Primary and secondary resource pulses in an alpine ecosystem: snow tussock grass (Chionochloa spp.) flowering and house mouse (Mus musculus) populations in New Zealand
Deborah J. Wilson A B and William G. Lee AA Landcare Research, Private Bag 1930, Dunedin 9054, New Zealand.
B Corresponding author. Email: wilsond@landcareresearch.co.nz
Wildlife Research 37(2) 89-103 https://doi.org/10.1071/WR09118
Submitted: 9 September 2009 Accepted: 13 January 2010 Published: 16 April 2010
Abstract
Context. Rodent populations in many parts of the world fluctuate in response to resource pulses generated by periodic high seed production (masting) by forest trees, with cascading effects on predation risk to other forest species. In New Zealand forests, populations of exotic house mice (Mus musculus) irrupt after periodic heavy beech (Nothofagus spp.) seedfall. However, in alpine grasslands, where snow tussock grasses (Chionochloa spp.) also flower and set seeds periodically, little is known about house mouse population dynamics.
Aims. Our primary objective was to test for an increase in alpine mouse density following a summer when snow tussocks flowered profusely. We also estimated mouse density in adjacent montane forest over 2 years, and assessed mouse diet, to predict their potential impacts on native species.
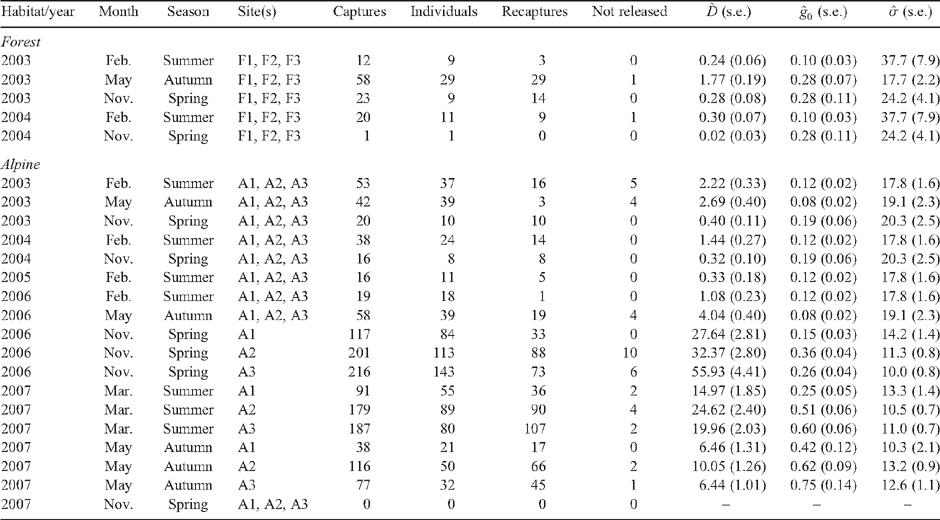
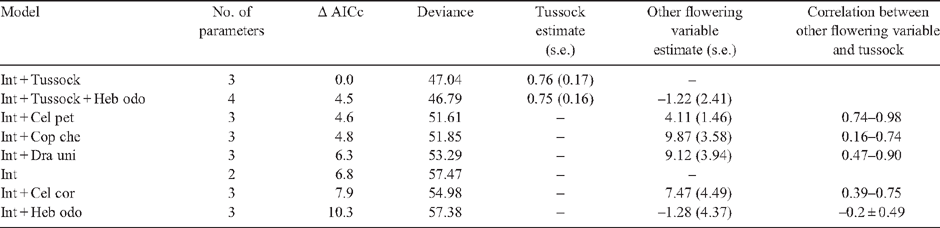
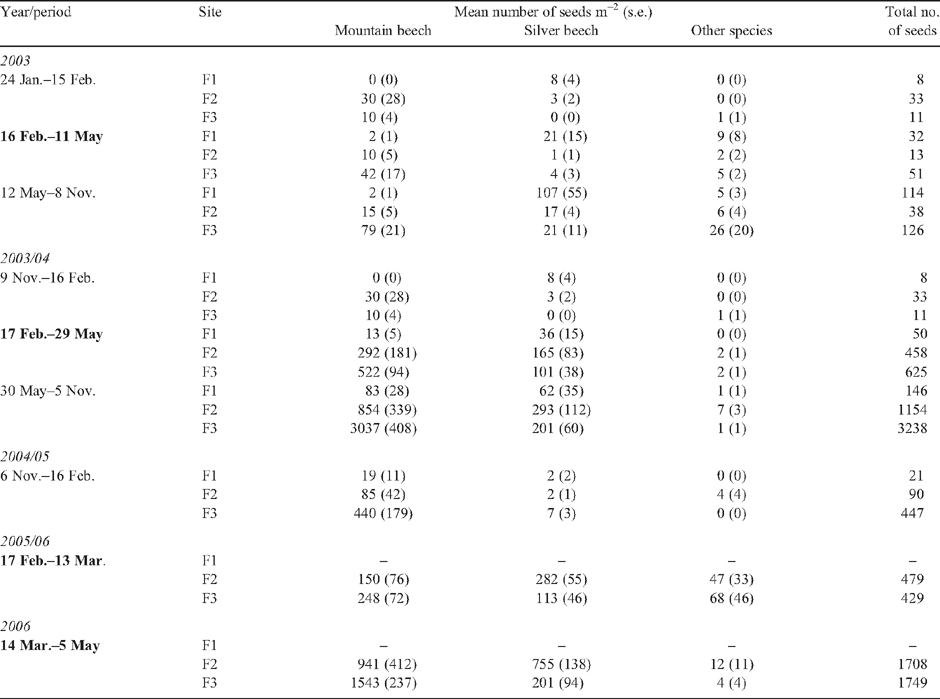
Methods. Flowering intensity of Chionochloa was assessed by counting flowering tillers on permanent transects (2003–06). Mouse density was estimated with capture–mark–recapture trapping in alpine (2003–07) and forest (2003–04) habitats. Mice were also collected and their stomach contents analysed. Flowering or fruiting of alpine shrubs and herbs, and beech seedfall at forest sites, were also measured.
Key results. Chionochloa flowered profusely in austral summer 2005/06. Between autumn (May) and spring (November) 2006, mean alpine mouse density increased from 4 ha–1 to 39 ha–1, then declined to 8 ha–1 by autumn (May 2007). No mice were captured in 768 trap-nights during the following spring (November 2007). Prior to the mouse irruption, mouse density was consistently higher at alpine (0.4–4.0 mice ha–1) than at montane forest (0.02–1.8 mice ha–1) sites (in 2003–04). Alpine mouse diet was dominated by arthropods before mast flowering, and by seeds during it.
Conclusions. The density and dynamics of alpine mice in relation to intensive snow-tussock flowering were similar to those in New Zealand beech forest in relation to beech masts.
Implications. We predict the timing and duration of periods of heightened predation risk to native alpine fauna, as the result of pulses in mouse density and likely associated pulses in the density of stoats (Mustela erminea), a key exotic predator.
Acknowledgements
We thank Gary McElrea, Lisa McElrea, Peter Lei, Rachel Peach and Mike Perry for organising field trips, and many Landcare Research staff and students for fieldwork and laboratory work. Murray Efford, Mike Fitzgerald, Chris Gillies, Kelvin Lloyd, Elaine Murphy and Clare Veltman gave scientific advice. Corinne Watts, John Dugdale, Leonie Clunie and Robert Hoare assisted with invertebrate identification. Southland Conservancy, DOC, supplied research permits, and the Borland Lodge provided accommodation and other assistance. Craig Briggs prepared the base map for Fig. 1. Christine Bezar, Dave Kelly, C. M. (Kim) King, Adrian Monks and Des Smith gave very valuable comments on earlier versions of this paper. The research was done with the approval of the Landcare Research Animal Ethics Committee (AEC 03/01/01 and 04/12/03) and was funded by the New Zealand Department of Conservation, the Miss E. L. Hellaby Indigenous Grasslands Research Trust, and the New Zealand Foundation for Research, Science and Technology.
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control 19, 716–723.
| Crossref | GoogleScholarGoogle Scholar |
Chown, S. L. , and Smith, V. R. (1993). Climate change and the short-term impact of feral house mice at the sub-Antarctic Prince Edward Islands. Oecologia 96, 508–516.
| Crossref | GoogleScholarGoogle Scholar |
Dugdale, J. S. (1996). Natural history and identification of litter-feeding Lepidoptera larvae (Insecta) in beech forests, Orongorongo valley, New Zealand, with especial reference to the diet of mice (Mus musculus). Journal of the Royal Society of New Zealand 26, 251–274.
Efford, M. G. , Dawson, D. K. , and Robbins, C. S. (2004). Density: software for analysing capture-recapture data from passive capture–mark– recapture arrays. Animal Biodiversity and Conservation 27, 217–228.
Elliott, G. P. , Dilks, P. J. , and O’Donnell, C. F. J. (1996). The ecology of yellow-crowned parakeets (Cyanoramphus auriceps) in Nothofagus forest in Fiordland, New Zealand. New Zealand Journal of Zoology 23, 249–265.
Hansson, L. (1970). Methods of morphological diet micro-analysis in rodents. Oikos 21, 255–266.
| Crossref | GoogleScholarGoogle Scholar |
Hurvich, C. M. , and Tsai, C. (1989). Regression and time series model selection in small samples. Biometrika 76, 297–307.
| Crossref | GoogleScholarGoogle Scholar |
Jędrzejewski, W. , Jędrzejewska, B. , Zub, Z. , Ruprecht, A. L. , and Bystrowski, C. (1994). Resource use by tawny owls Strix aluco in relation to rodent fluctuations in Białowieża National Park, Poland. Journal of Avian Biology 25, 308–318.
| Crossref | GoogleScholarGoogle Scholar |
Jones, C. G. , Ostfeld, R. S. , Richard, M. P. , Schauber, E. M. , and Wolff, J. O. (1998). Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease dynamics. Science 279, 1023–1026.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Kolesik, P. , Sarfati, M. S. , Brockerhoff, E. G. , and Kelly, D. (2007). Description of Eucalyptodiplosis chionochloae sp. nov., a cecidomyiid feeding on inflorescences of Chionochloa (Poaceae) in New Zealand. New Zealand Journal of Zoology 34, 107–115.
McKone, M. J. , Kelly, D. , and Lee, W. G. (1998). Effect of climate change on mast-seeding species – frequency of mass flowering and escape from specialist insect seed predators. Global Change Biology 4, 591–596.
| Crossref | GoogleScholarGoogle Scholar |
Murphy, E. C. (1992). The effects of a natural increase in food supply on a wild population of house mice. New Zealand Journal of Ecology 16, 33–40.
Purdey, D. C. , King, C. M. , and Lawrence, B. (2004). Age structure, dispersion and diet of a population of stoats (Mustela erminea) in southern Fiordland during the decline phase of the beechmast cycle. New Zealand Journal of Zoology 31, 205–225.
Rees, M. , Kelly, D. , and Bjornstad, O. N. (2002). Snow tussocks, chaos, and the evolution of mast seeding. American Naturalist 160, 44–59.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Ruscoe, W. A. , Goldsmith, R. , and Choquenot, D. (2001). A comparison of population estimates and abundance indices for house mice inhabiting beech forests in New Zealand. Wildlife Research 28, 173–178.
| Crossref | GoogleScholarGoogle Scholar |
Ruscoe, W. A. , Wilson, D. , McElrea, L. , McElrea, G. , and Richardson, S. J. (2004). A house mouse (Mus musculus) population eruption in response to heavy rimu (Dacrydium cupressinum) seedfall in southern New Zealand. New Zealand Journal of Ecology 28, 259–265.
Smith, D. H. V. , Wilson, D. J. , Moller, H. , Murphy, E. C. , and van Heezik, Y. (2007). Selection of alpine grasslands over beech forest by stoats (Mustela erminea) in montane southern New Zealand. New Zealand Journal of Ecology 31, 88–97.
Solomon, M. E. (1949). The natural control of animal populations. Journal of Animal Ecology 18, 1–35.
| Crossref | GoogleScholarGoogle Scholar |
Whitaker, J. O. (1966). Food of Mus musculus, Peromyscus maniculatus bairdi and Peromyscus leucopus in Vigo County, Indiana. Journal of Mammalogy 47, 473–486.
| Crossref | GoogleScholarGoogle Scholar |
White, P. C. L. , and King, C. M. (2006). Predation on native birds in New Zealand beech forests: the role of functional relationships between stoats Mustela erminea and rodents. The Ibis 148, 765–771.
| Crossref | GoogleScholarGoogle Scholar |
Wilson, D. J. , Efford, M. G. , Brown, S. J. , Williamson, J. F. , and McElrea, G. J. (2007). Estimating the density of ship rats in New Zealand forests with capture–mark–recapture trapping. New Zealand Journal of Ecology 31, 47–59.
|


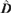 ) and spatial detection parameters (
) and spatial detection parameters (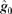 and
and 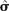 ) estimated by program D
) estimated by program D