Importance of promoting HIV testing for preventing secondary transmissions: modelling the Australian HIV epidemic among men who have sex with men
David P. Wilson A B , Alexander Hoare A , David G. Regan A and Matthew G. Law A
+ Author Affiliations
- Author Affiliations
A National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, NSW 2010, Australia.
B Corresponding author. Email: Dwilson@nchecr.unsw.edu.au
Sexual Health 6(1) 19-33 https://doi.org/10.1071/SH08081
Submitted: 29 October 2008 Accepted: 11 December 2008 Published: 23 February 2009
Abstract
Background: We address the research questions: (i) what proportion of new HIV infections is transmitted from people who are (a) undiagnosed, (b) in primary HIV infection (PHI), (c) on antiretroviral therapy?; and (ii) what is the expected epidemiological impact of (a) increasing the proportion of newly acquired HIV infections receiving early treatment, and (b) increasing HIV testing rates?
Methods: We used a mathematical model to simulate HIV transmission in the population of men who have sex with men (MSM) in Australia. We calibrated the model using established biological and clinical data and a wide range of Australian MSM epidemiological and behavioural data sources.
Results: We estimate that ~19% of all new HIV infections are transmitted from the ~3% of Australian HIV-infected MSM who are in PHI; ~31% of new HIV infections are estimated to be transmitted from the ~9% of MSM with undiagnosed HIV. We estimate that the average number of infections caused per HIV-infected MSM through the duration of PHI is ~0.14–0.28.
Conclusions: The epidemiological impact of increasing treatment in PHI would be modest due to insufficient detection of newly-infected individuals. In contrast, increases in HIV testing rates could have substantial epidemiological consequences. The benefit of testing will also increase over time. Promoting increases in the coverage and frequency of testing for HIV could be a highly-effective public health intervention, but the population-level impact of interventions based on promoting early treatment of patients diagnosed in PHI is likely to be small. Treating PHI requires further evaluation of its long-term effects on HIV-infected individuals.
Additional keywords: Australia, early treatment, HIV/AIDS, men who have sex with men, testing.
Acknowledgements
DPW conceived the analyses, conducted the literature survey, supervised the technical project, and wrote the manuscript, AH carried out all analyses and produced results and figures, DGR was involved in conceptualisation, literature survey, and manuscript editing, MGL was involved in conceptualisation, facilitating steering of the project and the reference group, and manuscript editing. DPW, AH, and DGR developed the model framework. The authors would like to thank the members of the Reference Group that provided advice and guidance through the development of this work. Members of the Reference Group, and the bodies they represented, were: Professor Frank Bowden, HIV/AIDS and STIs Subcommittee of the Ministerial Advisory Committee on AIDS, Sexual Health and Hepatitis; Ms Sharon Flanagan, Ms Karen Fox, Australian Government Department of Health and Ageing; Associate Professor John Imrie, National Centre in HIV Social Research, UNSW; Mr Philip Keen, AIDS Council of New South Wales; Dr Rosemary Lester, Communicable Diseases Network Australia; Dr Kelly Shaw, Blood Borne Virus Surveillance System; Mr Bill Whittaker, National Association of People Living with HIV/AIDS. The authors would also like to thank the following colleagues and collaborators at the National Centre in HIV Epidemiology and Clinical Research for making data available, and also for numerous discussions and advice on interpretation: Professor John Kaldor, Dr Handan Wand, Ms Linda Gelgor, Ms Kathleen Glenday, Professor Andrew Grulich, Associate Professor Anthony Kelleher, Ms Ann McDonald, Ms Melanie Middleton, Dr Garrett Prestage. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, University of New South Wales. Dr David Wilson is funded by a Vice Chancellor’s Fellowship from the University of New South Wales and by grant number DP0771620 from the Australian Research Council; Mr Alex Hoare is funded by a University Postgraduate Award from the University of New South Wales; Dr David Regan is funded by grant number 358425 from the Australian National Health and Medical Research Council; Associate Professor Matthew Law is funded by grant numbers 1-U01-AI6994–01, 1-U01-AI068641, 1-U01-A1069907–01, and 5-U19-AI05371 from the National Institutes of Health, USA.
References
[1]
Fisher M, Pao D, Murphy G, Dean G, McElborough D, Homer G, et al. Serological testing algorithm shows rising HIV incidence in a UK cohort of men who have sex with men: 10 years application. AIDS 2007; 21 2309–14.
| PubMed |
to obtain the following trends over time:
In regular relationships that are serodiscordant we assume that average condom usage is high. Based on the Futures study
29
we assume condoms are used in 75–85% of anal intercourse acts between discordant men who have sex with men (MSM). However, in regular relationships that are seroconcordant we assume that average condom usage is relatively low; we assume condoms are used in 5–10% of acts.
29
In casual relationships, serological disclosure is not as common as in regular relationships, but if the MSM in a casual relationship determine the relationship is serodiscordant then we assume condoms are used in 95–100% of acts. We assume that condoms are used more frequently in casual partnerships than in regular partnerships.
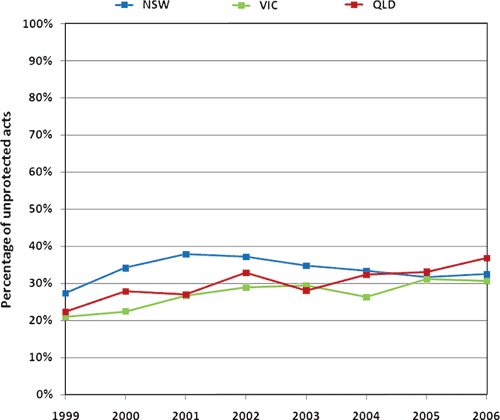
|
Appendix 4
d: There is evidence to suggest that the percentage of treated patients with undetectable virus has increased over time.
34
This could be due to numerous factors such as greater adherence or different drug regimes. We use data from the Australian HIV Observational Database (AHOD) database34:
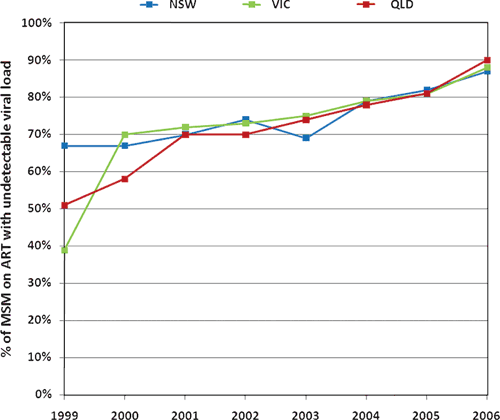
|
Appendix 5
e: We use the established relationship from
8
,
108
,
109
to determine the change in transmission probability as viral load changes, namely, 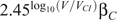 if the viral load (V), is greater than the baseline during chronic infection (V
CI
), where β
C
is the transmission probability associated with the baseline viral load, and
if the viral load (V), is greater than the baseline during chronic infection (V
CI
), where β
C
is the transmission probability associated with the baseline viral load, and 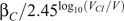 if V < V
CI
. Although this relationship was originally determined for heterosexual penile–vaginal intercourse, we use a greater baseline transmission probability for homosexual penile–anal intercourse, β
C
, and assume that the same multiplicative increase in transmission holds with changes in viral load. This relationship was established based on empirical data in a cohort of African heterosexual couples. In the absence of information to the contrary, we assume that this relationship holds for penile–anal transmission and that it applies across the range of viral loads, including those that are below detectable levels, regardless of whether or not a person is under treatment with antiretroviral therapy. However, the baseline transmission probability differs between the different modes of transmission.
108
if V < V
CI
. Although this relationship was originally determined for heterosexual penile–vaginal intercourse, we use a greater baseline transmission probability for homosexual penile–anal intercourse, β
C
, and assume that the same multiplicative increase in transmission holds with changes in viral load. This relationship was established based on empirical data in a cohort of African heterosexual couples. In the absence of information to the contrary, we assume that this relationship holds for penile–anal transmission and that it applies across the range of viral loads, including those that are below detectable levels, regardless of whether or not a person is under treatment with antiretroviral therapy. However, the baseline transmission probability differs between the different modes of transmission.
108
f: In order to model the impact of sexually transmissible infections (STIs) on HIV transmission it is necessary to estimate the proportion of MSM with other STIs as well as trends over time and by state. This is problematic for several reasons. First, while there are indications that the prevalence of some STIs, notably syphilis, is increasing in MSM in Australia, most data are reported only as notifications, not as the proportion of tests that are positive. Furthermore, the National Centre for HIV Social Research reports significant increases in testing (10–20%) in the past few years. Second, much of the published data on STIs in MSM in Australia is from the HIM (Health in Men) study and the incidence of STIs has decreased in this Sydney-based HIV-negative cohort over the past few years. Third, there are little data on trends in STI incidence and prevalence in MSM for the other states. Fourth, the most prevalent STI associated with HIV transmission is herpes simplex virus-2 (HSV-2) with prevalence in the HIM cohort estimated at ~23% masking any trends that might be occurring with other STIs. Of course, HSV-2 is latent for significant proportions of time in infected people and virus is shed periodically; thus, the effective prevalence of HSV-2 for which it increases HIV transmissibility is reduced. Given the uncertainty, we assume that the average proportion of MSM with STIs (ulcerative or non-ulcerative, that contribute to increasing HIV transmissibility) is in the range 5–15% initially (that is, at 1999). To investigate national HIV trends we do not distinguish STI levels between states. Although there are currently no published data, the prevailing perception among epidemiologists and sexual health clinicians is that there has been a rise in the number of STIs in recent years. However, because the magnitude of increase is different between different STIs we do not use STI data. Our initial analyses are based on an initial uncertainty range of 5–15% but constant over time. We then investigate numerous rates of increase in the prevalence of other STIs to determine the influence of increasing STIs on the HIV epidemic.
g: There is strong evidence that both ulcerative and non-ulcerative STIs can increase the probability of HIV transmission by augmenting HIV infectiousness and susceptibility. Reciprocally, HIV infection can enhance the transmission of other STIs. This is a complex synergy and the results of several prospective studies estimate the relative risks of HIV infection due to infection with other STIs in the range 2 to 24, but largely clustering between 2 and 5. We therefore assume that the multiplicative increase in transmission probability due to concurrent infection with another STI is in the range 2–5.
h: Data from the Gay periodic surveys over time for the percentage of MSM who tested for HIV in the past 12 months are used as shown in the graph below:
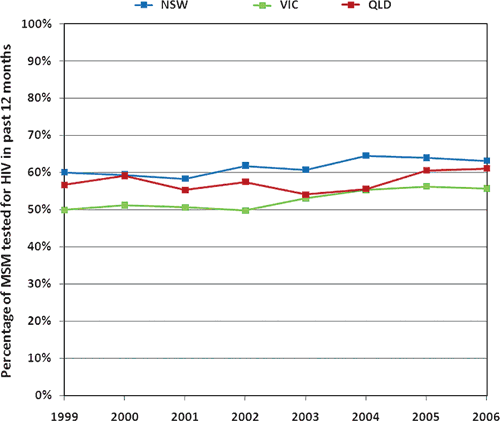
|
Appendix 6
i: We evaluated available data from primary infection cohorts of the percentage of HIV-infected MSM who commenced antiretroviral therapy (ART) within 1 year of HIV diagnosis, including patients recruited to the Acute Infection and Early Disease Research Program (CORE 01) protocol established by the National Institute of Health, and the Primary HIV and Early Disease Research: Australian Cohort (PHAEDRA) established by the National Centre in HIV Epidemiology and Clinical Research. These data have large uncertainty (summarised in Glenday et al.
16
), is limited in time and only includes NSW and Vic. Sample sizes are also not sufficient (as low as four in some years for Vic. and six for NSW). Consequently, this has been used as a rough guide but we make assumptions in the trends in early treatment based on personal communications with clinicians (e.g. Professor Tony Kelleher). We estimate the basic anecdotal trends observed over the past few years by the figure below. But since there are no firm data for the trends, we include greater uncertainty bounds on this time-dependent parameter than on the others (we use a multiplicative uncertainty range on these trends of 0.6–1.2.
We then assume that the initial dosing schedule for these patients who commence treatment in primary infection is 6–12 months, after which time 60–70% of these patients will continue ART and the remaining patients will discontinue therapy until a later time.
j: We assume that individuals who have not been diagnosed with HIV infection, but are infected, will have the same lifetime duration (30–35 years) of sexual activity (in terms of choosing new partners) as those that are uninfected or at different disease stages. However, the number of partners chosen will differ between some disease stages.
k: This leads to ~150 000–175 000 MSM nationally. The proportion of new MSM in NSW/ACT, Vic., Qld each year as a subset of the total national number are indicated.
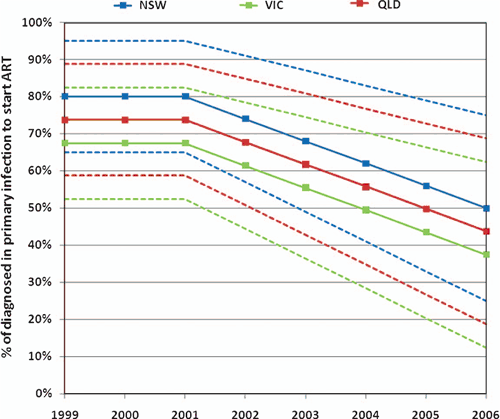
|






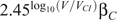 if the viral load (V), is greater than the baseline during chronic infection (V
CI
), where β
C
is the transmission probability associated with the baseline viral load, and
if the viral load (V), is greater than the baseline during chronic infection (V
CI
), where β
C
is the transmission probability associated with the baseline viral load, and 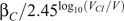 if V < V
CI
. Although this relationship was originally determined for heterosexual penile–vaginal intercourse, we use a greater baseline transmission probability for homosexual penile–anal intercourse, β
C
, and assume that the same multiplicative increase in transmission holds with changes in viral load. This relationship was established based on empirical data in a cohort of African heterosexual couples. In the absence of information to the contrary, we assume that this relationship holds for penile–anal transmission and that it applies across the range of viral loads, including those that are below detectable levels, regardless of whether or not a person is under treatment with antiretroviral therapy. However, the baseline transmission probability differs between the different modes of transmission.
if V < V
CI
. Although this relationship was originally determined for heterosexual penile–vaginal intercourse, we use a greater baseline transmission probability for homosexual penile–anal intercourse, β
C
, and assume that the same multiplicative increase in transmission holds with changes in viral load. This relationship was established based on empirical data in a cohort of African heterosexual couples. In the absence of information to the contrary, we assume that this relationship holds for penile–anal transmission and that it applies across the range of viral loads, including those that are below detectable levels, regardless of whether or not a person is under treatment with antiretroviral therapy. However, the baseline transmission probability differs between the different modes of transmission.