Re-testing for chlamydia at sexual health services in Australia, 2004–08
Rebecca Guy A I , Handan Wand A , Neil Franklin A , Christopher K. Fairley B C , Marcus Y. Chen B , Catherine C. O’Connor D , Lewis Marshall E , Andrew E. Grulich A , John M. Kaldor A , Margaret Hellard F , Basil Donovan A G and on behalf of the ACCESS Collaboration HA National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, NSW 2034, Australia.
B Melbourne Sexual Health Centre, Carlton, Vic. 3053, Melbourne, Australia.
C School of Population Health University of Melbourne, Melbourne, Vic. 3053, Australia.
D Sexual Health Service, Community Health, Royal Prince Alfred Hospital, Sydney South West Area Health Service, Camperdown, NSW 2050, Australia.
E Fremantle Hospital, Fremantle, WA 6869, Australia.
F Centre for Population Health, Burnet Institute, Melbourne, Vic. 3181, Australia.
G Sydney Sexual Health Centre, Sydney Hospital, Sydney, NSW 2001, Australia.
H See Box 1.
I Corresponding author. Email: Rguy@nchecr.unsw.edu.au
Sexual Health 8(2) 242-247 https://doi.org/10.1071/SH10086
Submitted: 11 July 2010 Accepted: 24 August 2010 Published: 18 May 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Objective: To describe the frequency of the 3-month test for re-infection among sexual health service patients in Australia. Methods: We assessed the re-testing rates at 30–120 days after chlamydia infection in men who have sex with men (MSM), heterosexual males and females attending sexual health services across Australia between 2004 and 2008. A χ2-test was used to determine significant differences in re-testing rates according to demographic characteristics and trends over time. Results: In the 5-year period, 10 207 MSM, 28 530 heterosexual males and 31 190 heterosexual females were tested for chlamydia. Of those tested, 9057 (13.0%) were positive. The proportion of patients with chlamydia infection who were re-tested in 30–120 days was 8.6% in MSM, 11.9% in heterosexual males and 17.8% in heterosexual females. Among MSM, chlamydia re-testing rates were lower in men aged <30 years (8.4%) than ≥30 years (12.5%) (P = 0.04) and lower in travellers and migrants (2.9%) than non-travellers (9.9%) (P = 0.002). In heterosexual males, chlamydia re-testing rates were lower in men in regional and rural areas (10.5%) than metropolitan areas (13.5%) (P = 0.017). There was no increasing trend in re-testing rates between 2004 and 2008 (P = 0.787). Of the patients re-tested, 44.1% of MSM were positive, 21.0% of heterosexual males and 16.1% of females. Discussion: The high chlamydia positivity at 30–120 days support recommendations that call for a 3-month test for re-infection following a positive test. The low re-testing rates highlight the need for innovative strategies to increase re-testing.
Additional keywords: re-infection, repeat testing, sexual health clinics.
Introduction
Chlamydia is a highly prevalent infection in young heterosexuals 1–4 and men who have sex with men (MSM) in Australia. 5 Chlamydia re-infections increase the risk of chlamydia-related sequelae such as pelvic inflammatory disease and infertility. 6 Also, in men who have sex with men (MSM), chlamydia re-infection of the rectum has been associated with an increased risk of HIV seroconversion. 7
| Box 1. ACCESS coordinating committee and representatives from participating services |
| ACCESS coordinating committee |
| Professor Basil Donovan (co-primary investigator), Dr Rebecca Guy, Professor John Kaldor, Mr Neil Franklin, Mr James Ward – the National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, NSW; Associate Professor Margaret Hellard (co-primary investigator), Ms Jane Goller, Mr Fabian Kong, Dr Isabel Bergeri – Burnet Institute, Melbourne, Vic.; Associate Professor Elizabeth Sullivan – Perinatal and Reproductive Epidemiology Research Unit, University of New South Wales, Sydney, NSW; Mr Wayne Dimech – National Serology Reference Laboratory, Fitzroy, Vic., Bridget Dickson – CaraData Pty Ltd. |
| Representatives from participating sexual health services |
| Mr Dy Kelaart, Mairead Hetherington – Alice Springs Clinic 34, Alice Springs, NT; Professor Frank Bowden, Dr Sarah Martin – Canberra Sexual Health Centre, Canberra, ACT; Associate Professor Anna McNulty, Mr Heng Lu – Sydney Sexual Health Centre, Sydney, NSW; Dr Brian Mulhall – Coffs Harbour Sexual Health, Coffs Harbour, NSW; Dr Peter Knibbs, Dr Catherine Pell – Darwin Clinic 34, Darwin, NT; Associate Professor Darren Russell, Ms Sandra Downing – Dolls’ House Sexual Health Clinic, Cairns, Qld; Dr Lewis Marshall – Fremantle Hospital, Fremantle, WA; Dr John Chua, Ms May Ngieng – Gold Coast Sexual Health, Gold Coast, Qld; Dr Michael Bolton, Ms Alison Kincaid – Greater Southern Area Health Service, NSW; Dr Maree O’Sullivan, Mr Houlihan Nives – Hobart Sexual Health Service, Hobart, Tas; Dr Debbie Allen, Mr Paul Maudlin – Holden St Sexual Health Centre, Gosford, NSW; Dr Catriona Ooi, Mr Martin O’Connor – Hunter New England Sexual Health Service, Newcastle, NSW; Dr Ingrid Van Beek, Dr Craig Rogers, Heng Lu – Kirketon Road Centre, Sydney, NSW; Dr David J Smith, Ms Cecily Gray – Lismore/Tweed Heads Sexual Health Service, Lismore, NSW; Professor Christopher Fairley, Dr Marcus Chen, Mr Afrizal Afrizal – Melbourne Sexual Health Centre, Melbourne, Vic.; Dr Tim Lynch, Ms Fiona D’Aquino – Orange Sexual Health Centre, Orange, NSW; Dr David Jardine, Ms Dee Archbold – Princess Alexandra Sexual Health, South Brisbane, Qld; Associate Professor Catherine O’Connor, Mr Seven Guney – RPA Sexual Health Clinic, Camperdown, NSW; Dr Stephen Davies, Ms Amanda Rickett – Royal North Shore Hospital Sexual Health, North Sydney, NSW; Dr Katerina Lagios, Ms Sangeetha Eswarappa – SWAHS: Eastern Division, NSW; Ms Jane Shakeshaft – SWAHS: Western Division, NSW; Associate Professor Katherine Brown, Ms Victoria McGrath – Sydney South Illawarra Health Service, Wollongong, NSW; Dr Pam Konecny – St George Short St Sexual Health Centre, Kogarah, NSW; Dr Arun Menon, Ms Angela Cooper – Townsville Sexual Health Service, Townsville, Qld. |
Chlamydia re-infection occurred in 22% of young women in a Melbourne cohort study in 2008–09, 4 and a clinical audit of 126 MSM in Melbourne in 2002–03 found re-infection rates of 47% in HIV-positive MSM and 25% in HIV-negative men re-tested at 12–18 months. 8 Re-infection is associated with unprotected sex with new partners, sex with a partner who has not yet been treated, or sex within a network of partners where there remains a high prevalence of chlamydia infection because treatment of partners is incomplete. 9
Due to the high chlamydia re-infection rates, clinical guidelines in Australia recommend that for all people diagnosed with chlamydia, a repeat test is conducted in 3 months to detect chlamydia re-infections. 9 , 10 In this paper, we assessed the observance of the clinical guideline recommending a 3-month test for re-infection and the extent of chlamydia positivity of re-testing among MSM, heterosexual women and heterosexual men attending sexual health services across Australia between 2004 and 2008.
Methods
The Australian Collaboration for Chlamydia Enhanced Sentinel Service (ACCESS) methods have been described in detail elsewhere 11 and more information can also be found at www.access-study.org (verified September 2010). In summary, the Australian Government funded the National Centre in HIV Epidemiology and Clinical Research (NCHECR) and the Burnet Institute to implement six sentinel networks for surveillance of chlamydia testing and positivity in collaboration with the National Serological Reference Laboratory and the National Perinatal Statistics Unit.
One of the six networks involves 25 sexual health services and is managed by NCHECR in collaboration with a steering committee including representation from sexual health services. This network includes most of the largest sexual health services in Australia. The services are located across all states and territories, except South Australia; 16 are located in metropolitan areas and nine in regional or remote areas.
All of these sexual health services use computerised medical records systems to collect information as part of routine care. On a 6-monthly basis, the services provide a core set of data to NCHECR including but not limited to the patient unique identifier, sex, age, postcode, country of birth, the gender of sexual partner(s) in the past 12 months, and the date and outcome of the chlamydia test.
Information extracted from sites are de-identified before being forwarded in a line-listed format to a central database at NCHECR.
The project was approved by 24 Human Research Ethics Committees.
Statistical analysis
We analysed data on all MSM, heterosexual males and heterosexual females attending 19 sexual health services that were able to provide data during the 5-year period from 1 January 2004 to 31 December 2008. The term MSM was used to describe men reporting sex with men in the last 12 months. Heterosexual was defined based on reporting a sexual partner of the opposite sex only in the last 12 months. Traveller or migrant status was defined as arrival in Australia in the current or previous calendar year.
Re-testing after a chlamydia infection
The proportion of patients diagnosed with chlamydia who were re-tested any time in the study period 30–120 days following a diagnosis with chlamydia infection was calculated for MSM, heterosexual males and females, and select demographic characteristics within these three patient subgroups. A χ2-test was used to determine if there was a significant difference in re-testing rates according to these demographic characteristics.
We also calculated the proportion of patients diagnosed with chlamydia who were re-tested in 30 and 120 days following a diagnosis with chlamydia infection by year, from 2004 to 2008. A χ2-test for trend was used to determine if there was a significant change in annual re-testing rates in 30–120 days over time.
Chlamydia positivity at re-test
The proportion of the patients diagnosed with chlamydia who were re-tested at anytime in the study period between 30 and 120 days and found to be positive was also calculated for MSM, heterosexual males and females.
Stata statistical software (StataCorp, College Station, TX, USA) was used to conduct all analyses. 12
Results
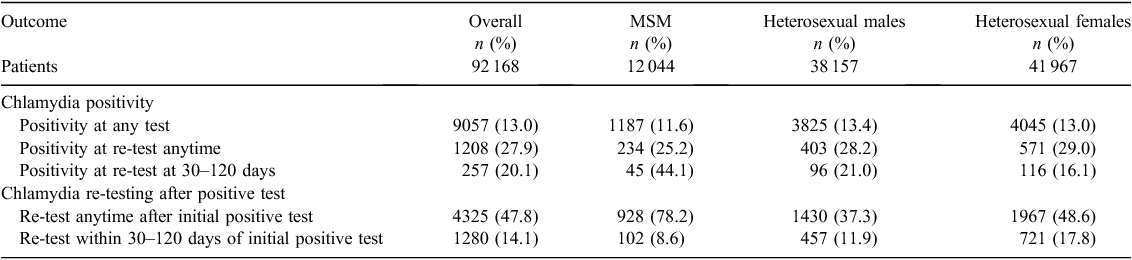
Between January 2004 and December 2008, 69 927 patients were tested for chlamydia and 9057 (13.0%) of all tests were positive. There were 10 207 MSM tested for chlamydia and 1187 (11.6%) of these tests were positive; 28 530 heterosexual males tested for chlamydia and 3825 (13.4%) of these tests were positive; 31 190 heterosexual females tested for chlamydia and 4045 (13.0%) tests were positive (Table 1).
|
Re-testing after a chlamydia infection
The proportion of patients with chlamydia infection re-tested any time in the study period was 47.8% overall; 78.2% in MSM, 37.3% in heterosexual males and 48.6% in heterosexual females (Table 1).
The re-testing rates within 30–120 days of chlamydia infection was 14.1% overall, 8.6% in MSM, 11.9% in heterosexual males and 17.8% in heterosexual females (Table 1). There was no significant increasing trend in annual re-testing rates within 30–120 days over time (P = 0.787): 14.5% in 2004, 14.7% in 2006 and 12.9% in 2008 (Table 2).

|
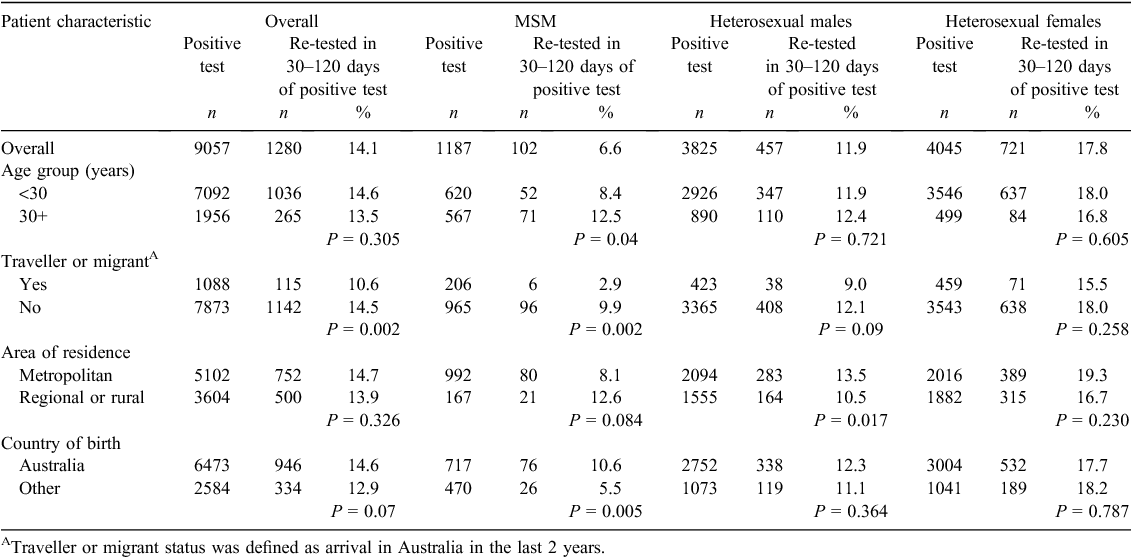
Among MSM, the chlamydia re-testing rates within 30–120 days of chlamydia diagnosis was lower in men aged less than 30 years (8.4%) compared with older men (12.5%) (P = 0.04), lower in men who were travellers (2.9%) compared with non-travellers (9.9%) (P = 0.002) and lower in men born overseas (5.5%) compared with Australian-born men (10.6%) (P = 0.005). In heterosexual males, chlamydia re-testing rates within 30–120 days of chlamydia were lower in men living in regional and rural areas (10.5%) compared with metropolitan areas (13.5%) (P = 0.017) (Table 3).
|
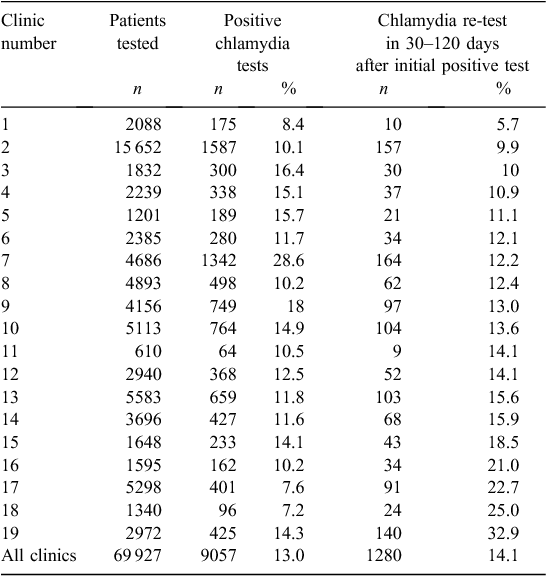
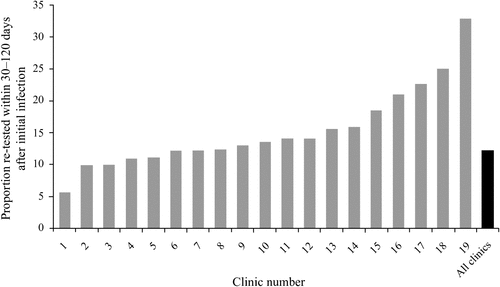
The re-testing rates within 30–120 days of chlamydia infection were <20% in 15 of the 19 clinics, and varied from a low of 5.7% to a high of 32.9% per clinic (Table 4, Fig. 1).
|
|
Chlamydia positivity at 30–120 days re-test
Of the patients diagnosed with chlamydia and re-tested within 30–120 days, 20.1% of re-tests were positive: 44.1% in MSM, 21.0% in heterosexual males and 16.1% in heterosexual females (Table 1).
Discussion
This study is the first national attempt to investigate the extent of follow-up testing after a chlamydia diagnosis in sexual health services in Australia. Our analysis demonstrated low levels of repeat testing around 3 months following a chlamydia diagnosis, despite being recommended in clinical guidelines 9 , 10 Only 14% of patients had a follow-up test in 30–120 days, with re-testing rates being highest in young heterosexual females (17.8%), followed by heterosexual males (11.9%) and MSM (8.6%).
The chlamydia re-testing rates within 30–120 days of infection was 8.6% in MSM, which is consistent with a recent analysis of HIV-negative MSM attending three primary care clinics in Melbourne, in which only 9% of HIV-negative MSM diagnosed at two general practice clinics with a high case load of MSM returned for a chlamydia re-test within 3 months of the initial infection, and 7% of MSM were re-tested at a large urban sexual health clinic. 13 Our study also showed that the lowest re-testing rates were in MSM aged less than 30 years, which was the age group where most chlamydia diagnoses occurred. Other analyses of MSM have demonstrated that being younger is strongly associated with chlamydia infection.5
The higher re-testing rate in heterosexual females than heterosexual males is also consistent with other studies. 14 , 15 Malotte et al. found re-testing rates of 8% in males and 14–18% in females. 14 Paneth-Pollak et al. found re-testing rates of 7% in males and 11% in females. 15 These findings are possibly a reflection of poorer health-seeking behaviour by men.
We found the lowest re-testing rates in the groups with the highest re-infection rates. In MSM, only 8.6% were re-tested within 30–120 days after a chlamydia diagnosis but 44.1% of these re-tests were positive. In heterosexual females, a much higher proportion (17.8%) were re-tested in 30–120 days but only 16.1% of these re-tests were positive. These findings have several possible explanations, including that MSM may be at higher risk of re-infection than other groups by recommencing sex with a partner who has not yet been treated. Also, selective re-testing by higher-risk MSM would introduce a bias.
Some of the apparent re-infections may have been treatment failures. In MSM, a recent study in the USA found a 13% treatment failure rate for anal chlamydia infections treated with azithromycin. 16
Even if patients are reminded about the need for re-testing by their clinician, patients may not consider themselves at risk or perceive testing as a priority, and thus there will be considerable variation in patient adherence to this recommendation. Studies have examined various strategies for increasing return for re-testing after a chlamydia infection, including cash incentives, phone and letter reminders, motivational counselling, a brief recommendation by the clinician and sending urine samples in the mail. 14 , 17 , 18 Malotte et al. trialled a range of initiatives and found a reminder phone call was the most effective method to increase re-testing rates. Financial incentives did not increase return rates compared with a brief recommendation by the treating doctor. 14 Paneth-Pollak et al. evaluated the impact of postcard reminders in the mail using a controlled observational study and found re-testing rates doubled from 7% to 15%. 15 However, in neither of these interventions did re-testing rates get above 25%, which suggests other innovative strategies or combinations of interventions may be needed.
The ACCESS system has some methodological limitations. First, we did not collect behavioural data to address the question of selective re-testing of high-risk patients, and therefore the positivity rate at re-test may be artificially high. Second, the analysis may have underestimated re-testing rates, as some patients could have been re-tested at other services. Most people are linked in with a regular general practice clinic for most of their health needs but will sometimes attend sexual health clinics for STI testing. On return to their general practice clinic, it is possible some MSM in particular will be re-tested, as many of the general practice clinics they attend specialise in gay men’s health and conduct regular asymptomatic STI screening. 13 Young heterosexuals, however, are less likely to be re-tested at their next general practice clinic visit as only a very smaller proportion (7%) of 16–29-year-olds are offered chlamydia testing at such clinics. 19 Third, symptomatic people are more likely to return than asymptomatic people, so this could cause an overestimate of the true positivity rate at re-test. Fourth, we did not collect treatment data or detailed sexual behaviour data to discriminate between re-infections and treatment failures. Finally, the analysis included travellers, which, as demonstrated among MSM, underestimated the re-testing rates, as some travellers may only plan to stay in Australia for a few weeks or months, and so would not be eligible to return for a re-test.
Overall, the high chlamydia positivity rates at re-test shown in our analysis support the recommendations of clinical guidelines for a 3-month test for re-infection. The analysis was also able to describe less-than-ideal chlamydia re-testing rates, highlighting the need for innovative strategies to increase the frequency of re-testing. Given limited resources, targeting previously infected patients for re-testing might disproportionately reduce the transmission of chlamydia in the population who are at highest risk of developing chlamydia-related morbidity such as pelvic inflammatory disease and infertility. 6
Conflicts of interest
None declared.
Acknowledgements
We thank all sentinel sites which provided data for ACCESS (Box 1). ACCESS was funded by the Australian Government Department of Health and Ageing through the Chlamydia Targeted Grants Program. The opinions expressed here are not necessarily those of the Department of Health and Ageing.
References
[1] Vajdic CM, Middleton M, Bowden FJ, Fairley CK, Kaldor JM. The prevalence of genital Chlamydia trachomatis in Australia 1997–2004: a systematic review. Sex Health 2005; 2 169–83.| The prevalence of genital Chlamydia trachomatis in Australia 1997–2004: a systematic review.Crossref | GoogleScholarGoogle Scholar | 16335545PubMed |
[2] Kong F, Hocking J, Kyle-Link C, Chen MY, Hellard ME. Sex and sport: chlamydia screening in rural sporting clubs. BMC Infect Dis 2009; 9 73
| Sex and sport: chlamydia screening in rural sporting clubs.Crossref | GoogleScholarGoogle Scholar | 19470183PubMed |
[3] Hocking JS, Willis J, Tabrizi S, Fairley CK, Garland SM, Hellard M. A chlamydia prevalence survey of young women living in Melbourne, Victoria. Sex Health 2006; 3 235–40.
| A chlamydia prevalence survey of young women living in Melbourne, Victoria.Crossref | GoogleScholarGoogle Scholar | 17112433PubMed |
[4] Hocking J, Fairley C, Bradshaw C, Chen M, Tabrizi S, et al. Chlamydia Incidence and Re-Infection Rates Study (CIRIS). Melbourne: University of Melbourne (commissioned by the Chlamydia Targetted Grants Program, Commonwealth Department of Health & Ageing); 2009.
[5] Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, Donovan B, et al Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study. Sex Transm Infect 2007; 83 113–9.
| Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s3it12ltQ%3D%3D&md5=6a4d88055217c09accd02887fa7d3cdeCAS | 17005541PubMed |
[6] Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010; 201 134–55.
| Risk of sequelae after Chlamydia trachomatis genital infection in women.Crossref | GoogleScholarGoogle Scholar |
[7] Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53 537–43.
| Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion.Crossref | GoogleScholarGoogle Scholar | 19935075PubMed |
[8] Lister NA, Smith A, Tabrizi SN, Garland S, Fairley CK. Re-infection of Neisseria gonorrhoeae and Chlamydia trachomatis infections among men who have sex with men. Int J STD AIDS 2006; 17 415–7.
| Re-infection of Neisseria gonorrhoeae and Chlamydia trachomatis infections among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zgslKqtA%3D%3D&md5=6b7a8bda47694c81416c96a7d25cd771CAS | 16734967PubMed |
[9] Sexually Transmissible Infections in Gay Men Action Group. Sexually transmitted infection testing guidelines for men who have sex with men (STIGMA guidelines). Sydney: Australasian Chapter of Sexual Health Medicine; 2008.
[10] Australasian Chapter of Sexual Health Medicine. National management guidelines for sexually transmissible infections. Melbourne: Royal Australasian College of Physicians; 2008.
[11] Guy R, Kong F, Goller J, Franklin N, Bergeri I, Dimech W, et al A new national chlamydia sentinel surveillance system in Australia: evaluation of the first stage of implementation. Commun Dis Intell 2010; in press.
| 21090187PubMed |
[12] StataCorp. Intercooled Stata. 9.0 ed. College Station: Stata Corp; 2004.
[13] Guy R, Goller JL, Spelman T, El-Hayek C, Gold J, Lim M, et al Does the frequency of HIV and STI testing among MSM in primary care adhere with Australian guidelines? Sex Transm Infect 2010;
| Does the frequency of HIV and STI testing among MSM in primary care adhere with Australian guidelines?Crossref | GoogleScholarGoogle Scholar | 20460263PubMed |
[14] Malotte CK, Ledsky R, Hogben M, Larro M, Middlestadt S, St. Lawrence JS, et al Comparison of methods to increase repeat testing in persons treated for gonorrhea and/or chlamydia at public sexually transmitted disease clinics. Sex Transm Dis 2004; 31 637–42.
| Comparison of methods to increase repeat testing in persons treated for gonorrhea and/or chlamydia at public sexually transmitted disease clinics.Crossref | GoogleScholarGoogle Scholar | 15502669PubMed |
[15] Paneth-Pollak R, Klingler EJ, Blank S, Schillinger JA. The elephant never forgets: piloting a chlamydia and gonorrhea retesting reminder postcard in an STD clinic setting. Sex Transm Dis 2010; 37 365–8.
| The elephant never forgets: piloting a chlamydia and gonorrhea retesting reminder postcard in an STD clinic setting.Crossref | GoogleScholarGoogle Scholar | 20473247PubMed |
[16] Steedman NM, McMillan A. Treatment of asymptomatic rectal Chlamydia trachomatis: is single-dose azithromycin effective? Int J STD AIDS 2009; 20 16–8.
| Treatment of asymptomatic rectal Chlamydia trachomatis: is single-dose azithromycin effective?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M%2FksVaquw%3D%3D&md5=46e4c4eaeb8edf0fe7e895fbc3f725a4CAS | 19103887PubMed |
[17] Sparks R, Helmers JR, Handsfield HH, Totten PA, Holmes KK, Wroblewski JKH, et al Rescreening for gonorrhea and chlamydial infection through the mail: a randomized trial. Sex Transm Dis 2004; 31 113–6.
| Rescreening for gonorrhea and chlamydial infection through the mail: a randomized trial.Crossref | GoogleScholarGoogle Scholar | 14743074PubMed |
[18] Bloomfield PJ, Steiner KC, Kent CK, Klausner JD. Repeat chlamydia screening by mail, San Francisco. Sex Transm Infect 2003; 79 28–30.
| Repeat chlamydia screening by mail, San Francisco.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s%2Fms12htQ%3D%3D&md5=4ef066a166c77c00ea2937d700f3e564CAS | 12576609PubMed |
[19] Kong FYS, Guy R, Bergeri I, Boyle DJ, Hocking JS, Merritt T, et al. Chlamydia testing rates in general practices across Australia: the Australian Collaboration for Chlamydia Enhanced Sentinel Surveillance (ACCESS). Australasian Sexual Health Conference; 7–9 September 2009; Brisbane.


