Increasing HIV diagnoses in Australia among men who have sex with men correlated with the growing number not taking antiretroviral therapy
John M. Murray A B D , Garrett Prestage B , Jeffrey Grierson C , Melanie Middleton B and Ann McDonald BA School of Mathematics and Statistics, University of New South Wales, Sydney, NSW 2052, Australia.
B National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, NSW 2052, Australia.
C Australian Research Centre in Sex, Health and Society, La Trobe University, 215 Franklin Street, Melbourne, Vic. 3000, Australia.
D Corresponding author. Email: J.Murray@unsw.edu.au
Sexual Health 8(3) 304-310 https://doi.org/10.1071/SH10114
Submitted: 13 September 2010 Accepted: 31 January 2011 Published: 23 May 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Background: Australia has experienced rising notifications of HIV diagnoses despite widely available combination antiretroviral therapy (CART). New HIV diagnoses have also been younger than the average age of those living with HIV. We investigated the degree to which several risk factors could explain this rise in notifications and the younger age profile. Methods: Numbers and ages of men who have sex with men (MSM) living with HIV in Australia from 1983 to 2007 were calculated from notifications of HIV diagnoses and deaths. We compared the trend over time as well as the average ages of MSM newly diagnosed with HIV infection from 1998 to 2007 with those for: notifications of gonorrhoea and syphilis, total MSM living with HIV infection, and the component not on CART. Results: The percentage of younger MSM not taking CART has increased since 1998 (aged <30 years P < 0.001; 30–39 years P = 0.004). The trend of new HIV diagnoses was most significantly correlated with the total number of MSM living with HIV infection and the sector not taking CART (P < 0.0001). Based on similarity of average ages, MSM living with HIV infection and not taking CART was the best predictor of the increasing trend in new HIV diagnoses (99.9999% probability, Akaike information criterion). Conclusions: Our analyses suggest MSM living with HIV infection and not taking CART could be the source of the increase in HIV infections. Consequently, greater CART enrolment should decrease HIV incidence, especially in younger MSM.
Additional keywords: age, AIDS, combined antiretroviral therapy, epidemiology.
Introduction
It is of considerable concern that numbers of new HIV diagnoses have risen over the past decade in countries where it was hoped that the widespread availability of combination antiretroviral therapy (CART) would have led to decreasing numbers of HIV infections.1 In the UK, new HIV diagnoses have doubled from 2001 to 2006; in Western Europe, the number of new HIV diagnoses attributed to unsafe sex between men also doubled from 1999 to 2006; new HIV diagnoses among men who have sex with men (MSM) in the USA experienced an 11% increase from 2001 to 2005;2 and Australia has also experienced increasing HIV diagnoses3 (Fig. 1b).
|
CART significantly reduces HIV RNA concentrations in blood and semen, and should therefore reduce the risk of HIV transmission in a corresponding manner to that seen in the reduced risk of HIV transmission to heterosexual partners of untreated individuals with lower HIV RNA concentrations.4,5 However, CART also significantly reduces the mortality and morbidity associated with HIV, and in developed countries, this has meant the lifespan of those living with HIV is approaching lifespans for the uninfected population.6 This has resulted in an increasing number of individuals living with HIV in countries where effective CART is widely available. In Australia, the number of MSM living with HIV infection increased by more than one-third from 1995 to 2005, whereas before CART became available at the beginning of this period, numbers with HIV infection seemed to plateau.7
Expanding populations of individuals living with HIV infection, as a consequence of CART-induced decreased mortality, were also evident in other countries. From 2001 to 2005 the number of people living with HIV infection increased by 24% in the USA.8 Numbers of people diagnosed with HIV infection grew even more dramatically in the UK, from 20 000 in 1999 to over 60 000 in 2008.9
Although CART does not eliminate the risk of HIV transmission, it is expected that individuals taking CART are much less likely to transmit the infection than those who are not on CART.4,10 Therefore the expanding percentage of individuals enrolled on CART in several countries is encouraging,9,11 not only in terms of improved health for those individuals but also, hopefully, in terms of lowering numbers of new infections in their communities.
However, an expanding population living with HIV can also result in an increasing number of individuals not on CART despite growing trends in percentage enrolment. Moreover, percentages enrolled on CART can differ by age group, resulting in more untreated young persons and subsequent greater transmission of HIV to other young persons. This may contribute to the observed younger average age of new HIV diagnoses compared with those living with HIV.7 This disparity in ages between new diagnoses and those living with HIV infection has not been previously analysed in detail, and needs to be incorporated into explanations for new infections. We investigate this situation, especially addressing the disparity in ages, among MSM in Australia.
Methods
Since comparisons are to be based on age as well as numbers of cases, data sources were obtained that reported notifications for different age groups over the period of investigation.
HIV diagnoses for MSM in Australia were obtained from the Australian National HIV and AIDS Registry (NHAR) for cases where the exposure categories were ‘male homosexual contact’, ‘male homosexual contact and injecting drug use’, and male ‘other or undetermined’. As previously described,7 these exposure categories best determine new HIV diagnoses among MSM in Australia, where at least 80% of notifications are through homosexual sex.
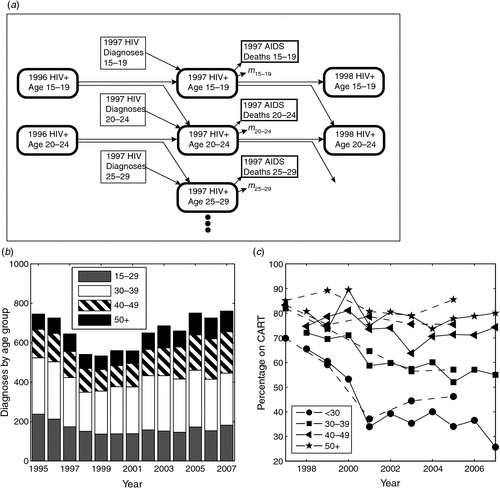
These diagnoses were tracked numerically over time, assuming aging of individuals so that they progressed over 5-year age groups from 15 to 60 years of age and above, as previously described.7 Numbers of deaths following AIDS among homosexual men, also listed under the above exposure categories, were obtained from the NHAR. The number of MSM living with HIV was then estimated from the diagnoses progressing with age, minus individuals in each group dying following AIDS, and also subject to natural annual mortality rates for males in the general community in each age group (m15–19, m20–24 …) obtained from the Australian Bureau of Statistics.12 The model for 1 year’s calculation is depicted in Fig. 1a and allows estimation of the number of MSM living with HIV infection at any year starting from 1983, as well as the number in each age group.7
Numbers and age groups of diagnoses of gonorrhoea and infectious syphilis in Australia were obtained from the National Notifiable Disease Surveillance System, which is maintained by the Australian Government Department of Health and Aging. Due to changes in the syphilis case definitions, data for infectious syphilis were only available from 2004 onwards.
In Australia, infectious syphilis and gonorrhoea occur in two main populations: Aboriginal and Torres Strait Islanders, and MSM.3 Aboriginal and Torres Strait Islander people represent a significant proportion of diagnoses only in rural and remote regions of Australia, with 10-fold higher rates in remote regions compared with rates in major cities.3 We therefore limited the diagnoses included in this study to urban men, using the remoteness structure of the Australian Standard Geographical Classification.13
CART usage for MSM living with HIV infection in Australia was obtained from two sources: the Gay Community Periodic Surveys (GCPS11) 1998–2007, and The HIV Futures Group.14 The GCPS annually survey MSM living with HIV infection in the capital cities (Sydney, Melbourne, Brisbane, Perth, Adelaide and Canberra) and assess the percentage taking antiretroviral therapy in the following age groups: under 30, 30–39, 40–49 and 50 and older. On average in the respective age groups, 68, 303, 255 and 90 HIV-positive MSM were surveyed each year from 1998. Biennially from 1997, the HIV Futures Group have surveyed MSM living with HIV infection in the states of New South Wales, Victoria and Queensland, these being the Australian states with the largest communities of MSM living with HIV. On average, the number surveyed in each of the age groups (under 30, 30–39, 40–49, 50–59 and 60 and older) were 19, 162, 236, 107 and 36 respectively. The percentage on CART used in estimates of numbers and ages was determined from the total number surveyed across Australia living with HIV infection and the subset enrolled on CART in each survey. The data from the GCPS were used for estimates of numbers enrolled on CART, since this survey covered more time points and a greater number of individuals. To remove fluctuations between years due to sample size, these estimates were averaged over a 3-year window centred on each year.
Results
Numbers of new HIV diagnoses among MSM in Australia have increased since 1999 (Fig. 1b). Over 80% of all diagnoses occur in this group so that this has also resulted in a growing total number of new HIV diagnoses. The average age of new diagnoses among MSM was also ~4 years younger than the estimated age of all MSM living with HIV infection in 2005, and this difference in age had widened over time.7 To investigate the part that MSM not on CART may play in increasing HIV diagnoses with this age disparity, we first determined whether individuals not enrolled on CART predominantly belong to younger age groups and whether their total has been increasing over time.
Number of MSM living with HIV infection who are not on CART
The percentage of MSM living with HIV in Australia taking CART has decreased since 1999 for younger age groups, but not for older individuals (Fig. 1c), where these values were determined from two sources: the GCPS11 and The HIV Futures Group.14 Combining the percentage estimates of MSM in these age groups on CART from the GCPS (since the number surveyed was highest and more frequent from this source), with the estimates from our model of the number of MSM living with HIV infection in the same age groups over these years (see Methods7), we obtain estimates of numbers of MSM living with HIV infection who are either taking CART or not (Table 1).
Although percentages of HIV-positive MSM enrolled on CART decreased significantly for the two younger age groups (P < 0.001 and P = 0.004) and showed a non-significant decrease for older ages, the estimated number of individuals taking CART increased from 7162 in 1998 to 8660 in 2007 (Table 1). This increase, however, was confined to the two older age groups: 40–49 and 50 years and above. The numbers of individuals in the younger age groups taking CART decreased considerably. Furthermore, the estimated number of MSM living with HIV infection and not taking CART increased for each of the age groups. Hence this increasing number of individuals not on CART is consistent with the hypothesis that they may be contributing to the increasing trend of new HIV diagnoses over this same period.
Comparison of trends
Although the number of individuals not on CART increases over time, consistent with the rise in numbers of new HIV diagnoses, other risk factors also exhibit an increase and have been suggested as possible explanations. These include other sexually transmissible infections (STI)1,15 and the rise in numbers of people living with HIV infection in general.16 Increased risky behaviour by individuals may also be a factor. We used notifications of new diagnoses of gonorrhoea and infectious syphilis in urban men, not only for the higher likelihood of transmission and acquisition of HIV when these STI are prevalent, but also as a surrogate marker for risky behaviour. In Australia, the majority of infectious syphilis and gonorrhoea notifications in urban regions occur in MSM.3
We investigated how well each of these explained the growth in numbers of new HIV diagnoses by first comparing the trend in observations of each risk factor with the trend in diagnoses. We hypothesised that a better predictor of new diagnoses would be a factor that showed a similar rate of change to that of new diagnoses.
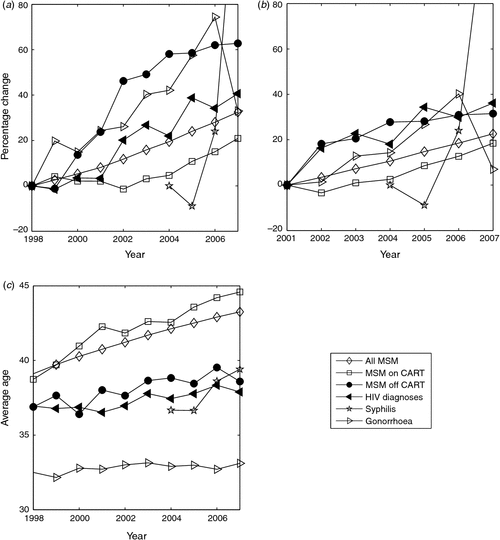
The values of each of these factors since 1998, the period over which data on CART and gonorrhoea were available, were compared with numbers of HIV diagnoses (Fig. 2a). Spearman rank correlations of each of these factors with numbers of new HIV diagnoses showed the greatest correlations with the total number of MSM living with HIV infection (P < 0.0001) and with the number of MSM not on CART (P < 0.0001). Syphilis notifications were not correlated with numbers of new HIV diagnoses (P = 0.75), whereas the other factors showed significant but intermediate levels of significance: numbers on CART (P = 0.03) and numbers of gonorrhoea notifications (P = 0.01). Percentage changes from 2001 show that HIV diagnoses are almost identical with the number of individuals not on CART (Fig. 2b).
|
Hence, solely based on trends over time, the growing total number of MSM living with HIV and the subgroup not on CART are most correlated with the trend shown by numbers of new HIV diagnoses.
Comparison of ages
If a risk factor significantly contributes to the sexual transmission or acquisition of HIV infection then not only should changes in numbers over time of observations of the risk factor parallel those of HIV diagnoses, but it would also be expected that the average ages of individuals observed for both the risk factor and the diagnoses would be similar. For example, if a risk factor has notifications where the individuals are, on average, 20 years of age, but HIV diagnoses are, on average, 40 years of age, then it is unlikely that the risk factor is a major contributor. Although MSM do not have sexual partnerships solely within their own age groups, on average this will be the case so that the average age of new HIV diagnoses should reflect the average age of the infecting individuals.
Although the estimated average age of MSM living with HIV increased from 39 to 43 years of age from 1998 to 2007, the average age of new diagnoses increased much more slowly, from 37 to 38 years of age (Fig. 2c). Individuals taking CART tended to be older so that this group of individuals was even further from duplicating the age profile of new HIV diagnoses. Urban men with gonorrhoea were much younger, around 32 or 33 years of age; however, urban men with infectious syphilis had average ages consistent with the ages of HIV diagnoses from 2004 to 2007.
By far the best predictor of the average age of new HIV diagnoses over this 10-year period was the average age of MSM living with HIV but not on CART (Fig. 2c). Using the Akaike information criterion,17 on the sum-of-squares differences between average age of HIV diagnoses over this period and for each of the possible predictors, individuals not on CART were the best model with a probability of 99.9999%. Hence individuals not on CART are the best predictor, based on similar ages to HIV diagnoses as well as having a consistent trend in numbers with new HIV diagnoses.
Discussion
Our calculations show that the number of MSM living with HIV infection who are not on CART is the best predictor, compared with all MSM living with HIV or MSM diagnosed with other STI, of the increasing number of new HIV diagnoses in MSM in Australia, in terms of both trends over time as well as consistent average ages (Fig. 2). As has been pointed out in other analyses,1,15 STI notifications have risen over the same period as increasing new HIV diagnoses and this naturally leads to the hypothesis that either by acting as cofactors in HIV transmission or acquisition, they have contributed to more HIV infections or they are reflective of increased risky behaviour with a similar result. However, notifications of gonorrhoea and syphilis show less or no significant correlation with HIV diagnoses from 1998 to 2007, and the average age of notifications of these two STI are very different to HIV notifications. Hence they are less likely to be major drivers of the expanding number of new HIV diagnoses.
We cannot, by its very nature, present an analysis of the numbers and ages of individuals with undiagnosed HIV infection or determine its correlation with new diagnoses. Other modelling has estimated that 31% of new HIV infections in Australia are due to transmission from individuals with undiagnosed infection,18 and this group is unlikely to be the main contributor to new HIV infections. An analysis of CD4+ T-cell numbers for MSM at HIV diagnosis in Australia (J. Murray, unpubl. data) suggests that older individuals are more likely to be undiagnosed compared with younger men, since the median CD4+ T-cell count at diagnosis decreases with age. This has also been observed elsewhere.19 Hence individuals who are undiagnosed will, on average, be older than new diagnoses. Therefore a comparison of individuals who are undiagnosed in terms of age would provide a poorer model than individuals who are diagnosed and not on CART.
Here, we have used HIV diagnoses as a surrogate marker for HIV infections. In general, there can be a considerable delay between these events. However, HIV testing in Australia is frequent among MSM,20 so that the time from infection to diagnosis should be considerably smaller than that observed in other comparable countries and, subsequently, HIV diagnoses should reasonably reflect HIV infections. Another limitation of this analysis is that we use notifications of gonorrhoea and syphilis in urban men. This should reflect levels of these STI mostly in MSM, but not necessarily in those living with HIV or those likely to become HIV infected.
The most notable limitation, however, is that we use correlations in numbers and ages not on CART as evidence for causation of new HIV diagnoses. Although this need not be the case, the converse should be true, that causation enforces correlation. For the other factors we investigated, there was significantly less correlation and therefore less evidence of these factors contributing to new HIV infections.
Previous investigations of the causes of increased HIV diagnoses in Australia have argued that changing levels of antiretroviral therapy are not responsible.21 Their arguments are based on the comparison of trends in HIV diagnoses from different Australian states against estimates of numbers of persons living with HIV in each state. Their estimates of each state’s number of persons living with HIV were determined from diagnoses in each state, rather than from those still residing there. There has been considerable movement of HIV-infected individuals within Australia.22 Hence comparisons based on estimates of numbers of HIV-infected persons diagnosed in individual states can be very inaccurate.
The percentage on CART has significantly declined for younger individuals (Fig. 1). The underlying reason for this preferential disenrollment from CART of younger individuals has not been conclusively described. The clinical incentive to commence CART early in HIV infection has lessened over the past decade, as the initial hope that CART would eradicate HIV infection in an individual has faded.23 Individuals are now more likely to stay off therapy while their CD4+ T-cell counts remain above suitable levels,24 since the Strategies for Management of Antiretroviral Therapy (SMART) trial indicated that treatment should not be interrupted once it is commenced.25 Older individuals are more likely to present with lower CD4+ T-cell counts at HIV diagnosis,26 and so it is understandable that a larger proportion of older individuals with diagnosed HIV infection will be on CART. Conversely, younger individuals maintain their CD4+ T-cell counts for longer periods of time and hence are more likely not to take CART when treatment is guided by CD4+ T-cell counts.
An increase in the length of time following infection at which CART treatment was initiated is supported by findings from analysis of the first decade of CART in Europe and North America, where median CD4+ T-cell counts at commencement of CART increased from 170 cells per mm3 in 1995–96 to 269 cells per mm3 in 1998 but then decreased to ~202 cells per mm3 in 2002–03.27 Our findings that decreased usage of CART may contribute to increased HIV diagnoses in Australia are also relevant to other countries.
The converse of this statement, that increasing CART levels reduces the numbers of new HIV infections, is of most interest to those advocating antiretroviral-based secondary prevention of HIV transmission. It has been observed that expanding CART coverage has been associated with a decrease in new HIV diagnoses in British Columbia.28 Our results are consistent with these findings, although they are more focussed on MSM rather than the injecting drug user community. In addition, we note the increasing numbers of individuals not on CART.
If decreased usage of CART underlies the rise in new HIV diagnoses, as suggested here, then it will be important to assess the level of CART that is necessary to limit and hopefully reduce new HIV infections. While the primary consideration for commencing CART with any patient should be clinical – and we do not propose otherwise – it is important to understand the implications of treatment guidelines at a population level for the secondary prevention of HIV transmission. Our analyses indicate that not only may earlier commencement of CART be beneficial to those currently living with HIV infection, it may also have a significant impact on secondary prevention.
Conflicts of interest
John Murray has received funding from Merck, and Johnson & Johnson.
Acknowledgements
The National Centre in HIV Epidemiology and Clinical Research (NCHECR) is funded by the Australian Government Department of Health and Aging, and is affiliated with the Faculty of Medicine, The University of New South Wales. Its work is overseen by the Ministerial Advisory Committee on AIDS, Sexual Health and Hepatitis. The NCHECR Surveillance Program is a collaborating unit of the Australian Institute of Health and Welfare. HIV Futures at the Australian Research Centre in Sex, Health and Society, La Trobe University, is funded by the Australian Government Department of Health and Aging.
References
[1] Sullivan PS, Hamouda O, Delpech V, Geduld JE, Prejean J, Semaille C, et al Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol 2009; 19 423–31.| Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005.Crossref | GoogleScholarGoogle Scholar |
[2] UNAIDS. North America, Western and Central Europe: AIDS epidemic update regional summary; 2007. WHO Library Cataloguing-in-Publication Data, UNAIDS: Geneva; 2007.
[3] National Centre in HIV Epidemiology and Clinical Research. HIV/AIDS, viral hepatitis and sexually transmissible infections in Australia annual surveillance Report 2007. Sydney: The University of New South Wales; 2007.
[4] Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23 1397–404.
| Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[5] Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342 921–9.
| Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group.Crossref | GoogleScholarGoogle Scholar |
[6] Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007; 146 87–95.
[7] Murray JM, McDonald AM, Law MG. Rapidly ageing HIV epidemic among men who have sex with men in Australia. Sex Health 2009; 6 83–6.
| Rapidly ageing HIV epidemic among men who have sex with men in Australia.Crossref | GoogleScholarGoogle Scholar |
[8] Centers for Disease Control and Prevention. HIV/AIDS surveillance report, 2005. Volume 17, revised edn. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2007.
[9] Health Protection Agency. HIV in the United Kingdom: 2009 report. London: Health Protection Agency, Centre for Infections; 2009.
[10] Vernazza P, Hirschel B, Bernasconi E, Flepp M. Les personnes séropositives ne souffrant d’aucune autre MST et suivant un traitment antirétroviral efficace ne transmettent pas le VIH par voie sexuelle. Bull Med Suisses 2008; 89 165–9.
[11] Zablotska I, Prestage G, Frankland A, Chong S, Sutherland R, Corrigan N, et al. Gay community periodic survey: Sydney, February 2007. Sydney: National Centre in HIV Social Research, The University of New South Wales; 2007.
[12] Jain SK. Trends in mortality: by causes of death in Australia, the states and territories during 1971–92, and in statistical divisions and sub-divisions during 1991–92. Contract No.: 3313.0. Canberra: Australian Bureau of Statistics; 1994.
[13] Australian Standard Geographical Classification, Australian Bureau of Statistics, Catalogue no. 1216.0. Canberra: Commonwealth of Australia; 2010.
[14] Grierson JW, Pitts MK, Thorpe RD. State of the (positive) nation: findings from the fourth national Australian HIV futures survey. Int J STD AIDS 2007; 18 622–5.
| State of the (positive) nation: findings from the fourth national Australian HIV futures survey.Crossref | GoogleScholarGoogle Scholar |
[15] Hoare A, Wilson DP, Regan DG, Kaldor J, Law MG. Using mathematical modelling to help explain the differential increase in HIV incidence in New South Wales, Victoria and Queensland: importance of other sexually transmissible infections. Sex Health 2008; 5 169–87.
| Using mathematical modelling to help explain the differential increase in HIV incidence in New South Wales, Victoria and Queensland: importance of other sexually transmissible infections.Crossref | GoogleScholarGoogle Scholar |
[16] Ginige S, Chen MY, Hocking JS, Grulich AE, Fairley CK. Rising HIV notifications in Australia: accounting for the increase in people living with HIV and implications for the HIV transmission rate. Sex Health 2007; 4 31–3.
| Rising HIV notifications in Australia: accounting for the increase in people living with HIV and implications for the HIV transmission rate.Crossref | GoogleScholarGoogle Scholar |
[17] Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev 2004; 11 192–6.
| AIC model selection using Akaike weights.Crossref | GoogleScholarGoogle Scholar |
[18] Wilson DP, Hoare A, Regan DG, Law MG. Importance of promoting HIV testing for preventing secondary transmissions: modelling the Australian HIV epidemic among men who have sex with men. Sex Health 2009; 6 19–33.
| Importance of promoting HIV testing for preventing secondary transmissions: modelling the Australian HIV epidemic among men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[19] Chadborn TR, Baster K, Delpech VC, Sabin CA, Sinka K, Rice BD, et al No time to wait: how many HIV-infected homosexual men are diagnosed late and consequently die? (England and Wales, 1993–2002). AIDS 2005; 19 513–20.
| No time to wait: how many HIV-infected homosexual men are diagnosed late and consequently die? (England and Wales, 1993–2002).Crossref | GoogleScholarGoogle Scholar |
[20] Prestage G, Jin F, Zablotska IB, Imrie J, Grulich AE, Pitts M. Trends in HIV testing among homosexual and bisexual men in eastern Australian states. Sex Health 2008; 5 119–23.
| Trends in HIV testing among homosexual and bisexual men in eastern Australian states.Crossref | GoogleScholarGoogle Scholar |
[21] Marrone J, Fairley CK, Chen M, Hocking JS. Comparisons of trends in antiretroviral use and HIV notification rates between three Australian states. Aust N Z J Public Health 2007; 31 131–4.
| Comparisons of trends in antiretroviral use and HIV notification rates between three Australian states.Crossref | GoogleScholarGoogle Scholar |
[22] Prestage G, Ferris J, Grierson J, Thorpe R, Zablotska I, Imrie J, et al Homosexual men in Australia: population, distribution and HIV prevalence. Sex Health 2008; 5 97–102.
| Homosexual men in Australia: population, distribution and HIV prevalence.Crossref | GoogleScholarGoogle Scholar |
[23] Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, et al Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997; 387 188–91.
| Decay characteristics of HIV-1-infected compartments during combination therapy.Crossref | GoogleScholarGoogle Scholar |
[24] Gazzard BG. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med 2008; 9 563–608.
| British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008.Crossref | GoogleScholarGoogle Scholar |
[25] SMART. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis 2008; 197 1133–44.
| Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study.Crossref | GoogleScholarGoogle Scholar |
[26] Zingmond DS, Wenger NS, Crystal S, Joyce GF, Liu H, Sambamoorthi U, et al Circumstances at HIV diagnosis and progression of disease in older HIV-infected Americans. Am J Public Health 2001; 91 1117–20.
| Circumstances at HIV diagnosis and progression of disease in older HIV-infected Americans.Crossref | GoogleScholarGoogle Scholar |
[27] May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, et al HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet 2006; 368 451–8.
| HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis.Crossref | GoogleScholarGoogle Scholar |
[28] Montaner JS, Wood E, Kerr T, Yip B, Lima V, Shannon K, et al. Abstract 88LB: association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly among injection drug users in British Columbia, Canada. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco; 27 February 2010.