Short-term physiological effects of smoke on grapevine leaves
T. L. Bell A B D , S. L. Stephens C and M. A. Moritz CA Faculty of Agriculture and Environment, University of Sydney, Sydney, NSW 2015, Australia.
B Bushfire Cooperative Research Centre, East Melbourne, Vic. 3002, Australia.
C Department of Environmental Science, Policy, and Management, University of California, Berkeley, CA 94720, USA.
D Corresponding author. Email: tina.bell@sydney.edu.au
International Journal of Wildland Fire 22(7) 933-946 https://doi.org/10.1071/WF12140
Submitted: 21 August 2012 Accepted: 15 March 2013 Published: 23 July 2013
Abstract
In recent years, bushfires and prescribed burns have caused substantial economic loss to the wine industry due to smoke taint, which makes wine unpalatable. Considerable research is being done to ameliorate smoke taint but the information available about the effect of smoke on grapevines is limited. We examined the physiological effects of short-term exposure to smoke on leaves of several varieties of grapevines. Gas exchange was measured before and after short-term exposure of leaves to smoke that was produced by combustion of two different fuels. For most varieties, short-term exposure to smoke had little effect on leaf physiology. For varieties that were affected by smoke, patterns of recovery of leaf physiology depended on fuel type. Short-term exposure to smoke had, at best, no significant effect and, at worst, only temporary effects on functioning of leaves. All varieties had recovered to pre-smoke functioning within 48 h. This study will contribute to the growing body of information relevant to fire and land management agencies and the wine industry in fire-prone areas including Australia, North and South America and Europe.
Additional keywords: bushfire, gas exchange, photosynthesis, stomatal conductance, transpiration.
Introduction
On average, ~5 × 107 ha of land have burnt each year between 1997 and 2003 in Australia as a result of both planned and unplanned fires (Ellis et al. 2004). The economic loss associated with bushfire events in Australia has amounted to hundreds of millions of dollars over the last decade. In particular, bushfires during the summer of 2006–07 in Victoria caused an estimated loss of revenue of AU$7.5–9.0 × 107 due to smoke taint in wine (Whiting and Krstic 2007). Grapes exposed to smoke from prescribed burns or bushfires often produce wine and juice that is unpalatable and is therefore unprofitable. Such financial losses have prompted inquiry into the chemistry involved in smoke taint in wine and a comprehensive research program has been developed around this topic by the Australian wine industry (Jiranek 2011). In contrast, research into the direct effects of smoke on grapevine physiology is lacking and the information currently available is anecdotal or poorly replicated. In light of predictions for increased bushfire risk in Australia (Beer and Williams 1995; Williams et al. 2001; Pitman et al. 2007), the recent spate of landscape-scale wildfires in California and southern Europe, and the potential economic impact of fires (e.g. the wine industry in the US is estimated to be worth US$9 × 1010, MKF Research 2006), national and international need for such research is likely to escalate.
It is well known that smoke from bushfires can alter atmospheric composition and can affect human health and visibility, but environmental effects of smoke are largely unknown. Consequently there is only a small body of literature exploring the effects of smoke on plant physiology and growth in Australia and elsewhere (Bell and Adams 2009). Of the publications that are available, most are concerned with the effect of smoke on seed germination (e.g. see Dixon et al. 2009; Light et al. 2009) with little information available on the direct effects of smoke on the physiology and biochemistry of plants (e.g. Taylor and Van Staden 1998; Sparg et al. 2005; Jain and Van Staden 2006; Kulkarni et al. 2010). In contrast, the adverse effect of air pollution on vegetation has long been described (see review by Ward and Hardy 1991). Many of the reactive chemicals found in air pollution are also commonly found in bushfire smoke making the substantive literature dealing with the effects of air pollution on plant physiology a potential source of comparative information. Smoke from burning of vegetation contains a complex mixture of the visible products of burning (particulate matter and water vapour) and primary and secondary gaseous products including considerable quantities of CO and CO2 and variable amounts of CH4, NH3, SO2, oxides of nitrogen (NO and NO2), volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (Crutzen and Andreae 1990; Ward 1999; Andreae and Merlet 2001; Stephens et al. 2007). It can be hypothesised that differing leaf-level responses would result from exposure to smoke produced from different fuel types based on the extensive range of emissions produced during combustion of vegetation. This hypothesis is supported by variation among plant species in type and quantity of naturally emitted biogenic VOCs (Guenther et al. 1995; Winters et al. 2009). In addition, it should be noted that the composition and quantity of smoke produced depends on moisture content and structural arrangement of fuel (Ward 1999).
It is commonly reported that grapevines (Vitis vinifera L.) have a wide range of tolerance to stressful environmental conditions such as high temperature and high atmospheric vapour pressure deficit (Kadir 2006; Soar et al. 2006), drought or limited water supply (e.g. Regina and Carbonneau 1999; Medrano et al. 2003; Stevens et al. 2008; Santesteban et al. 2009; Edwards et al. 2011), high irradiance and UV-B radiation (Schultz 2000; Moutinho-Pereira et al. 2007) and salinity (Stevens et al. 1999; Ben-Asher et al. 2006). Such tolerance is no doubt due to a long history of selection for varieties to withstand a multiplicity of climatic and soil conditions and changing agricultural practices, but also evolving consumer and market demand (see review by Jackson and Lombard 1993). Indeed, grapevines are commonly grown in areas with Mediterranean-type climates where there is seasonal drought and in some circumstances may be cultivated under water- and nutrient-limited conditions to improve grape quality (Keller 2005). Variation in yield and quality even occurs within a single vineyard and from year to year, due to subtle differences in climatic and soil conditions (Bramley and Hamilton 2004; Bramley 2005). Given the tolerance and variability of this species, it can therefore be hypothesised that grapevines will be able to withstand short-term contact with smoke, at least in terms of leaf functioning.
The broad aim of the study was to determine physiological effects of short-term exposure of grapevines to smoke produced from combustion of known types of leaf litter. Two related studies were conducted – one in the laboratory using a smoke chamber and the second in a small glasshouse. Using measures of gas exchange of grapevine leaves before and after exposure to smoke, we set out to address three questions: (i) will leaf physiology be affected by short-term exposure to smoke, (ii) is there variation in response to smoke according to grapevine variety and (iii) will smoke from different fuel types cause different patterns of physiological response? With such insight, the effect of smoke on grapevines can be better understood and planned fires can be managed accordingly to reduce potential economic losses.
Materials and methods
Establishment of smoke chamber study
Six grapevine varieties grafted onto known rootstocks (Cabernet Franc: clone FPS11/ rootstock 3309G, Cabernet Sauvignon: FPS07/5BB, Chardonnay: FPS04/SO4, Durif: FPS03/SO4, Pinot Noir: FPS37/5C and Syrah: TCVS/F, 120-mm canes) were sourced from a local supplier (Novavine, Santa Rosa, CA). Four weeks before experimentation, rootlings were transplanted into 180-mm diameter × 200-mm depth plastic pots in a mix of sand, peat and vermiculite (1 : 1 : 1 v/v), and were maintained in glasshouse facilities at the University of California, Berkeley, CA. The glasshouse was naturally lit and held at a constant temperature of 25°C and ambient humidity. Grapevine plants were supplied with slow-release fertiliser at the time of potting and were watered as required. Routine pest and fungal control measures were practiced fortnightly and plants were pruned as required to maintain a manageable size and to promote new leaf growth. All experimental plants were of similar size and age having been grown from canes and, as phenological stages are important in smoke taint studies (e.g. Kennison et al. 2011), no flowers or fruits were present.
The smoke chamber was a modified sealed plastic cooler (370 × 370 × 940 mm) fitted with a dispersal manifold at the top allowing smoke to enter and a vacuum line at the bottom to draw smoke through to the chamber (see Calder et al. 2010). A Perspex window (200 × 250 mm) was fitted into the front of the chamber to expose plants to ambient light. Smoke was produced by combusting 200 mg of leaf litter (see below) in a glass funnel with a steel-screen. The funnel was fitted into the top of a filter flask that was connected to the vacuum line of the smoke chamber. The flask was cooled to 25°C during combustion using a water bath. Leaf litter was burnt to ash and the smoke produced was pulled into the chamber under vacuum and held there for periods of 15 min by closing a tap fitted into the vacuum line. This particular time interval was used as it corresponded with exposure times already used in published (e.g. Calder et al. (2010) used 20-min exposure) and unpublished studies (P. Cowan, pers. comm.) to allow a broad comparison of plant responses. Two plants of the same variety were treated per day and photosynthetic measures were made between 0800 and 1600 hours.
The leaf litter used for production of smoke for the chamber study came from Coast Live Oak (Quercus agrifolia Née) and Tasmanian Bluegum (Eucalyptus globulus Labill.). The advantage of using these species as fuel is that they are likely to be a significant contributor to smoke emissions in the winegrowing regions of California as they occupy a sizeable area of the surrounding vegetation or as plantations and windbreaks (CalFlora 2012). Tasmanian Bluegum is a common forest tree in Victoria and Tasmania (Boland et al. 2006) and is therefore also expected to be a major contributor to smoke emissions during wildfires in these Australian states. Leaves from both species were collected from small plantings of mature trees on the University of California, Berkeley campus. Whole leaves were collected from the surface litter layer, air-dried and ground using a small electric grinder. Ground leaves were sieved to obtain the 2-mm fraction. All six grapevine varieties were treated once with smoke produced by combustion of leaf litter from Coast Live Oak but only four varieties (Cabernet Franc, Cabernet Sauvignon, Pinot Noir and Syrah) were treated with smoke produced from combustion of Tasmania Bluegum owing to poor growth of replicate plants of Chardonnay and Durif. Two grapevine varieties (Cabernet Franc and Pinot Noir) were exposed to smoke from combustion of Coast Live Oak a second time.
Establishment of glasshouse study
Grapevines were treated with smoke in a small enclosed glasshouse as a means of keeping the light environment high during smoke treatment and avoiding the initial reduction in photosynthesis and gas exchange encountered in the smoke chamber. Six grapevine varieties (Cabernet Sauvignon, Chardonnay, Muscat Gordo, Pinot Noir, Shiraz and Verdelho, 120-mm canes) were obtained as own-rooted plants from a local supplier (Boulevarde Nurseries, Irymple, Vic.) and maintained in glasshouse facilities at the University of Melbourne (Creswick, Vic.). The glasshouse was naturally lit and held at a constant temperature range of 20–25°C and ambient humidity. Twelve months before experimentation, rootlings were transplanted into 250-mm diameter × 300-mm depth plastic pots in a commercially available potting mix (Hortopine, Vic.). Five replicates per variety were prepared. Grapevines were supplied with slow-release fertiliser at the time of potting and again after 6 months of growth. Plants were watered as required and pesticide and fungicide applied when needed. Plants were pruned every 3 months to manage their size and to promote new leaf growth. Plants were of a similar age and did not have flowers or fruits at any stage of the experiment.
Smoke was produced for the glasshouse study using a 100-mm diameter bee smoker (Beeco Bee Smoker) sourced from a local beekeeping supply company (Pender Beekeeping Supplies, NSW). Whole leaves for combustion were collected from the litter layer produced by mature Eucalyptus globulus trees growing in a small plantation on the edge of the Creswick State Forest near the University of Melbourne campus in Creswick. Leaves were air-dried and cut into 20 × 20-mm pieces. The bee smoker was loosely packed with a 50–60 mm thick layer of leaf litter and lit with a butane lighter. As flaming combustion reduced, more leaf litter was added and the smoke produced was pumped around the leaves of the grapevine plants as uniformly as possible for 15 min. Replicates (n = 5) of each grapevine variety were treated together on the same day and photosynthetic measures made between 0900 and 1300 hours.
Photosynthetic and gas exchange measurements
For both studies, gas exchange was measured and rates of net photosynthesis (A), stomatal conductance to water vapour (gs) and transpiration (E) were calculated using an infrared gas analyser (LI-6400 Portable Photosynthesis System, Li-Cor Biosciences, Lincoln, NE) equipped with a clamp-on leaf cuvette. A photosynthetic photon flux density of 1000 μmol m–2 s–1 was supplied by a 6400–04 LED blue-red light source and temperatures of 25°C and 30% humidity were used throughout both studies.
For the smoke chamber study, grapevines were transferred from the glasshouse to a growth room with a 12-h dark–light cycle providing light levels of 800–900 μmol m–2 s–1 and constant temperature (25°C) and kept there for 2–3 days before and after smoke treatment. A young fully expanded leaf was selected on each plant and gas exchange was measured intensively on this leaf during two main time periods: 240 min (4 h) before exposure (‘pre-smoke’) and 240 min (4 h) after exposure to smoke (‘post-smoke’). To account for the effect of the smoke chamber itself (i.e. reduced light availability), plants were placed into the chamber for 15 min before the start of pre-smoke measurements. The gas exchange of leaves was then measured every 10 min for the first hour then every 30 min for the next 3-h period. Plants were then transferred to the smoke chamber and exposed to smoke for 15 min produced by burning one of the two fuel types (Coast Live Oak or Tasmanian Bluegum). Gas exchange was measured immediately after removal of the plant from the smoke chamber (post-smoke) and at the same frequency of measurement as before exposure to smoke (i.e. 10- and 30-min intervals). Gas exchange was measured again after 24 and 48 h. Four replicate plants of each grapevine variety were used.
To determine the response of leaves to repeated exposure to smoke, two grapevine varieties, Cabernet Franc and Pinot Noir, were exposed to smoke a second time in the smoke chamber (4 weeks after the first exposure) using the same treatment and measurement protocols as above (n = 4). The two varieties selected for this comparison was based on the most marked short-term physiological response to smoke during the first exposure.
For the glasshouse study, grapevines were transferred to a small glasshouse (~15-m2 floor space) at least 24 h before treatment to allow the plants to adjust to any change in ambient light conditions. The three youngest fully expanded leaves were tagged on each plant and rates of gas exchange were measured using these leaves. Measurements of gas exchange were made before smoke exposure (time = 0 min), at 30, 60 and 90 min after exposure to smoke and again after 24 and 48 h. Five replicate plants of each grapevine variety were used.
Chlorophyll content of leaves
As the experimental period for both studies spanned 6–10 weeks in autumn, chlorophyll content of leaves was used to indicate if plants were entering winter dormancy. A leaf chlorophyll meter (SPAD-501, Minolta Corp., Osaka, Japan) was used to measure chlorophyll content of at least 20 of the youngest fully expanded leaves of each variety every fortnight for the smoke chamber study (four measurement times) and at the start and end of the glasshouse study (two measurement times).
Statistical analyses
Photosynthesis, gas exchange and chlorophyll data collected for each grapevine variety from both studies were compared using two-way repeated-measures ANOVA (PASW Statistics 21, SPSS Inc.). Sphericity of data was tested using Mauchly’s test statistic and in all cases it was non-significant so variances of differences were assumed to be not significantly different (P > 0.05) and no correction tests were required.
Results
Smoke chamber study
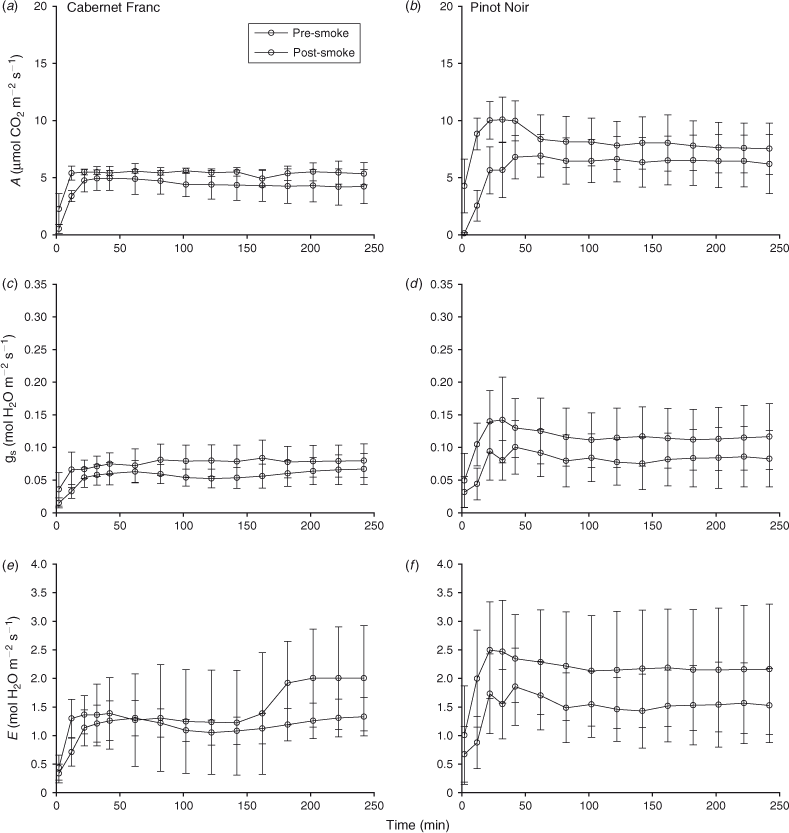
All of the grapevine varieties tested in the smoke chamber study had low rates of photosynthesis, stomatal conductance and transpiration for the first 10–20 min of measurement regardless of whether they were exposed to smoke or not (Fig. 1; note that only data for Pinot Noir and Chardonnay are shown). This is most likely due to low light conditions in the smoke chamber (250–300 μmol m–2 s–1). Leaves quickly adjusted to higher light conditions in the cuvette and after the first 30–60 min of measurement, mean rates of photosynthesis were between 8 and 13 µmol CO2 m–2 s–1, stomatal conductance ranged from 0.15 to 0.20 mol H2O m–2 s–1 and transpiration rates were 2.0–2.5 mol H2O m–2 s–1 before treatment with smoke (Fig. 1).
In the 4-h (240-min) period immediately after treatment with smoke from combustion of Coast Live Oak leaf litter there were three general patterns of gas exchange: (1) lower rates of gas exchange were maintained for the entire period of measurement (e.g. Cabernet Franc, Chardonnay; see Fig. 1), (2) rates quickly returned to pre-smoke levels and thereafter remained constant (e.g. Pinot Noir; see Fig. 1, Syrah) or (3) rates of gas exchange gradually recovered to be close to pre-smoke values after 4 h (e.g. Cabernet Sauvignon, Durif). Despite this, three of the six grapevine varieties (Cabernet Franc, Cabernet Sauvignon and Pinot Noir) showed no significant difference in rates of gas exchange when pre- and post-smoke treatments were compared over the 4-h period of intensive measurement (two-way repeated-measures ANOVA; P > 0.05). The exceptions were for Durif, which had a significantly lower rate of photosynthesis after smoke exposure (F1,3 = 24.318, P < 0.001), Syrah, which had a significantly lower rate of stomatal conductance (F1,3 = 19.127, P = 0.022), and both Chardonnay (F1,3 = 194.104, P < 0.001) and Syrah, which had significantly lower rates of transpiration (F1,3 = 46.348, P = 0.006) after exposure to smoke.
Similar patterns were evident for rates of gas exchange of leaves from four grapevine varieties when treated with smoke produced from combustion of leaf litter from Tasmanian Bluegum (Fig. 2; note that only data for Cabernet Franc and Cabernet Sauvignon are shown). Three of the varieties (Cabernet Franc, Cabernet Sauvignon, Pinot Noir) showed no significant difference in rates of gas exchange within the 4-h period of intensive measurement after exposure to smoke (two-way repeated-measures ANOVA; P > 0.05). Syrah was the only variety to have a significantly lower transpiration rate after exposure to smoke (F1,3 = 13.353, P = 0.035).
Two of the grapevine varieties, Cabernet Franc and Pinot Noir, were treated a second time with smoke from combustion Coast Live Oak leaf litter, 4 weeks after initial exposure. Prior to the second smoke treatment, mean rates of photosynthesis (5 µmol CO2 m–2 s–1, Fig. 3), stomatal conductance (0.07 mol H2O m–2 s–1) and transpiration (1.3–2.0 mol H2O m–2 s–1) were generally low compared with the first smoke treatment, but were not significantly different (two-way repeated-measures ANOVA; P > 0.05). Similarly, there were no significant differences between rates of gas exchange of these two varieties before and after treatment with smoke a second time (two-way repeated-measures ANOVA; P > 0.05).
As an indicator of short-term recovery of leaves, rates of gas exchange were compared for all grapevine varieties before (pre-smoke exposure) and at 4, 24 and 48 h after exposure to smoke produced from both types of leaf litter (Tables 1, 2, 3). When compared with rates of photosynthesis before smoke treatment, some grapevine varieties had mean rates that were similar throughout the entire period of measurement (e.g. Cabernet Sauvignon) whereas other varieties had rates that were lower (e.g. Cabernet Franc) or higher (e.g. Chardonnay) at 24 and 48 h after exposure (Table 1). Regardless, there were no significant difference in photosynthesis among pre- or post-smoke exposure time (two-way repeated-measures ANOVA; F3,9 = 1.297, P = 0.334) with lowest rates measured 4 h after exposure to smoke from Coast Live Oak. Similarly, mean rates of photosynthesis were not significantly different among grapevine varieties (F5,15 = 2.246, P = 0.103), but there was a significant interaction of exposure time and variety (F15,45 = 3.907, P < 0.001). When Tasmanian Bluegum leaves were used as a fuel to produce smoke there were no significant difference among rates of photosynthesis for exposure time (F3,9 = 1.552, P = 0.267) or grapevine variety (F3,9 = 1.609, P = 0.255), nor with the interaction of these two variables (F9,27 = 0.718, P = 0.688). When plants from two grapevine varieties were exposed to smoke from Coast Live Oak a second time, there was a significant difference for rates of photosynthesis for exposure time (F3,9 = 4.847, P = 0.028), but not for variety (F1,3 = 0.136, P = 0.737).
Prior to exposure to smoke, stomatal conductance ranged from 0.12 to 0.21 mol H2O m–2 s–1 (Table 2) and transpiration ranged from 1.85 to 2.63 mol H2O m–2 s–1 depending on grapevine variety (Table 3). As with photosynthesis, the lowest mean rate of both measures of gas exchange was for 4 h after exposure to smoke but all plants had returned to pre-smoke rates after 48 h. For stomatal conductance, significant differences were found among exposure time when grapevines were subjected to smoke from both Coast Live Oak (F3,9 = 7.614, P = 0.008) and Tasmanian Bluegum (F3,9 = 15.955, P = 0.001), but only among grapevine varieties when exposed to smoke from Tasmanian Bluegum (F3,9 = 9.428, P = 0.004). Similar patterns were evident for rates of transpiration where significant differences were found among interval time when exposed to smoke from both Coast Live Oak (F3,9 = 6.196, P = 0.014) and Tasmanian Bluegum (F3,9 = 11.290, P = 0.002), and for grapevine variety when exposed to smoke from Coast Live Oak (F5,15 = 3.347, P = 0.031) but not when exposed to smoke from Tasmanian Bluegum leaf litter.
Glasshouse study
Apart from Pinot Noir, rates of photosynthesis and transpiration immediately after treatment with smoke (0 min) were similar to pre-smoke rates of photosynthesis (Fig. 4a, e; note that only data for Pinot Noir and Chardonnay are shown). After 48 h, all varieties had returned to pre-smoke levels of gas exchange, and in some cases (Verdelho, Muscat Gordo; data not shown) rates of gas exchange were higher than before exposure to smoke. These patterns however were only significant for rates of stomatal conductivity (two-way repeated-measures ANOVA; F5,20 = 14.894, P < 0.001). In contrast, there were consistent significant differences among grapevine varieties for rates of photosynthesis (F6,24 = 17.218, P < 0.001), stomatal conductivity (F6,24 = 29.048, P < 0.001), and transpiration (F6,24 = 17.118, P < 0.001). Pinot Noir and Chardonnay (Fig. 4) generally had lower rates of gas exchange (i.e. photosynthesis: 6–9 µmol CO2 m–2 s–1, stomatal conductivity: 0.08–0.15 mol H2O m–2 s–1 and transpiration: 1.8–2.5 mol H2O m–2 s–1) than the other four grapevine varieties (photosynthesis: 10–12 µmol CO2 m–2 s–1, stomatal conductivity: 0.10–0.20 mol H2O m–2 s–1 and transpiration: 2.0–3.0 mol H2O m–2 s–1).
Chlorophyll content of leaves
The chlorophyll content of leaves increased in some varieties and decreased in others throughout the study periods for the smoke chamber and glasshouse studies. During the smoke chamber study, the chlorophyll content of grapevine leaves varied significantly during the course of the experiment (two-way repeated-measures ANOVA; F3,12 = 12.779, P < 0.001) for all of the grapevine varieties (F5,20 = 11.415, P < 0.001), and there was a significant interaction between measurement time and grapevine variety (F15,60 = 7.785, P < 0.001; data not shown). During the glasshouse study, the chlorophyll content of leaves did not vary significantly between the start and end of the experimental period (F1,4 = 1.654, P = 0.268) for any of the grapevine varieties, but chlorophyll content did vary among varieties (F5,20 = 6.961, P = 0.001), and there was a significant interaction between the two variables (F5,20 = 5.903, P = 0.002; data not shown).
Discussion
In general, short-term exposure of leaves to smoke had little effect on photosynthesis, stomatal conductance or transpiration for an array of grapevine varieties. Most of the varieties tested quickly returned to pre-smoke levels and certainly all varieties had recovered within 48 h. Working with a range of deciduous angiosperm and evergreen coniferous species and 30-min periods of exposure, Calder et al. (2010) also found rates of photosynthesis of plants exposed to smoke to be comparable with control (unsmoked) plants after 48 h. In their study, deciduous plants recovered more slowly than coniferous plants but for all species tested no long-term differences in seedling growth or leaf chemistry were evident. Similarly, when Chrysanthemoides monilifera (Bitou Bush, Asteraceae) was exposed to smoke for 1 min, rates of photosynthesis and gas exchange recovered to levels measured for control plants within 24 h (Gilbert and Ripley 2002). It is obvious that physiological responses of leaves of grapevines to short-term smoke exposure are relatively short-lived and are similar to responses of other woody species, albeit only a small number of species have been tested.
There is little published information available that can be used for direct comparison with the current study exploring the leaf-level effects of exposure of grapevines to smoke. The lack of information is quite surprising as it has been reported that grapevines are one of the most economically important and widely grown fruit species (Vivier and Pretorius 2002), and interest in this species is increasing with the development of new technologies such as gene sequencing, mass spectrometry and proteomics (e.g. Hayasaka et al. 2005; Troggio et al. 2008; Marangon et al. 2009; Giribaldi and Giuffrida 2010). This research gap is also intriguing given the recent interest in smoke taint in wine, particularly in Australia (Kennison et al. 2007; Jiranek 2011). Much of the smoke-related work in Australia involving grapevines has concentrated on the chemistry of production processes and amelioration of smoke taint in wine (e.g. Fudge et al. 2011; Kennison et al. 2011; Ristic et al. 2011) whereas the physiological effects of smoke on the plants is yet to be considered. However, the body of knowledge that describes the variation within Vitis vinifera as a commercial production species can be used as an indication of the responses to help explain the variability shown in physiological response to smoke.
Photosynthesis is very sensitive to prolonged stress such as high temperature or drought, particularly during vegetative and reproductive stages of development in grapevines (Kadir 2006). Heat stress can reduce rates of net CO2 assimilation, stomatal conductance and transpiration and ultimately lead to a reduction in plant growth and fruit size, changes in berry development and colour and a reduction in acidity and sugar content of juice (Regina and Carbonneau 1999; Medrano et al. 2003; Kadir 2006; Greer and Weston 2010). In contrast, short pulses of heat stress can have minimal effect on rates of photosynthesis and transpiration and little effect on longer-term fruit development and quality (Soar et al. 2009). Grapevines subject to water stress were more severely affected by additional short-term temperature stress than plants undergoing short-term temperature stress alone but all plants had the capacity to recover quickly (Edwards et al. 2011). On the basis of such tolerance, it is not surprising that grapevines recovered rapidly after short-term exposure to smoke, particularly as the plants were not otherwise stressed (i.e. well watered and fertilised). It may also be reasonable to expect that there would be no long-term consequences for plant growth and yield after short-term exposure to smoke. Nonetheless, as bushfires mostly occur in hot dry summer months, the relatively benign physiological effects of short-term exposure to smoke demonstrated here may be exacerbated under seasonally stressful environmental conditions. The effect may be further influenced by higher temperatures and increased emissions of CO2 and other greenhouse gases as predicted under future climate change scenarios (Webb et al. 2007; Keller 2010). However, responses to smoke under future climatic scenarios are likely to be complex as grapevines have the ability to respond at the leaf-level by altering stomatal density when subjected to changed levels of atmospheric CO2 (Moutinho-Pereira et al. 2009; Rogiers et al. 2011).
Stomata are the main point of entry for gaseous contaminants, so regulation of the opening and closing of stomata will play a key role in determining plant sensitivity to smoke (Darrall 1989). Reduction in stomatal conductance is one of the first responses associated with drought or water stress in grapevine plants as it relates to conservation of leaf water status and regulation of internal CO2 supply (see reviews by Chaves et al. 2010; Lovisolo et al. 2010). Leaf stomata responses of grapevines to other forms of stress such as temperature vary widely from minimal or limited response to being strongly correlated with temperature according to cultivar or genotype, timing and intensity of heat stress and light and water availability (e.g. Sepúlveda and Kliewer 1986; Ferrini et al. 1995; Palliotti et al. 2009; Edwards et al. 2011; Luo et al. 2011). As the plants in this study were not stressed in relation to water availability, temperature or nutrient supply, any short-term reduction in gas exchange was most probably due to some chemical component in smoke. There were some changes in chlorophyll content of leaves during the 8-week course of the smoke chamber study, but not the glasshouse study, so we cannot discount that seasonal changes and variety may have had some role in response to smoke exposure.
Many of the components of smoke are also atmospheric pollutants and countless studies have shown that air pollution can impinge on plant physiology (see general reviews by Krupa and Manning 1988; Darrall 1989; Robinson et al. 1998; and Weinstein 1984 for a specific review of the effect of pollution on grapevines). For example, there are many VOCs present in smoke from smouldering wood (McKenzie et al. 1995), and burning vegetation (Andreae and Merlet 2001) and this group of compounds is known to influence stomatal conductance and other leaf physiological processes (Cape 2003). Dust, which has a size and physical structure similar to particulate matter produced during bushfires (i.e. diameter range from 0.5 to 43 µm, Ward and Hardy 1991), has been shown to reduce stomatal conductance, decrease photosynthesis and increase leaf temperatures (Hirano et al. 1995; Grantz et al. 2003). Net photosynthesis may be reduced during short-term exposure to SO2 (2–24-h exposure) with relatively rapid recovery ranging from immediately after exposure to several days after removal of the pollutant (Darrall 1989). Ozone is particularly toxic to plants (Krupa and Manning 1988; Black et al. 2000), even at levels near ambient (Bergmann et al. 1995), but is a secondary gaseous product and found in greater concentrations as the smoke plume ages. Even CO2 can disrupt normal cellular functioning in high concentrations (Robinson et al. 1998). In contrast, when the gases NOx and NH3 are deposited onto internal leaf surfaces via stomata, they are rapidly dissolved in the apoplast to form NO2–, NO3– and NH4+ and can be utilised by plants through normal enzymatic pathways (Fangmeier et al. 1994; Stulen et al. 1998). Smoke from forest fires in Indonesia in 1997 caused increases in atmospheric levels of CO2, CO, SO2 and CH4 and, although concomitant reductions in photosynthesis and stomatal conductance of saplings trees were largely attributed to reduction of photosynthetically active radiation, atmospheric pollutants are likely to have had a role (Davies and Unam 1999).
Given the potential for the chemical components of smoke to influence rates of leaf functioning, the type of fuel used to produce smoke is likely to have a role in determining physiological response of leaves. In this study, grapevines showed greater sensitivity to smoke from burning leaf litter from Coast Live Oak compared with Tasmanian Bluegum. It is difficult to extrapolate from other smoke exposure studies as only single fuel types were used ranging from a common grass (Gilbert and Ripley 2002) to a mixture of leaves representing the experimental species (Calder et al. 2010). Similarly, research investigating smoke taint in wine has only used fruit exposed to smoke produced from combustion of dry barley straw (e.g. Kennison et al. 2011 and references therein; Ristic et al. 2011; Wilkinson et al. 2011) or fruit collected after being opportunistically exposed to smoke from summer bushfires (e.g. Fudge et al. 2011; Singh et al. 2011) or planned burning (Hayasaka et al. 2010). The amount of fuel burnt will also determine the concentration of the gaseous and particulate components of smoke. An obvious weakness of both the smoke chamber and glasshouse studies was a lack of control of the density of smoke, particularly in the glasshouse setup. All attempts were made towards consistency during smoke production by drying, homogenising and weighing fuel. Further experimentation involving more elaborate chamber design with analytical capacity such as mass spectrometry (see Maleknia et al. 2007, 2009) would be required to describe and quantify the smoke used. This challenge is being addressed in our current studies and results from this research agenda will be more comparable to the effects of smoke from planned fires and small-scale bushfires.
The length of time of smoke exposure may be critical for plant functioning. In our experiments 15 min of exposure to smoke produced no visible leaf injury. Rates of photosynthesis, stomatal conductance and transpiration of Chrysanthemoides monilifera returned to control (unsmoked) levels when plants were exposed to smoke for 1 min but exposure for 5 min caused leaf necrosis and shoot death (Gilbert and Ripley 2002). Smoke from bushfires may be present for hours to days depending on the type and extent of the fire, fuel loads, fire behaviour, topography and prevailing winds. As an illustration of the potential time and extent of exposure, the smoke generated during the 59 days that the Alpine bushfires in Victoria in 2003 burnt was trapped locally in the valley system in the area but also blanketed the greater Melbourne metropolitan area prompting smog alerts and health warnings (Wareing and Flinn 2003). More realistically, smoke from smaller bushfires and planned fires generally only persists for the duration of the fire apart from localised smouldering of fallen wood and tree stumps.
In this study, white varieties of grapevine (Chardonnay and Verdelho) were generally more sensitive to smoke than red varieties (Pinot Noir, Shiraz, Cabernet Sauvignon), but this largely depended on the source of the smoke. Comparison and classification of varieties of grapevines according to some feature or behaviour is common and can be of practical use. For example, several grapevine varieties have been classified as being isohydric (e.g. Grenache) or anisohydric (e.g. Syrah) according to their ability to maintain or vary leaf water potential (Schultz 2003). Water use and gas exchange of three contrasting grapevine varieties measured by Moutinho-Pereira et al. (2007) also indicated that there are morphological, biochemical and physiological differences that confer tolerance to abiotic stress such as drought and high light intensity. Differences in tolerance of grapevine rootstocks to water limitation have been demonstrated and the significance of such information for management purposes was highlighted (Koundouras et al. 2008). Other research indicates that certain wine grapevine varieties can cope better with drought (e.g. Chardonnay > Semillon, Regina and Carbonneau 1999), disease (e.g. Cabernet Sauvignon > Merlot, Christen et al. 2007) and heat (e.g. Cabernet Sauvignon > Chardonnay > Pinot Noir, Kadir 2006) or to respond to irrigation (e.g. Tempranillo > Manto Negro, Medrano et al. 2003), but such trends are difficult to extrapolate for tolerance of smoke. However, the response of herbaceous species with mesophytic leaves to smoke compared with native Australian species with sclerophyllous leaves has indicated greater sensitivity of the former (V. Aerts, unpubl. data). Understanding the susceptibility of different varieties of grapevine and other agricultural or horticultural crops to smoke damage will help land managers work together with local producers to determine optimal timing and extent of planned fire events to reduce the effect of smoke.
We can conclude that smoke from burning vegetation has little effect on the short-term leaf physiology of grapevines. This is irrespective of fuel type and grapevine variety. However, in this study only a single species of plant was investigated and only two fuel types were used. The combustion products from vegetation fires are highly complex and vary considerably according to fuel type, weather conditions and fire behaviour so there is great potential for other agricultural and non-agricultural species to be affected differently by smoke. Length of exposure time, concentration of smoke components, plant phenology and the interplay of other plant stresses such as drought, nutrient limitation, competition and high temperature may also play an important role in plant recovery after exposure to smoke and further studies should take these variables into consideration.
Acknowledgements
Funding for this research was from a Professional Scholar award from the Australian-American Fulbright Commission. Additional support was provided by the Bushfire Cooperative Research Centre, the University of Melbourne and the University of Sydney. Thanks are owed to Tom Nemcik for advice and supplying grapevine plants in California and to Amanda Ashton, Jennifer Brooks and Barbara Rotz for their help with maintaining plants and taking measurements. Thanks to Malcolm Possell for comments on an earlier draft of the manuscript.
References
Andreae MO, Merlet P (2001) Emissions of trace gases and aerosols from biomass burning. Global Biogeochemical Cycles 15, 955–966.| Emissions of trace gases and aerosols from biomass burning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtV2iuw%3D%3D&md5=97bbef209e6448c582d57158ccda567eCAS |
Beer T, Williams AA (1995) Estimating Australian forest fire danger under conditions of doubled carbon dioxide concentrations. Climatic Change 29, 169–188.
| Estimating Australian forest fire danger under conditions of doubled carbon dioxide concentrations.Crossref | GoogleScholarGoogle Scholar |
Bell TL, Adams MA (2009) Smoke from wildfires and prescribed burning in Australia: effects on human health and ecosystems. In ‘Developments in Environmental Science. Vol. 8: Wildland fires and air pollution’. (Eds A Bytnerowicz, MJ Arbaugh, AR Riebau, C Andersen) pp. 289–316. (Elsevier: Amsterdam, the Netherlands)
Ben-Asher J, Tsuyuki I, Bravdo B-A, Sagih M (2006) Irrigation of grapevines with saline water. I. Leaf area index, stomatal conductance, transpiration and photosynthesis. Agricultural Water Management 83, 13–21.
| Irrigation of grapevines with saline water. I. Leaf area index, stomatal conductance, transpiration and photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Bergmann E, Bender J, Weigel HJ (1995) Growth responses and foliar sensitivities of native herbaceous species to ozone exposures. Water, Air, and Soil Pollution 85, 1437–1442.
| Growth responses and foliar sensitivities of native herbaceous species to ozone exposures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xit1ejtLs%3D&md5=dd058b1a6207054c6f8a6b1c8fa14db2CAS |
Black VJ, Black CR, Roberts JA, Stewart CA (2000) Impact of ozone on the reproductive development of plants. Tansley Review No. 115 New Phytologist 147, 421–447.
| Impact of ozone on the reproductive development of plants. Tansley Review No. 115Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnsVKqs70%3D&md5=05086196eda6259bc3bef91adaef8009CAS |
Boland DJ, Brooke MIH, Chippendale GM, Hall N, Hyland BPM, Johnston RD, Kleinig DA, McDonald MW, Turner JD (2006) ‘Forest Trees of Australia’. (CSIRO Publishing: Melbourne)
Bramley RGV (2005) Understanding variability in winegrape production systems. 2. Within vineyard variation in quality over several vintages. Australian Journal of Grape and Wine Research 11, 33–42.
| Understanding variability in winegrape production systems. 2. Within vineyard variation in quality over several vintages.Crossref | GoogleScholarGoogle Scholar |
Bramley RGV, Hamilton RP (2004) Understanding variability in winegrape production systems. 1. Within vineyard variation in yield over several vintages. Australian Journal of Grape and Wine Research 10, 32–45.
| Understanding variability in winegrape production systems. 1. Within vineyard variation in yield over several vintages.Crossref | GoogleScholarGoogle Scholar |
Calder WJ, Lifferth G, Moritz MA, St Clair SB (2010) Physiological effects of smoke exposure on deciduous and conifer tree species. International Journal of Forestry Research 2010, 438930 https://doi.org/10.1155/2010/438930
CalFlora (2012) Information of California plants for education, research and conservation. (The Calflora Database: Berkeley, CA) Available at http://www.calflora.org [Verified 14 May 2013]
Cape JN (2003) Effects of airborne volatile organic compounds on plants. Environmental Pollution 122, 145–157.
| Effects of airborne volatile organic compounds on plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtFOrsQ%3D%3D&md5=de73f466bcfa8eefe73c55dc057dd2c9CAS | 12535603PubMed |
Chaves MM, Zarrouk O, Francisco R, Costa JM, Santos T, Regalado AP, Rodrigues ML, Lopes CM (2010) Grapevine under deficit irrigation: hints from physiological and molecular data. Annals of Botany 105, 661–676.
| Grapevine under deficit irrigation: hints from physiological and molecular data.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3ovFGnsg%3D%3D&md5=c8d1a757d554904a767b521c9bb7837fCAS | 20299345PubMed |
Christen D, Schönmann S, Jermini M, Strasser RJ, Défago G (2007) Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environmental and Experimental Botany 60, 504–514.
| Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntFyhtbY%3D&md5=59636ebfd27e2350c7cddb19361bb38dCAS |
Crutzen PJ, Andreae MO (1990) Biomass burning in the tropics: impacts on atmospheric chemistry and biogeochemical cycles. Science 250, 1669–1678.
| Biomass burning in the tropics: impacts on atmospheric chemistry and biogeochemical cycles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmsF2htQ%3D%3D&md5=c08a9472273ac1fc82ecd35989835895CAS | 17734705PubMed |
Darrall NM (1989) The effect of air pollutants on physiological processes in plants. Plant, Cell & Environment 12, 1–30.
| The effect of air pollutants on physiological processes in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXitVOht7g%3D&md5=ba5241df14d1092baa375fc03bbc33d8CAS |
Davies SJ, Unam L (1999) Smoke-haze from the 1997 Indonesian forest fires: effects on pollution levels, local climate, atmospheric CO2 concentrations, and tree photosynthesis. Forest Ecology and Management 124, 137–144.
| Smoke-haze from the 1997 Indonesian forest fires: effects on pollution levels, local climate, atmospheric CO2 concentrations, and tree photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Dixon KW, Merritt DJ, Flematti GR, Ghisalberti EL (2009) Karrikinolide – a phytoreactive compound derived from smoke with application in horticulture, ecological restoration and agriculture. Acta Horticulturae 813, 155–170.
Edwards EJ, Smithson L, Graham DC, Clingeleffer PR (2011) Grapevine canopy response to a high-temperature event during deficit irrigation. Australian Journal of Grape and Wine Research 17, 153–161.
| Grapevine canopy response to a high-temperature event during deficit irrigation.Crossref | GoogleScholarGoogle Scholar |
Ellis S, Kanowski P, Whelan R (2004) National inquiry on bushfire mitigation and management. (Commonwealth of Australia: Canberra) Available at http://www.coagbushfireenquiry.gov.au/findings.htm [Verified 15 May 2013]
Fangmeier A, Hadwiger-Fangmeier A, Van der Eerden L, Jäger H-J (1994) Effects of atmospheric ammonia on vegetation – a review. Environmental Pollution 86, 43–82.
| Effects of atmospheric ammonia on vegetation – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXkslGnsrc%3D&md5=74f2d719c2ca1c149da534d8e68e3751CAS | 15091648PubMed |
Ferrini F, Mattii GB, Nicese FP (1995) Effects of temperature on key physiological responses of grapevine leaf. American Journal of Enology and Viticulture 46, 375–379.
Fudge AL, Ristic R, Wollan D, Wilkinson KL (2011) Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Australian Journal of Grape and Wine Research 17, S41–S48.
| Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGnuro%3D&md5=ead73af55be487f4caa6c04c2ba22feaCAS |
Gilbert ME, Ripley BS (2002) The effect of smoke on the photosynthetic gas exchange of Chrysanthemoides monilifera. South African Journal of Botany 68, 525–531.
Giribaldi M, Giuffrida G (2010) Heard it through the grapevine: proteomic perspective on grape and wine. Journal of Proteomics 73, 1647–1655.
| Heard it through the grapevine: proteomic perspective on grape and wine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovFygt7c%3D&md5=884824266b228af07b4f6174ab94a559CAS | 20580953PubMed |
Grantz DA, Garner JHB, Johnson DW (2003) Ecological effects of particulate matter. Environment International 29, 213–239.
| Ecological effects of particulate matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXis1arur0%3D&md5=8752d4870f731968e9517fbab9859e71CAS | 12676209PubMed |
Greer DH, Weston C (2010) Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in controlled environment. Functional Plant Biology 37, 206–214.
| Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in controlled environment.Crossref | GoogleScholarGoogle Scholar |
Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P (1995) A global model of natural volatile organic compound emissions. Journal of Geophysical Research Atmosphere 100, 8873–8892.
| A global model of natural volatile organic compound emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmvFKrsb0%3D&md5=977e19d5b04bfca5c596c7888610c4c5CAS |
Hayasaka Y, Baldock GE, Pollnitz AP (2005) Contributions of mass spectrometry in the Australian Wine Research Institute to advances in knowledge of grape and wine constituents. Australian Journal of Grape and Wine Research 11, 188–204.
| Contributions of mass spectrometry in the Australian Wine Research Institute to advances in knowledge of grape and wine constituents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsVarurw%3D&md5=ce71d7e53ec379bcc7db17859d17d765CAS |
Hayasaka Y, Baldock GA, Parker M, Pardon KH, Black CA, Herderich MJ, Jeffery DW (2010) Glycosylation of smoke-derived volatiles in grapes as a consequence of grapevine exposure to bushfire smoke. Journal of Agricultural and Food Chemistry 58, 10 989–10 998.
Hirano T, Kiyota M, Aiga I (1995) Physical effects of dust on leaf physiology of cucumber and kidney bean plants. Environmental Pollution 89, 255–261.
| Physical effects of dust on leaf physiology of cucumber and kidney bean plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXms1emt78%3D&md5=b8336326a6b5cfbe399556dd163f2c74CAS | 15091515PubMed |
Jackson DI, Lombard PB (1993) Environmental and management practices affecting grape composition and wine quality – a review. American Society for Enology and Viticulture 44, 409–430.
Jain N, Van Staden J (2006) A smoke-derived butenolide improves early growth of tomato seedlings. Plant Growth Regulation 50, 139–148.
| A smoke-derived butenolide improves early growth of tomato seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Gnu7zL&md5=29664476f68c7041a7c7529c23cf0772CAS |
Jiranek V (2011) Smoke taint compounds in wine: nature, origin, measurement and amelioration of affected wines. Australian Journal of Grape and Wine Research 17, S2–S4.
| Smoke taint compounds in wine: nature, origin, measurement and amelioration of affected wines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGntbs%3D&md5=d76c9d1e06181d6db12b3c22a7738541CAS |
Kadir S (2006) Thermostability of photosynthesis of Vitis aestivalis and V. vinifera. Journal of the American Society for Horticultural Science 131, 476–483.
Keller M (2005) Deficit irrigation and vine mineral nutrition. American Journal of Enology and Viticulture 56, 267–283.
Keller M (2010) Managing grapevines to optimise fruit development in a challenging environment: a climate change primer for viticulturists. Australian Journal of Grape and Wine Research 16, 56–69.
| Managing grapevines to optimise fruit development in a challenging environment: a climate change primer for viticulturists.Crossref | GoogleScholarGoogle Scholar |
Kennison KR, Wilkinson KL, Williams HG, Smith JH, Gibberd MR (2007) Smoke-derived taint in wine: effects of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. Journal of Agricultural and Food Chemistry 55, 10 897–10 901.
| Smoke-derived taint in wine: effects of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlynu7rE&md5=86507e4b8bfcdc2cb9d4508d3a2e2f7fCAS |
Kennison KR, Wilkinson KL, Pollnitz AP, Williams HG, Gibberd MR (2011) Effect of smoke application to field-grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties. Australian Journal of Grape and Wine Research 17, S5–S12.
| Effect of smoke application to field-grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGntbg%3D&md5=1df0a6fefa9bcfb435e5ef374babff59CAS |
Koundouras S, Tsialtas IT, Zioziou E, Nikolaou N (2008) Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. cabernet-sauvignon) under contrasting water status: leaf physiological and structural responses. Agriculture, Ecosystems & Environment 128, 86–96.
| Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. cabernet-sauvignon) under contrasting water status: leaf physiological and structural responses.Crossref | GoogleScholarGoogle Scholar |
Krupa SV, Manning WJ (1988) Atmospheric ozone: formation and effects on vegetation. Environmental Pollution 50, 101–137.
| Atmospheric ozone: formation and effects on vegetation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXhvVWms7Y%3D&md5=ef8e685c4687fc626c5ff9b717ef972dCAS | 15092655PubMed |
Kulkarni MG, Ascough GD, Verschaeve L, Baeten K, Arruda MP, van Staden L (2010) Effect of smoke-water and a smoke-isolated butenolide on the growth and genotoxicity of commercial onion. Scientia Horticulturae 124, 434–439.
| Effect of smoke-water and a smoke-isolated butenolide on the growth and genotoxicity of commercial onion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksFKjsrs%3D&md5=d6e1070cc91367b03325f525794a0376CAS |
Light ME, Daws MI, Van Staden J (2009) Smoke-derived butenolide: towards understanding its biological effects. South African Journal of Botany 75, 1–7.
| Smoke-derived butenolide: towards understanding its biological effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivVGqtr4%3D&md5=20c58919ea2aaf4407f0a28a336b38b9CAS |
Lovisolo C, Perrone I, Carra A, Ferrandino A, Flexas J, Medrano H, Schubert A (2010) Drought-induced changes in development and function of grapevine (Vitis spp.) organs and their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Functional Plant Biology 37, 98–116.
| Drought-induced changes in development and function of grapevine (Vitis spp.) organs and their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlyhsrs%3D&md5=87fde9864c23ad812d346a8864d79708CAS |
Luo H-B, Ma L, Xi H-F, Duan W, Li S-H, Loescher W, Wang J-F, Wang L-J (2011) Photosynthetic responses to heat treatments at different temperatures and following recovery in grapevine (Vitis amurensis L.) leaves. PLoS ONE 6, e23033
| Photosynthetic responses to heat treatments at different temperatures and following recovery in grapevine (Vitis amurensis L.) leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Sqt73N&md5=91824b97766e47355be1a15dc6d3341cCAS | 21887227PubMed |
Maleknia SD, Bell TL, Adams MA (2007) PTR-MS analysis of reference and plant-emitted volatile organic compounds. International Journal of Mass Spectrometry 262, 203–210.
| PTR-MS analysis of reference and plant-emitted volatile organic compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsVWms7w%3D&md5=c4b23672a1e409cabcdc853c7336f3d3CAS |
Maleknia SD, Bell TL, Adams MA (2009) Eucalypt smoke and wildfires: temperature dependent emissions of biogenic volatile organic compounds. International Journal of Mass Spectrometry 279, 126–133.
| Eucalypt smoke and wildfires: temperature dependent emissions of biogenic volatile organic compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVCnug%3D%3D&md5=2f32dc916ce6b4995db7aae84922a617CAS |
Marangon M, Van Sluyter SC, Haynes PA, Waters EJ (2009) Grape and wine proteins: their fractionation by hydrophobic interaction chromatography an identification by chromatographic and proteomic analysis. Journal of Agricultural and Food Chemistry 57, 4415–4425.
| Grape and wine proteins: their fractionation by hydrophobic interaction chromatography an identification by chromatographic and proteomic analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktF2gs7g%3D&md5=b34c354125f9db501b1f72bf7b25944fCAS | 19354294PubMed |
McKenzie LM, Hao WM, Richards GN, Ward DE (1995) Measurement and modelling of air toxins from smouldering combustion of biomass. Environmental Science & Technology 29, 2047–2054.
| Measurement and modelling of air toxins from smouldering combustion of biomass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmsFCkurs%3D&md5=49158ef0e2673fbcdf744238ef7aac31CAS |
Medrano H, Escalona JM, Cifre J, Bota J, Flexas J (2003) A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: effects of water availability from leaf photosynthesis to grape yield and quality. Functional Plant Biology 30, 607–619.
| A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: effects of water availability from leaf photosynthesis to grape yield and quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmvFSkt70%3D&md5=7a4ba0340a37eeb351234968951efab0CAS |
Moutinho-Pereira J, Magalhães N, Gonçalves B, Bacelar E, Brito M, Correia C (2007) Gas exchange and water relations of three Vitis vinifera L. cultivars growing under Mediterranean climate. Photosynthetica 45, 202–207.
| Gas exchange and water relations of three Vitis vinifera L. cultivars growing under Mediterranean climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktlSmt74%3D&md5=3dba20767b74901f0c77465b61c37414CAS |
Moutinho-Pereira JM, Gonçalves B, Bacelar E, Boaventura Cunha J, Coutinho J, Correia CM (2009) Effects of elevate CO2 on grapevine (Vitis vinifera L.): physiological and yield attributes. Vitis 48, 159–165.
Palliotti A, Silvestroni O, Petoumenou D (2009) Photosynthetic and photoinhibition behavor of two field-grown cultivars under multiple summer stresses. American Journal of Enology and Viticulture 60, 189–198.
Pitman AJ, Narisma GT, McAneney J (2007) The impact of climate change on the risk of forest and grassland fires in Australia. Climatic Change 84, 383–401.
| The impact of climate change on the risk of forest and grassland fires in Australia.Crossref | GoogleScholarGoogle Scholar |
Regina MD, Carbonneau A (1999) Gas exchanges in grapevines under water stress regime. II. Photorespiration and varietal behaviour. Pesquisa Agropecuaria Brasileira 34, 37–43.
MKF Research (2006) The impact of wine, grapes and grape products on the American economy. MKF Research Report. (St Helena, CA, USA) Available at www.cawg.org/images/stories/pdf/impactonamericaneconomyfinal.pdf [Verified 15 May 2013]
Ristic R, Osidacz P, Pinchbeck KA, Hayasaka Y, Fudge AL, Wilkinson KL (2011) The effect of winemaking techniques on the intensity of smoke taint in wine. Australian Journal of Grape and Wine Research 17, S29–S40.
| The effect of winemaking techniques on the intensity of smoke taint in wine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGnurw%3D&md5=8b8ca2a236ace396235209659fbb4cdaCAS |
Robinson MF, Heath J, Mansfield TA (1998) Disturbance in stomatal behaviour caused by air pollutants. Journal of Experimental Botany 49, 461–469.
Rogiers SY, Hardie WJ, Smith JP (2011) Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Australian Journal of Grape and Wine Research 17, 147–152.
| Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
Santesteban LG, Miranda C, Royo JB (2009) Effect of water deficit and rewatering on leaf gas exchange and transpiration decline of excised leaves of four grapevine (Vitis vinifera L.) cultivars. Scientia Horticulturae 121, 434–439.
| Effect of water deficit and rewatering on leaf gas exchange and transpiration decline of excised leaves of four grapevine (Vitis vinifera L.) cultivars.Crossref | GoogleScholarGoogle Scholar |
Schultz HR (2000) Climate change and viticulture: a European perspective on climatology, carbon dioxide and UV-B effects. Australian Journal of Grape and Wine Research 6, 2–12.
| Climate change and viticulture: a European perspective on climatology, carbon dioxide and UV-B effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjt1Sls70%3D&md5=b2c8e06ca7e6563e50780833a7f0b70bCAS |
Schultz HR (2003) Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant, Cell & Environment 26, 1393–1405.
| Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought.Crossref | GoogleScholarGoogle Scholar |
Sepúlveda G, Kliewer WM (1986) Stomatal response of three grapevine cultivars (Vitis vinifera L.) to high temperature. American Journal of Enology and Viticulture 37, 44–52.
Singh DP, Ching HH, Pitt KM, Cleary M, Dokoozlian NK, Downey MO (2011) Guaiacol and 4-methylguaiacol accumulate in wines made from smoke-affected fruit because of hydrolysis of their conjugates. Australian Journal of Grape and Wine Research 17, S13–S21.
| Guaiacol and 4-methylguaiacol accumulate in wines made from smoke-affected fruit because of hydrolysis of their conjugates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGntbY%3D&md5=6bd714090d157ae9479640e84ebe9517CAS |
Soar CJ, Spiers J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR (2006) Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Australian Journal of Grape and Wine Research 12, 2–12.
| Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksFSlsLc%3D&md5=fc138d386d82cc1445b746cfe8698626CAS |
Soar CJ, Collins MJ, Sadras VO (2009) Irrigated Shiraz vines (Vitis vinifera) upregulate gas exchange and maintain berry growth in response to short spells of high maximum temperature in the field. Australian Journal of Grape and Wine Research 8, 86–94.
Sparg SG, Kulkarni MG, Light ME, Van Staden J (2005) Imporving seedling vigour of indigenous medicinal plants with smoke. Bioresource Technology 96, 1323–1330.
| Imporving seedling vigour of indigenous medicinal plants with smoke.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisFyjsrk%3D&md5=31a8a4fa0de2bb66a6c60295f4c321c2CAS | 15792578PubMed |
Stephens SL, Martin RE, Clinton NE (2007) Prehistoric fire area and emissions from California’s forests, woodlands, shrublands, and grasslands. Forest Ecology and Management 251, 205–216.
| Prehistoric fire area and emissions from California’s forests, woodlands, shrublands, and grasslands.Crossref | GoogleScholarGoogle Scholar |
Stevens RM, Harvey G, Partington DL, Coombe BG (1999) Irrigation of grapevines with saline water at different growth stages. I. Effects on soil salinity and sodicity, vegetative growth and yield. Australian Journal of Agricultural Research 50, 343–355.
| Irrigation of grapevines with saline water at different growth stages. I. Effects on soil salinity and sodicity, vegetative growth and yield.Crossref | GoogleScholarGoogle Scholar |
Stevens RM, Pech JM, Gibberd MR, Walker RR, Jones JA, Taylor J, Nicholas PR (2008) Effect of reduced irrigation on growth, yield, ripening rates and water relations of Chardonnay vines grafted to five rootstocks. Australian Journal of Grape and Wine Research 14, 177–190.
Stulen I, Perez-Soba M, De Kok LJ, Van der Eerden L (1998) Impact of gaseous nitrogen deposition on plant functioning. New Phytologist 139, 61–70.
| Impact of gaseous nitrogen deposition on plant functioning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkt1egu7g%3D&md5=ebc3ce7730a4e8d93a9d12c7459826d9CAS |
Taylor JLS, Van Staden J (1998) Plant-derived smoke solutions stimulate the growth of Lycopersicon esculentum roots in vitro. Plant Growth Regulation 26, 77–83.
| Plant-derived smoke solutions stimulate the growth of Lycopersicon esculentum roots in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXisFGg&md5=7d87b9a1655e25150e7523658b5e7639CAS |
Troggio M, Vezzulli S, Pindo M, Malacarne G, Fontana P, Moreira FM, Costantini L, Grando MS, Viola R, Velasco R (2008) Beyond the genome, opportunities for a modern viticulture: a research overview. American Journal of Enology and Viticulture 59, 117–127.
Vivier MA, Pretorius IS (2002) Genetically tailored grapevines for the wine industry. Trends in Biotechnology 20, 472–478.
| Genetically tailored grapevines for the wine industry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotFyqur8%3D&md5=dc5da419fd890050ef846f1f4c707ea3CAS | 12413822PubMed |
Ward DE (1999) Smoke from wildland fires. In ‘Health Guidelines for Vegetation Fire Events: Background Papers’. (Eds KT Goh, D Schwela, JG Goldammer, O Simpson) pp. 70–85. (World Health Organization: Lima)
Ward DE, Hardy CC (1991) Smoke emissions from wildland fires. Environment International 17, 117–134.
| Smoke emissions from wildland fires.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXktlCltr4%3D&md5=ae87822b6df337a58f7d9878f173fd3eCAS |
Wareing K, Flinn D (2003) ‘The Victorian alpine fires, January–March 2003. Fire Management, Victorian Department of Sustainability and Environment. (Melbourne) Available at http://www.dse.vic.gov.au/fire-and-other-emergencies/the-victorian-alpine-fires-2003 [Verified 18 May 2013]
Webb LB, Whetton PH, Barlow EWR (2007) Modelled impact of future climate change on the phenology of winegrapes in Australia. Australian Journal of Grape and Wine Research 13, 165–175.
| Modelled impact of future climate change on the phenology of winegrapes in Australia.Crossref | GoogleScholarGoogle Scholar |
Weinstein LH (1984) Effects of air pollution on grapevines. Vitis 23, 274–303.
Whiting J, Krstic M (2007) Understanding the sensitivity to timing and management options to mitigation the negative impacts of bush fire smoke on grape and wine quality – scoping study. Victorian Department of Primary Industries. (Melbourne)
Wilkinson KL, Ristic R, Pinchbeck KA, Fudge AL, Singh DP, Pitt KM, Downey MO, Baldock GA, Hayasaka Y, Parker M, Herderich MJ (2011) Comparison of methods for the analysis of smoke related phenols and their conjugates in grapes and wine. Australian Journal of Grape and Wine Research 17, S22–S28.
| Comparison of methods for the analysis of smoke related phenols and their conjugates in grapes and wine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGnur4%3D&md5=cbe0ba25bf053d9dc1435cfccecc326aCAS |
Williams AA, Karoly DJ, Tapper N (2001) The sensitivity of Australian fire danger to climate change. Climatic Change 49, 171–191.
| The sensitivity of Australian fire danger to climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktVOht7c%3D&md5=0013432585efd6ccc9a567d197fd6bc2CAS |
Winters AJ, Adams MA, Bleby TM, Rennenberg H, Steigner D, Steinbrecher R, Kreuzwieser J (2009) Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia. Atmospheric Environment 43, 3035–3043.
| Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXls1emurw%3D&md5=14b78420e58b6347f87660331f1e689cCAS |