria: Version 3.1.’ (International Union for Conservation of Nature: Cambridge, UK).
Kitchener DJ, Stoddart J, Henry J (1983) A taxonomic appraisal of the genus Ningaui Archer (Marsupialia: Dasyuridae), including description of a new species. Australian Journal of Zoology 31, 361–379.
| A taxonomic appraisal of the genus Ningaui Archer (Marsupialia: Dasyuridae), including description of a new species.Crossref | GoogleScholarGoogle Scholar |
Lazarides M (1997) A revision of Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae). Australian Systematic Botany 10, 381–489.
| A revision of Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae).Crossref | GoogleScholarGoogle Scholar |
Lazarides M, Weiller C, McCusker A (2005) Triodia. In ‘Flora of Australia. Vol. 44B. Poaceae 3: Centothecoideae–Chloridoideae’. (Ed. K Mallett) pp. 203–256. (CSIRO Publishing: Melbourne, Vic., Australia).
Lundie-Jenkins G, Phillips C, Jarman P (1993) Ecology of the rufous hare-wallaby, Lagorchestes hirsutus Gould (Marsupialia: Macropodidae), in the Tanami Desert, Northern Territory. II. Diet and feeding strategy. Wildlife Research 20, 477–494.
| Ecology of the rufous hare-wallaby, Lagorchestes hirsutus Gould (Marsupialia: Macropodidae), in the Tanami Desert, Northern Territory. II. Diet and feeding strategy.Crossref | GoogleScholarGoogle Scholar |
Mant J, Bayer R, Crisp M, Trueman J (2000) A phylogeny of Triodieae (Poaceae: Chloridoideae) based on the ITS region of nrDNA: testing conflict between anatomical and inflorescence characters. In ‘Grasses: Systematics and Evolution’. (Eds S Jacobs, J Everett) pp. 213–217. (CSIRO: Melbourne, Vic., Australia).
Morton S, James S (1988) The diversity and abundance of lizards in arid Australia: a new hypothesis. American Naturalist 132, 237–256.
| The diversity and abundance of lizards in arid Australia: a new hypothesis.Crossref | GoogleScholarGoogle Scholar |
Padial JM, Aurélien M, De la Riva I, Vences M (2010) The integrative future of taxonomy. Frontiers in Zoology 7, 16
| The integrative future of taxonomy.Crossref | GoogleScholarGoogle Scholar |
Pritzel E (1918) Species novae ex Australia centrali. Repertorium Novarum Specierum Regni Vegetabilis 15, 356–361.
| Species novae ex Australia centrali.Crossref | GoogleScholarGoogle Scholar |
Reginato M (2016) monographaR: an R package to facilitate the production of plant taxonomic monographs. Brittonia 68, 212–216.
| monographaR: an R package to facilitate the production of plant taxonomic monographs.Crossref | GoogleScholarGoogle Scholar |
Rice B, Westoby M (1999) Regeneration after fire in Triodia R.Br. Australian Journal of Ecology 24, 563–572.
| Regeneration after fire in Triodia R.Br.Crossref | GoogleScholarGoogle Scholar |
Westoby M, Rice B, Griffin G, Friedel M (1988) The soil seed bank of Triodia basedowii in relation to time since fire. Australian Journal of Ecology 13, 161–169.
| The soil seed bank of Triodia basedowii in relation to time since fire.Crossref | GoogleScholarGoogle Scholar |
Yeates DK, Seago A, Nelson L, Cameron SL, Joseph L, Trueman JWH (2011) Integrative taxonomy, or iterative taxonomy? Systematic Entomology 36, 209–217.
| Integrative taxonomy, or iterative taxonomy?Crossref | GoogleScholarGoogle Scholar |