Root morphology and phosphorus requirements of 12 tropical pasture species grown in a controlled environment
Jonathan W. McLachlan A * , Richard J. Flavel A and Chris N. Guppy A
A * , Richard J. Flavel A and Chris N. Guppy A
A
Abstract
Tropical pasture species are often established in phosphorus (P)-deficient soils that can limit plant productivity and persistence. Little is known about the root traits and critical P requirements of many tropical grasses and legumes. Characterisation of these traits would allow P-efficient species to be used when soil fertility is poor and agricultural inputs are limited, or for important root traits to be considered if selecting for P efficiency.
To assess the shoot yield, root morphology, P acquisition and critical P requirements of a range of commonly grown tropical pasture species.
Five tropical grasses and seven tropical legumes were grown in pots containing a light-textured, low-P soil to investigate shoot growth and root traits in response to applied P (0–120 mg P kg−1 soil).
The shoot yield of each species increased in response to applied P, yet there were differences in maximum shoot yield (1.7–9.8 g DM pot−1) and critical external P requirements (12.8–38.0 mg P kg−1 soil) among the species. The acquisition of P was associated to varying degrees with the development of root length when plants were grown in soil that enabled near-maximum growth (e.g. R2 = 0.71–0.77 for the grasses and R2 = 0.14–0.43 for the legumes in the 15–30 mg P kg−1 soil treatments).
Longer roots were associated with higher shoot yields and better P-acquisition efficiency, and the grasses generally had comparable or lower critical P requirements than the legumes.
Phosphorus-efficient species should be used when soils are known to be P deficient, or where P fertilisation is not an option, and grasses and legumes could be paired on the basis of their P requirements.
Keywords: biomass allocation, C4 grasses, critical phosphorus requirements, pasture production, phosphorus-acquisition efficiency, phosphorus fertiliser, root traits, tropical legumes.
Introduction
The extensive grazing systems of northern Australia are based on pastures that are dominated by C4 grasses (e.g. Cenchrus ciliaris L., Chloris gayana Kunth and Panicum spp.) (Robertson et al. 1997; Haling et al. 2013; Bell and Sangster 2022). These grasses were often highly productive when first established because of relatively high available nitrogen (N) compared to present levels, but continual grazing and a lack of nutrient input mean that pasture productivity has generally declined over time as a result of the rundown of available N (Graham et al. 1981; Clem and Hall 1994; Jones et al. 1995). It is for these reasons that legumes are expected to improve the productivity of these pastures because they fix atmospheric nitrogen and increase forage quality (Clem and Hall 1994; Jones and Rees 1997; Hall and Walker 2005). However, the productivity and persistence of legumes are likely to be constrained if the species used are not adapted to the prevailing conditions or if other nutrient deficiencies cannot be managed appropriately. This is particularly important in northern Australia, where soils are commonly infertile, amendments are often not used at planting, and there are limited applications of maintenance fertiliser (McIvor et al. 2011).
Phosphorus (P) is important in grazing systems because it is strongly linked to the productivity and persistence of the legume component. Among temperate pasture species, it is well known that legumes such as subterranean clover (Trifolium subterraneum L.) require more P to achieve maximum yield than the grasses with which they are grown (Hill et al. 2005; Haling et al. 2016a). This means that P fertiliser applications must be based on the requirement of the legume component if optimal pasture productivity is to be maintained (Ozanne et al. 1976). Among tropical pasture species, it is well documented that Stylosanthes spp. can persist in very low-P soils (Jones 1974; Smith et al. 1990) and more recently it has been suggested that the critical external P requirements of tropical pasture legumes such as Desmanthus and Stylosanthes are relatively low (Macor et al. 2022). Recent work has also demonstrated that there are large differences in the early growth and critical P requirements of Desmanthus spp. genotypes (McLachlan et al. 2021). Despite all this work, little is known about the P requirements of many tropical pasture species, even though P deficiency is widespread across northern Australia. The comparative P requirements of many tropical grasses and legumes have also not been quantified. This has prompted one review to suggest that one of the priorities for northern Australia is to determine the critical P requirements of adapted tropical legumes (Peck et al. 2015). A greater understanding of the nutrient requirements of important grasses and legumes would inform species selection, including the most appropriate combinations of species, and possibly encourage nutrient management in tropical pasture systems.
Differences in plant critical P requirements are often associated with root morphological traits such as root length, specific root length and root hair length. These traits allow plants to develop a large root surface area for the acquisition of relatively immobile P (Hodge 2004; Richardson et al. 2009). Indeed, it is well known that temperate grasses generally have lower P requirements than temperate legumes because they produce relatively long thin roots with long root hairs (Evans 1977; Haling et al. 2016b). Recent research has demonstrated that there are similar differences in the root traits of several warm-season pasture species (namely Desmanthus spp., Digitaria eriantha Steud, Medicago sativa L. and Panicum coloratum L.), with the grasses generally producing longer roots than the legumes (McLachlan et al. 2023a). There are also reports of differences in root morphology among tropical pasture legumes. For example, early work by Crush (1974) reported that the roots of Centrosema and Stylosanthes were relatively thick (average root diameter of both species was 0.29 mm), root hairs were relatively short (root hair length of both species was 0.11 mm) and the proportion of roots with root hairs was relatively low (root hair coverage was 17 and 6% respectively). More recently, McLachlan et al. (2021) reported differences in the root traits of eight Desmanthus spp. genotypes. They found that root length was the main driver of differences in critical P requirements among the genotypes, whereas root hairs were short and highly patchy. Further work is required to understand the root traits that confer improved P-acquisition efficiency among a wider range of tropical pasture species. In particular, little is known about differences between tropical grasses and legumes.
A greater understanding of root morphology and P-efficiency may help inform the selection of tropical pasture legumes to be incorporated into rundown C4 pastures. Further information about critical P requirements would also assist species selection, depending on the inherent soil fertility of a paddock, or for the need to manage pasture nutrition through strategic application of fertilisers (e.g. at pasture establishment). It is hypothesised that tropical grasses will develop roots more efficiently than tropical legumes, which will result in the grasses having lower critical P requirements. The objectives of the present work were to (1) determine the critical P requirements of a range of commonly grown tropical pasture species, and (2) identify root traits that are associated with P acquisition among these species. This information will provide a greater understanding of how tropical pasture species respond to soil P supply, is expected to inform species selection for better pasture management in the extensive grazing systems of northern Australia, and possibly encourage awareness of the need to manage soil fertility, where possible, for optimum legume productivity and persistence.
Materials and methods
Plant material
Twelve tropical pasture species were grown to investigate shoot yield and root morphology in response to soil P supply. There were five grasses, including Bambatsi Panic (Panicum coloratum L.), Floren Bluegrass (Dichanthium aristatum (Poir.)), Gatton Panic (Panicum maximum), Katambora Rhodes (Chloris gayana Kunth cv. Katambora) and Premier Digit (Digitaria eriantha Steud cv. Premier). There were seven legumes, including Bundey Centro (Centrosema pascuorum cv. Bundey), Butterfly Pea (Clitoria ternatea), Cardillo Centro (Centrosema molle cv. Cardillo), Common Stylo (Stylosanthes guianensis var. V8), JCU 7 Desmanthus (Desmanthus leptophyllus cv. JCU 7), JCU 9 Desmanthus (Desmanthus pernambucanus cv. JCU 9) and Shrubby Stylo (Stylosanthes scabra cv. Seca). These species were selected because they are commonly grown in the pastures of northern Australia.
Soil and nutrient treatments
A sandy soil (Grey Tenosol; Isbell 1996) was collected from the upper 2–15 cm soil layer of a field at Newholme SMART Farm, Armidale, New South Wales (NSW), Australia (30°26′21.4″S, 151°39′55.5″E). This soil was used because it allows easy removal of plant roots for analysis. The soil had a Colwell-extractable P concentration of 3 mg P kg−1 (as measured by the method of Colwell (1963)), a phosphorus buffering index (PBI) of 29 (as measured by the modified method of Burkitt et al. (2008)), and a pH (1:5 w/v; 0.01 M CaCl2) of ~4.7. The soil was passed through a 5 mm sieve and a basal nutrient solution was applied, and included 34 mg kg−1 soil MgSO4.7H2O, 36 mg kg−1 CaSO4.2H2O, 141 mg kg−1 KNO3, 23 mg kg−1 (NH4)2SO4, 14 mg kg−1 NH4NO3, 99 μg kg−1 H3BO3, 635 μg kg−1 MnCl2.4H2O, 301 μg kg−1 ZnSO4.7H2O, 28 μg kg−1 CuSO4.5H2O, 60 μg kg−1 (NH4)2MoO4, 17 μg kg−1 CoCl2·6H2O and 1283 μg kg−1 FeNa-EDTA. Six P-amended soil treatments (0, 7.5, 15, 30, 60 and 120 mg P kg−1 soil, hereafter referred to as P0, P7.5, P15, P30, P60 and P120 respectively) were prepared by adding KH2PO4 to the nutrient solution before it was applied to the soil. Potassium chloride was also added to the nutrient solution that was applied to the P0–P30 treatments, to balance the K so that it was equivalent to that of the P60 treatment. On the basis of previous experimentation, the P120 treatment was expected to allow maximum growth, whereas the P0–P60 treatments were predicted to be within the P-responsive range of the legumes (McLachlan et al. 2021). After the addition of all nutrients, the Colwell extractable P concentrations of the P0–P120 soils were 3, 7, 14, 25, 38 and 96 mg Colwell P kg−1 soil. Cylindrical PVC pots (87 mm internal diameter; 200 mm height) were filled with 1.3 kg (oven-dry basis) of the P-amended soils. The total depth of soil was ~190 mm and the bulk density was ~1.15 g cm−3.
Plant growth conditions and experimental design
Micro-swards of each species were established by sowing seed (~5 mm depth) to achieve a population of six plants per pot. The seed of the two Desmanthus spp. was heat treated by submersing in 80°C water for 10 s, while the seed of Gatton Panic was mechanically scarified using sandpaper. These methods have been previously identified to satisfactorily break seed dormancy prior to sowing (Hopkinson and English 2004; McLachlan et al. 2021). Four replicate pots of each species in each P treatment were prepared. After planting, the pots were watered and moved to a glasshouse (natural daylight ~1800 μmol m−2 s−1 peak intensity; 30°C/25°C, day/night) in Armidale, NSW, Australia. Humidifiers were used to maintain the humidity within the glasshouse at ~50%. Pots were arranged in a randomised complete block design (blocks comprised the different replicates), which was generated using DiGGer (Coombes 2006). Soil moisture was maintained at 80–100% field capacity by watering daily to a predetermined weight. An additional 50 mg N kg−1 soil as CH4N2O was applied to the surface of each pot 4 weeks after planting because of early signs of N deficiency in some of the grasses.
Harvest and measurements
Plants were harvested after 5 weeks of growth because a longer growing period would likely have restricted the root development and growth of the most productive species because of the small volume of soil in the pots. Shoots were cut at the soil surface, oven-dried at 75°C for 72 h and weighed. Shoot samples were finely cut before a ~50 mg subsample was pre-digested in a glass tube with 1 mL deionised water and 4 mL 70% (v/v) nitric acid for at least 4 h. Samples were then digested using a Milestone UltraWAVE 640 (Milestone Srl, Sorisole, Italy). The P concentration of the digested samples was determined colorimetrically at 630 nm by using the malachite green method (Irving and McLaughlin 1990). Shoot P content was calculated by multiplying shoot P concentration and shoot dry mass. Shoot samples were also assessed for N concentration by using a LECO Trumac Analyser. Shoot N content was calculated by multiplying shoot N concentration and shoot dry mass, while the N:P ratio of the shoots was calculated as the shoot N concentration divided by the shoot P concentration.
The soil from each pot was removed intact and cut longitudinally into the following three sections: (1) one-quarter for measurement of root hair length and mycorrhizal colonisation, (2) one-half for drying, and (3) one-quarter for root length scanning and drying, meaning that three-quarters of the soil core would be dried for determination of root dry mass. The roots were then washed from the soil over a 2 mm sieve. There was no evidence of any disease on the roots at the time of collection. Root samples for the measurement of root hair length and mycorrhizal colonisation were stored in 70% (v/v) ethanol at 4°C. Root samples for drying were oven-dried at 75°C for 72 h and weighed. Root samples for scanning were scanned using an Epson Perfection V700 Photo flatbed scanner (Seiko Epson Corporation, Suwa, Japan) at 600 dpi. When a root sample was too large for scanning, a representative subsample was scanned and the remaining roots were dried. Root length and average root diameter were determined using WinRHIZO™ software (Regent Instruments Inc., Quebec, Canada) (Bouma et al. 2000). Scanned root samples were then oven-dried at 75°C for 72 h and weighed. The estimated total mass of roots in the entire soil core was calculated as 4/3 multiplied by the combined dry mass of the unscanned half and scanned quarter. Total root mass fraction was calculated as the estimated total mass of roots divided by total plant mass (i.e. combined dry mass of shoots and roots). Total root length was calculated by multiplying the specific root length of roots from the scanned quarter by the estimated total mass of roots. Root length densities were calculated as root length per unit soil volume; the total soil volume was 1129 cm3.
Root hairs were measured on two lengths of root selected at random from each of the stored samples. Roots were imaged using a Nikon SMZ25 stereomicroscope fitted with a high-resolution Nikon DS-Ri2 digital camera (Nikon Corporation, Tokyo, Japan). Root hairs approximately perpendicular to the line of vision were randomly selected and measured using ImageJ 1.52 (FIJI) (Schindelin et al. 2012). The lengths of five root hairs per image (i.e. 10 root hairs per pot) were measured. Root hair coverage (i.e. the proportion of root length covered with root hairs) was estimated for roots in the P7.5 treatment, using the root sample that had been scanned at 600 dpi. The percentage of root length covered by root hairs was estimated using the gridline intersect method (Giovannetti and Mosse 1980) within ImageJ (FIJI) (Schindelin et al. 2012).
The surface area of the root hair cylinder (SARHC) was calculated as follows:
where ARD = average root diameter, RHL = root hair length, and RL = root length.
Root length colonisation by mycorrhizal fungi was measured on the plants grown in the P7.5 treatment. Roots were cleared in 10% (w/v) KOH for 50 min at 90°C, rinsed in water and 0.1 M (v/v) HCl, and stained using a 5% (v/v) Schaeffer black ink/white vinegar solution for 2 h (Vierheilig et al. 1998). Colonisation % was determined using the gridline intersect method, by examining the presence/absence of mycorrhizal fungi at 100 intersects on the stained root samples (Giovannetti and Mosse 1980). Root hair coverage and mycorrhizal colonisation were assessed only in the P7.5 treatment. It was expected that the soil P supply in this treatment would be below the critical external P requirement of all the species and that their reliance on P acquisition strategies would be highest, whereas the soil P supply in the P0 treatment was expected to cause severe P deficiency that could limit the expression of their P acquisition strategies.
Statistical analyses
Measured parameters were analysed in R (ver. 4.0.2; R Core Team 2020) by fitting linear models and using an ANOVA with ‘species’ and ‘P treatment’ as predictor variables. When appropriate, the effect of ‘replicate’ from the randomised complete block design was included in the most parsimonious model. When included, the effect of ‘replicate’ accounted for the error associated with spatial variation in the glasshouse. Shoot yield responses to P application were determined by fitting a self-starting Weibull growth function (y = a − b × exp (−exp (c) × xd), where x is the P application rate and y is the shoot dry mass), as described by Crawley (2013). Critical external P requirements were calculated as the amount of P applied to achieve 90% of maximum yield on the basis of fitted Weibull growth functions, and the 95% confidence intervals of the critical P requirements were determined by bootstrapping residuals as described by Crawley (2013). Critical internal P requirements were calculated as the shoot P concentration that corresponded with the critical external P application rate (as defined by 90% relative shoot yield). The means and standard errors for root hair length, root hair coverage and mycorrhizal colonisation were calculated from the fitted linear models (R package emmeans, ver. 1.5.0, https://CRAN.R-project.org/package=emmeans; Lenth 2020) and means were compared using Tukey’s honest significant differences (HSD). The relative importance of root morphological traits for P uptake in the P7.5 treatment was determined using random forest modelling (R package randomForest, https://CRAN.R-project.org/doc/Rnews/; Liaw and Wiener 2002). The importance of each factor was estimated by the change in out-of-bag prediction error calculated when each factor was dropped from the model. The sensitivity of the proposed model to any one species was tested by sequentially removing individual species and assessing the scale and rank of importance factors. Importance factors were not influenced by species. Correlations between root length and shoot P content were analysed using linear regression (Crawley 2013). Normal quantile–quantile plots and Shapiro–Wilk tests were used to test the normality of the residuals for all fitted models. When required, these models were log-transformed to meet the assumptions of normality. A 5% level of significance was applied for all statistical tests.
Results
Biomass production and critical external P requirements
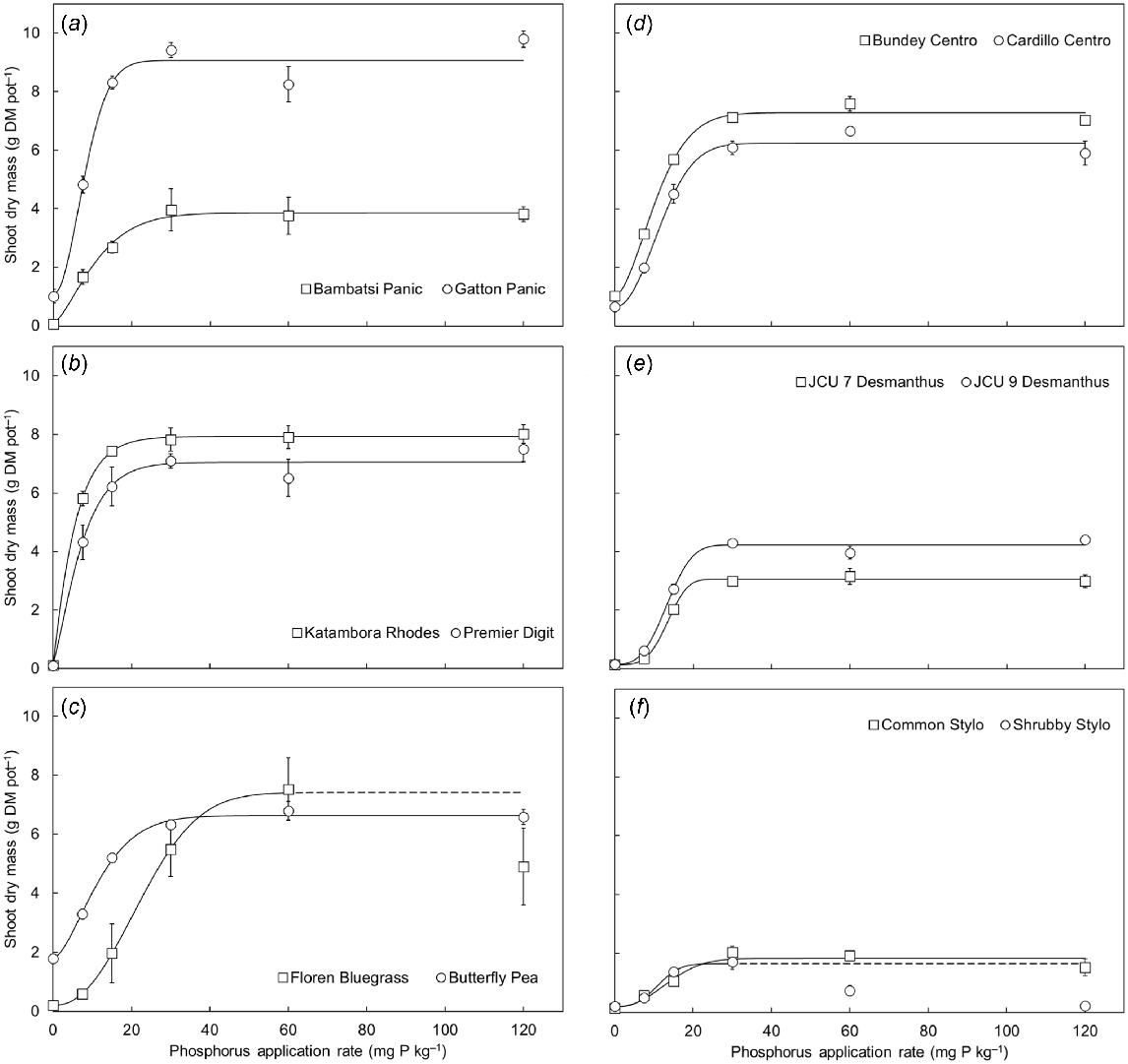
The shoot dry mass of each pasture species increased in response to applied P, with the largest shoot yields being generally produced in the P30–P120 treatments (P < 0.001; Fig. 1). The exceptions were Shrubby Stylo and Floren Bluegrass, both of which had lower shoot yields when grown above the P30 and P60 treatments respectively. Average canopy height also increased in response to soil P supply (P < 0.001), and reflected the shoot yields of the pasture species (R2 = 0.62, P < 0.001) (Supplementary material Fig. S1). In general, the grasses produced taller canopies than the legumes, with the relative difference being more pronounced at lower levels of soil P supply. Although increased soil P supply generally had a positive effect on shoot yield, there were substantial differences in how the species responded to P (P < 0.001; Fig. 1). In particular, maximum shoot yield ranged from 1.7 g DM pot−1 for Shrubby Stylo to 9.8 g DM pot−1 for Gatton Panic (5.7-fold difference; Table 1), and critical external P requirements ranged from 12.8 mg P kg−1 soil for Katambora Rhodes to 38.0 mg P kg−1 soil for Floren Bluegrass (3.0-fold difference; Table 1). On average, the shoot yields of the grasses were 1.6-fold higher than those of the legumes (P < 0.001), whereas the critical external P requirements of the grasses, except Floren Bluegrass, were equal to or lower than those of the legumes.
(a–f) The shoot dry mass of 12 tropical pasture species when grown in response to six rates of applied phosphorus (P) (0, 7.5, 15, 30, 60 and 120 mg P kg−1 soil). Values show the mean ± s.e. (n = 4). The curves that were fitted to the shoot yield data show Weibull growth functions (Crawley 2013). ANOVA results were as follows: species P < 0.001, P treatment P < 0.001, species × P treatment interaction P < 0.001. The yield achieved by the Shrubby Stylo in the P60 and P120 treatments was significantly lower than in the P30 treatment. To enable the Weibull growth function to be fitted, the shoot yield achieved in the P60 and P120 treatments was assumed to be equivalent to that of the P30 treatment. Similarly, the yield achieved by Floren Bluegrass in the P120 treatment was significantly lower than in the P60 treatment. To enable the Weibull growth function to be fitted, the shoot yield achieved in the P120 treatment was assumed to be equivalent to that of the P60 treatment. The dashed lines represent the assumed P response curves.
| Species | Maximum shoot yield (g DM pot−1) | Critical external P requirement (mg P kg−1 soil) | Critical internal P requirement (mg P g−1 DM) | |
|---|---|---|---|---|
| Bambatsi Panic | 3.96 | 22.6 (14.7–*) | 1.31 | |
| Gatton Panic | 9.80 | 14.4 (11.9–20.4) | 0.67 | |
| Katambora Rhodes | 8.02 | 12.8 (10.2–16.9) | 0.90 | |
| Premier Digit | 7.50 | 15.7 (11.2–29.1) | 1.28 | |
| Floren Bluegrass | 7.52 | 38.0 (29.5–*) | 1.55 | |
| Butterfly Pea | 6.79 | 21.2 (17.6–25.0) | 1.41 | |
| Bundey Centro | 7.58 | 19.7 (17.7–22.0) | 1.63 | |
| Cardillo Centro | 6.66 | 20.4 (17.7–25.4) | 1.77 | |
| JCU 7 Desmanthus | 3.16 | 18.4 (16.5–22.0) | 1.53 | |
| JCU 9 Desmanthus | 4.41 | 19.7 (17.7–22.5) | 1.81 | |
| Common Stylo | 2.04 | 22.5 (16.8–45.6) | 2.80 | |
| Shrubby Stylo | 1.72 | 17.1 (14.8–20.1) | 2.46 |
The critical external P requirements were calculated as the amount of P applied to achieve 90% of maximum yield on the basis of the self-starting Weibull growth functions that were fitted to the shoot yield data, with the value range in parentheses showing the 95% confidence intervals determined using bootstrap analysis (Crawley 2013). The critical internal P requirements were determined as the shoot P concentration that corresponded with the critical external P application rate. Asterisk indicates the values that could not be determined because of variable plant growth.
Shoot P concentration and critical internal P requirements
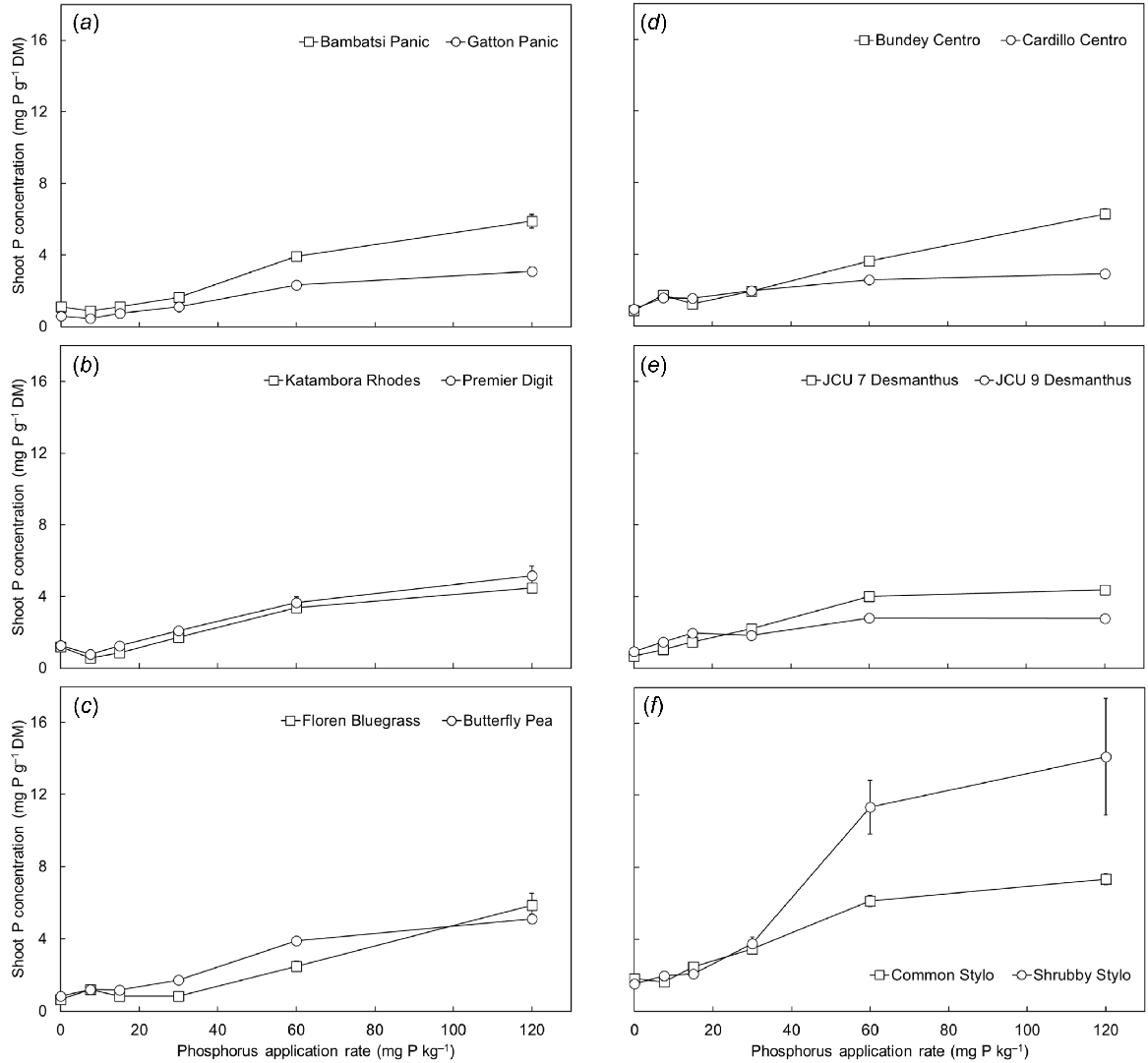
The shoot P concentration of the pasture species increased in response to applied P, particularly between the P30 and P120 treatments, with the highest concentrations achieved in the P120 treatment (P < 0.001; Fig. 2). Across the entire P treatment range, there were significant differences in shoot P among the pasture species (3.1-fold range in the P0 treatment to 4.7-fold range in the P120 treatment) (P < 0.001). There were also differences in the critical internal P requirement of the species. The grasses had critical internal P requirements that ranged between 0.67 and 1.55 mg P g−1 DM, whereas the requirements of the legumes ranged between 1.41 and 2.80 mg P g−1 DM (Table 1). Shoot P content increased with soil P supply (P < 0.001) and varied significantly among the pasture species (P < 0.001) (data not shown because they reflect the shoot dry mass and shoot P concentration results).
(a–f) The shoot phosphorus (P) concentration of 12 tropical pasture species when grown in response to six rates of applied P (0, 7.5, 15, 30, 60 and 120 mg P kg−1 soil). Values show the mean ± s.e. (n = 4). ANOVA results were as follows: species P < 0.001, P treatment P < 0.001, species × P treatment interaction P < 0.001.
Shoot N concentration and shoot N:P ratio
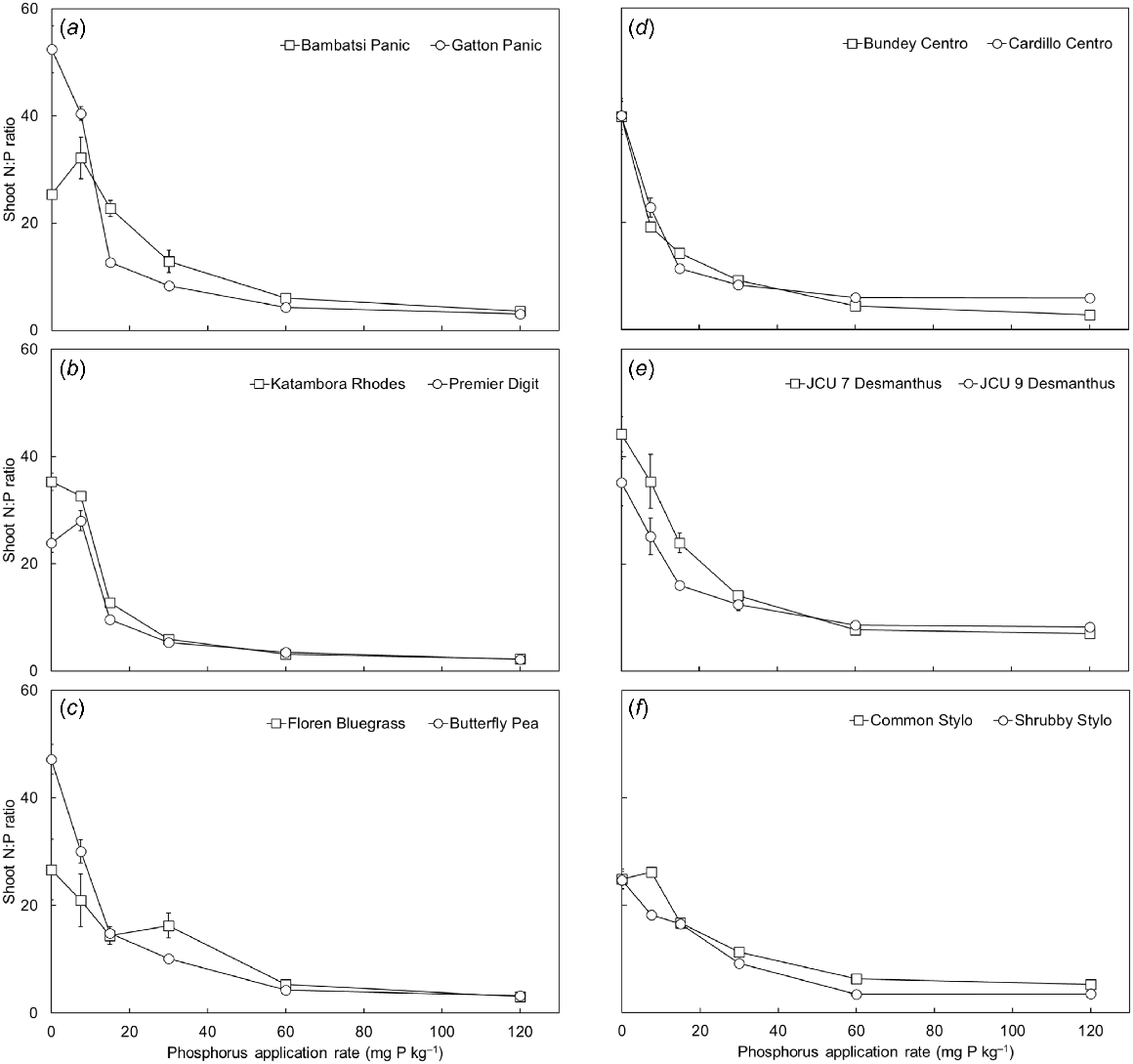
The shoot N concentration of the pasture species was highest in the P0 treatment and declined as soil P supply was increased (P < 0.001; Fig. S2). On average across the soil P supply treatments, shoot N concentrations were higher for the legumes (average 28 mg N g−1 DM) compared to the grasses (average 16 mg N g−1 DM) (P < 0.001). The N and P concentration data were used to calculate shoot N:P ratio. The N:P ratio of the shoots was inversely proportional to soil P supply (P < 0.001; Fig. 3). On average across the P treatments, the two Desmanthus spp. had the highest shoot N:P ratio, whereas Premier Digit and Katambora Rhodes had the lowest.
(a–f) The shoot nitrogen:phosphorus (P) ratio of 12 tropical pasture species when grown in response to six rates of applied P (0, 7.5, 15, 30, 60 and 120 mg P kg−1 soil). Values show the mean ± s.e. (n = 4). ANOVA results were as follows: species P < 0.001, P treatment P < 0.001, species × P treatment interaction P < 0.001.
Root traits
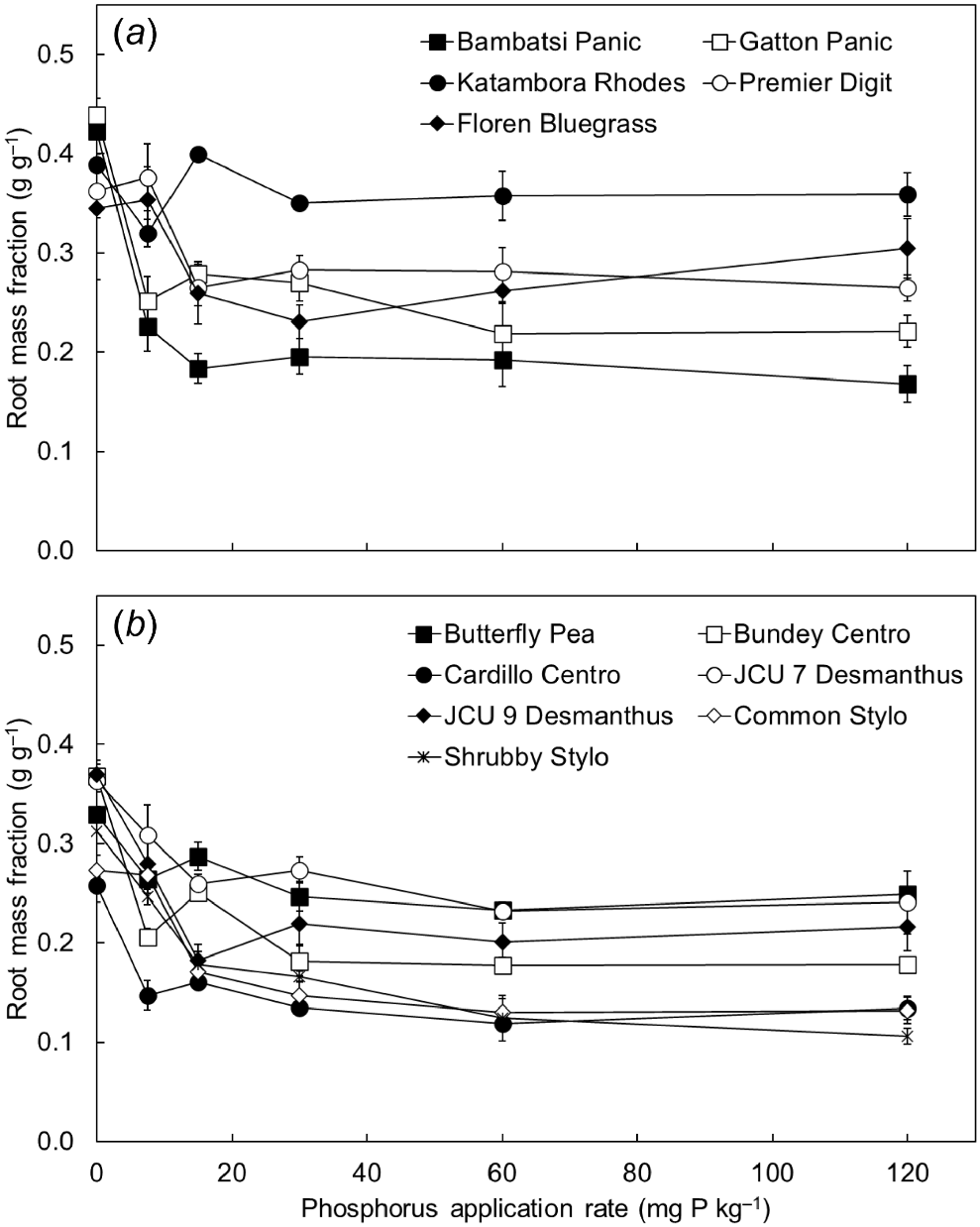
Root mass fraction was lowest when the pasture species were grown with an adequate supply of P (i.e. at or above their critical external P requirement) that allowed maximum growth (i.e. the P30–P120 treatments) (Fig. 4). At lower rates of soil P supply, the proportion of plant biomass allocated to roots increased significantly (P < 0.001), so that most of the species allocated the largest proportion of plant biomass to roots when grown in the P0 treatment. This pattern of response was the same for the grasses and the legumes. On average across the P treatments, there was a substantial difference in the proportion of biomass allocated to roots by the different pasture species (P < 0.001). In general, the grasses achieved higher root mass fractions than did the legumes.
The root mass fraction of (a) five tropical grasses and (b) seven tropical legumes when grown in response to six rates of applied phosphorus (P) (0, 7.5, 15, 30, 60 and 120 mg P kg soil−1). Values show the mean ± s.e. (n = 4). ANOVA results were as follows: species P < 0.001, P treatment P < 0.001, species × P treatment interaction P < 0.001.
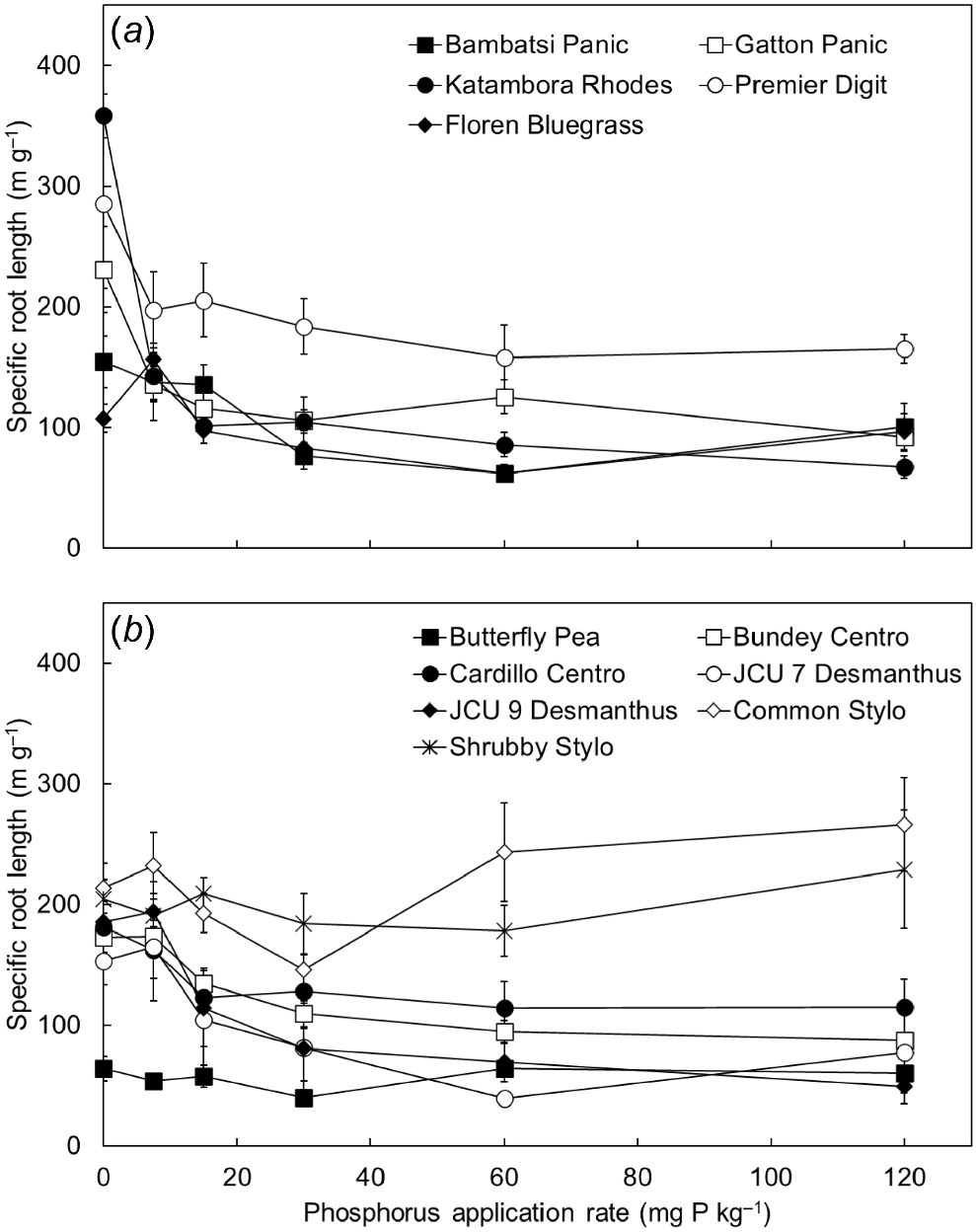
Specific root length was influenced by both the level of applied P (P < 0.001) and pasture species (P < 0.001; Fig. 5). Most of the pasture species achieved the highest specific root lengths in the low-P treatments (i.e. P0–P7.5 treatments), particularly the grasses (e.g. Gatton Panic, Katambora Rhodes and Premier Digit). Specific root lengths of some of the legumes were relatively unresponsive to P treatment. For example, the two Stylosanthes spp. had relatively high specific root lengths across the range of P treatments, whereas that of Butterfly Pea was relatively low. Average root diameters varied among the species, ranging from 0.15 mm for Premier Digit to 0.46 mm for Butterfly Pea (P < 0.001; data not shown). On average across the species, average root diameter declined at lower levels of soil P supply (P < 0.001) and was negatively correlated with specific root length (R2 = 0.25, P < 0.001).
The specific root length of (a) five tropical grasses and (b) seven tropical legumes when grown in response to six rates of applied phosphorus (P) (0, 7.5, 15, 30, 60 and 120 mg P kg soil−1). Values show the mean ± s.e. (n = 4). ANOVA results were as follows: species P < 0.001, P treatment P < 0.001, species × P treatment interaction P < 0.001.
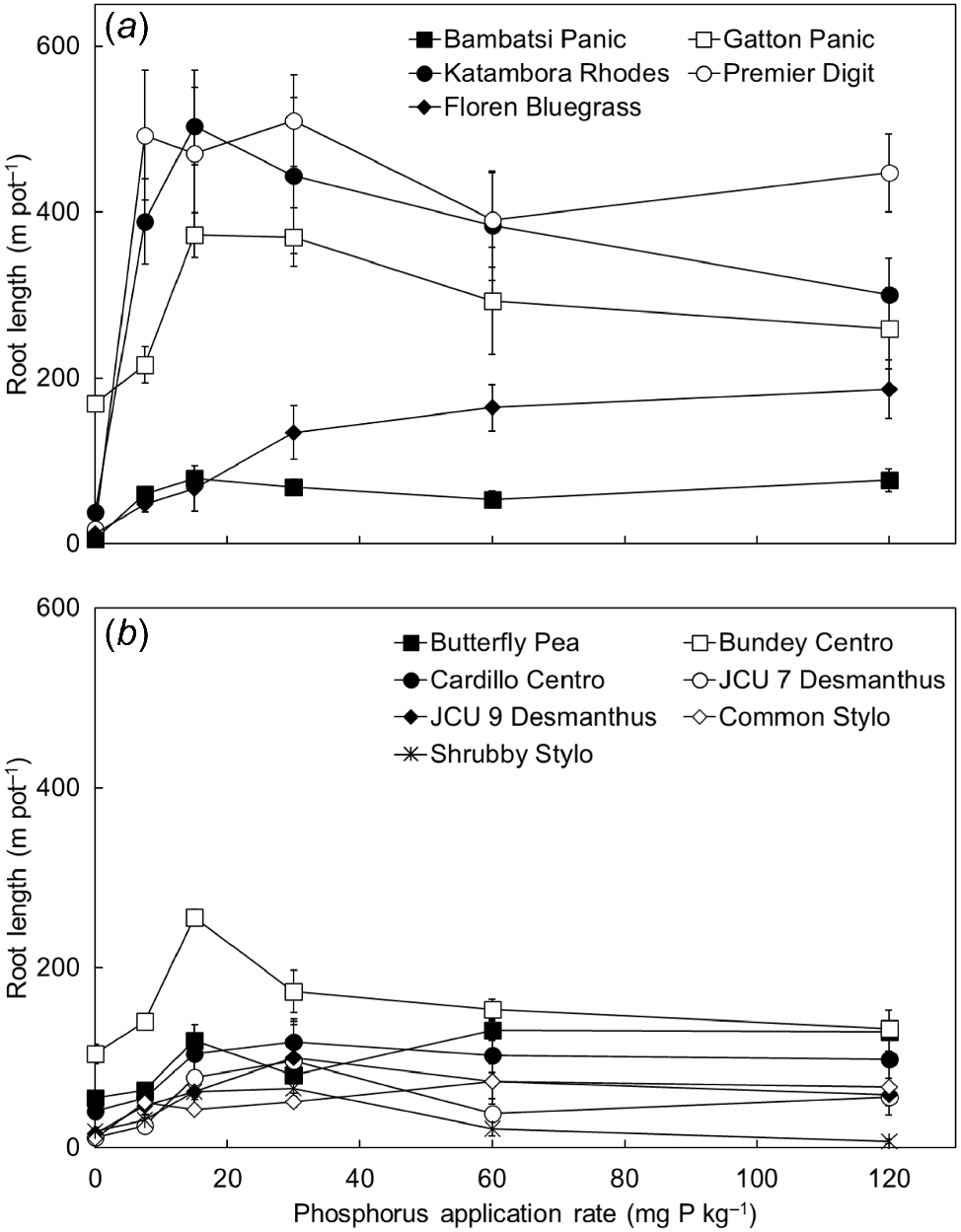
Root length was generally lowest under severe P constraint (in the P0–P7.5 treatments) and increased in response to soil P supply, with the longest roots generally being achieved in the P15–P120 treatments where P was less limiting (P < 0.001; Fig. 6). The development of root length varied significantly among the grasses and legumes (P < 0.001), because Gatton Panic, Katambora Rhodes and Premier Digit produced relatively long roots in the P7.5–P120 treatments, whereas all of the legumes produced relatively short roots in each of the P treatments.
The root length of (a) five tropical grasses and (b) seven tropical legumes when grown in response to six rates of applied phosphorus (P) (0, 7.5, 15, 30, 60 and 120 mg P kg soil−1). Values show the mean ± s.e. (n = 4). ANOVA results were as follows: species P < 0.001, P treatment P < 0.001, species × P treatment interaction P < 0.001.
Root hair length did not change substantially across the soil P treatments (P = 0.043). Consequently, the root hair lengths of each of the species were averaged across the P treatments (Table 2). Average root hair length varied by 3.4-fold among the pasture species (P < 0.001). The longest root hairs were produced by the two Stylosanthes spp. and the shortest were produced by the two Desmanthus spp. Root hair coverage ranged between 3% and 55% (P < 0.001) and mycorrhizal colonisation ranged between 3% and 51% (P < 0.001) among the pasture species (Table 2). There was a slight negative correlation between mycorrhizal fungi colonisation and root hair length (R2 = 0.12, P = 0.011), whereas there was no correlation between mycorrhizal fungi colonisation and either root hair coverage or average root diameter (P > 0.05) (data not shown).
| Species | Average RHL (mm) | RHC (%) | AMF (%) | |
|---|---|---|---|---|
| Bambatsi Panic | 0.27c | 55.0g | 43.3c | |
| Gatton Panic | 0.28c | 32.5de | 16.5a | |
| Katambora Rhodes | 0.35d | 39.0ef | 12.8a | |
| Premier Digit | 0.24bc | 47.8fg | 14.3a | |
| Floren Bluegrass | 0.19ab | 26.0cd | 37.5bc | |
| Butterfly Pea | 0.18ab | 16.8bc | 20.3ab | |
| Bundey Centro | 0.16a | 7.5ab | 51.0c | |
| Cardillo Centro | 0.18ab | 32.5de | 38.2c | |
| JCU 7 Desmanthus | 0.14a | 12.5ab | 5.8a | |
| JCU 9 Desmanthus | 0.15a | 8.3ab | 16.0a | |
| Common Stylo | 0.38d | 8.0ab | 6.7a | |
| Shrubby Stylo | 0.48e | 2.8a | 2.8a |
Root hair length was averaged across the six phosphorus (P) treatments (0, 7.5, 15, 30, 60 and 120 mg P kg−1 soil) because the main effect of soil P supply was minimal (P = 0.043). Root hair coverage and mycorrhizal colonisation were assessed only in the 7.5 mg P kg−1 soil treatment. Different letters denote significant differences at P = 0.05 for each trait.
Phosphorus acquisition
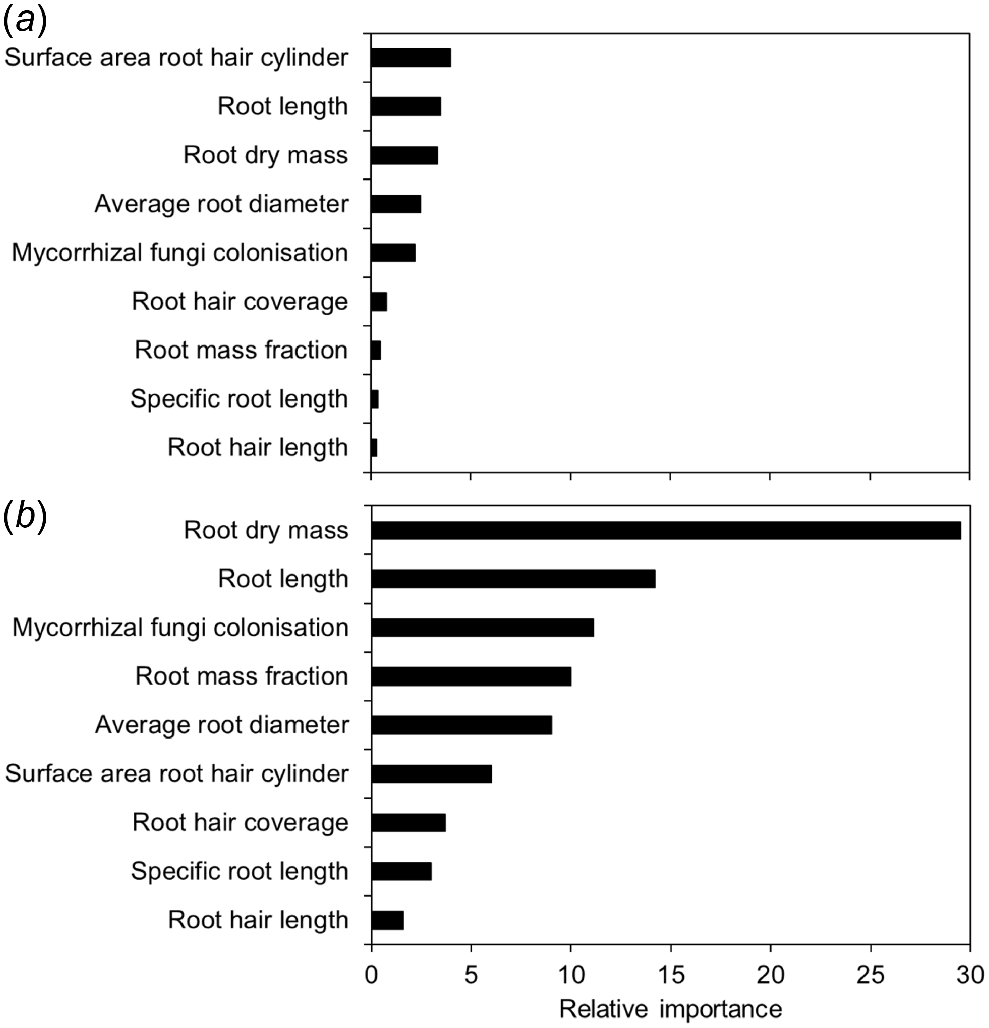
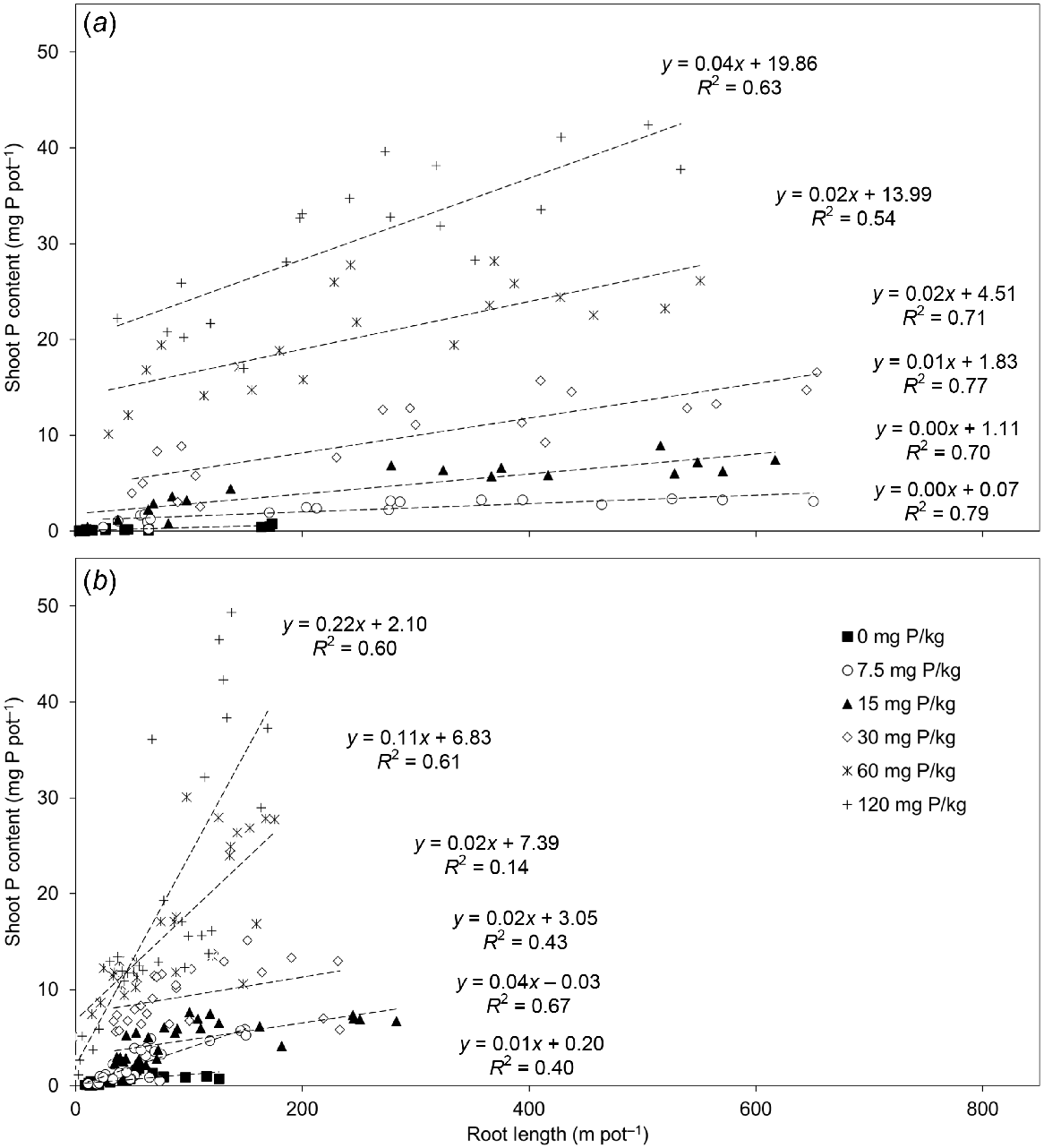
The relative importance of the different root traits for determining shoot P content was analysed using random forest modelling when the 12 pasture species were grown in the P7.5 treatment (Fig. 7). The modelling indicated that surface area of the root hair cylinder, root length and root dry mass were the three most important traits that influenced the P uptake of the grasses (Fig. 7a), whereas root dry mass, root length and mycorrhizal fungi colonisation were the three most important traits that influenced the P uptake of the legumes (Fig. 7b). Because root length had the highest shared relative importance for both the grasses and legumes, it was correlated with shoot P content at each level of soil P supply (Fig. 8). In the P0–P30 treatments, in which the tropical pasture species would have been foraging for P, the R2 coefficients of determination were strong for the grasses (R2 = 0.70–0.79; Fig. 8a) and generally moderate for the legumes (R2 = 0.14–0.67; Fig. 8b). There was an increase in the slope of the correlations with increasing soil P supply, which was more pronounced for the legumes, particularly in the P60–P120 treatments. This increase could be attributed to more P uptake per unit root length, yet the R2 coefficients of determination also indicated that there were significant differences in shoot P content per unit root length among both groups of species (i.e. the five grasses and the seven legumes; Fig. 8). Indeed, shoot P content per unit root length varied significantly (P < 0.001) among the pasture species (data not shown).
The relative importance of root morphological traits for phosphorus (P) acquisition for (a) five tropical grasses and (b) seven tropical legumes when grown with 7.5 mg P kg−1 applied to a low-P soil. The relative importance of each trait was determined using random forest modelling, by estimating the change in out-of-bag prediction error calculated when each factor was dropped from the model. This indicated the traits that were more closely linked among the species of grass and legume. The sensitivity of the proposed model to any one species was tested by sequentially removing individual species and assessing the scale and rank of importance factors. Importance factors were not influenced by species.
The relationship between root length and shoot phosphorus (P) content for (a) five tropical grasses and (b) seven tropical legumes that were grown in response to six rates of applied P (0, 7.5, 15, 30, 60 and 120 mg P kg soil−1). The regression lines and R2 coefficients of determination were fitted separately for each P treatment and include all data points of each species.
Discussion
Shoot yield responses, root morphological traits and critical P requirements varied among the tropical grasses and legumes examined in this study. In general, the grasses produced longer roots that were associated with higher shoot yields and comparable or lower critical external P requirements than the legumes. This difference in root length between the grasses and legumes is consistent with previous findings for temperate pasture species (Evans 1977; Haling et al. 2016b) and more recent work among several warm-season pasture species (McLachlan et al. 2023a). However, the difference in critical P requirements between the grasses and legumes is smaller than has generally been observed among temperate grasses and legumes (Hill et al. 2005; Haling et al. 2016a), which in part could be due to the experimental conditions because the entire soil profile had been amended with P. In addition, the critical external P requirements of most of the species tested may be lower than the benchmark range of 28–32 mg Colwell P kg−1 that is currently used in the temperate grazing systems of southern Australia (Sandral et al. 2019). This assumption is based on recent findings for tropical pasture legumes Desmanthus and Stylosanthes, which are suggested to have critical external P requirements of 12–14 mg Colwell P kg−1 (Macor et al. 2022). In the present study, only Floren Bluegrass had a significantly higher critical external P requirement than the Desmanthus spp.
Shoot growth and critical external P requirements
The tropical pasture grasses were generally more productive and, with the exception of Floren Bluegrass, had comparable or lower critical external P requirements than did the tropical pasture legumes examined in this study. In addition, the grasses had taller canopies than did the legumes, particularly at lower levels of soil P supply. These differences in height and productivity suggest that, if the legumes were grown in mixed pasture swards with the grasses, the legume component may be less competitive, which could lead to a grass-dominant pasture. Indeed, maintaining the balance between grasses and legumes often requires some form of ongoing management even in low-input systems (e.g. maintaining adequate soil fertility and limiting selective grazing) (Dear and Virgona 1996). Because the soils of northern Australia are generally nutrient-deficient and receive little nutrient input, plus grazing management is limited because of extensive paddock areas, these potential differences in competitiveness between grasses and legumes are likely to be more pronounced.
Differences in critical external P requirements among the tropical pasture species indicate that there is potential to use P-efficient selections in inherently low-P soil. For example, Katambora Rhodes had the lowest critical external P requirement and was the most P-efficient species; therefore, it may be more suited to relatively infertile soils compared to Floren Bluegrass, which had a relatively high critical external P requirement. This difference in P requirement is supported by known differences in preferred soil type, as Katambora Rhodes is suited to light sandy soils, whereas Floren Bluegrass is suited to heavier clay soils (Boschma et al. 2010). Differences in critical P requirements also allow species with similar requirements to be identified and paired for use in mixed-pasture swards. For example, Premier Digit and Bambatsi Panic are likely to be more suitable for pairing with tropical pasture legumes such as Desmanthus spp., because their critical P requirements are more similar and therefore the relative difference in competition for soil P is less likely to negatively affect the productivity and persistence of the legume component. Premier Digit and Bambatsi Panic are commonly-grown species on medium- and heavier-textured soils respectively (Boschma and McCormick 2008); so, the ability to pair these grasses with tropical pasture legumes would be advantageous. By comparison, Katambora Rhodes with its low critical external P requirement is likely to be highly competitive under low to moderate P conditions and outcompete other species. Indeed, Rhodes grass is known to establish quickly and be highly competitive, and will even outcompete other tropical grasses such as Bambatsi Panic and Premier Digit (Lodge et al. 2009).
Previous research has demonstrated that several Stylosanthes spp. can persist in very low-P soils (Jones 1974; Smith et al. 1990). In the present study, Shrubby Stylo had the lowest critical external P requirement of the legumes, but it was not substantially lower than that of the other legume species. This may be a result of the sandy soil that was used in the present experiment. Indeed, factors such as texture, pH and PBI are likely to influence the expression of root traits that facilitate P acquisition and improve plant productivity, and previous work has demonstrated that pH and PBI influence the critical P requirements of Premier Digit and JCU 9 Desmanthus (McLachlan et al. 2024a). Alternatively, the similarity between the Stylosanthes spp. and the other legumes may be because our assessment was conducted on plants during the early establishment phase and it is possible that Stylosanthes spp. either take longer to mature, or implement other P acquisition strategies later in their maturity. Nevertheless, their early lifecycle root traits indicated that they should have been more efficient at acquiring P. For example, their specific root length and root hair length were both relatively high compared with the other legumes. These are important traits for developing a large surface area for P acquisition (Richardson et al. 2009), so their relative P-acquisition efficiency may have improved with time. Indeed, the sensitivity of Shrubby Stylo to the P60 and P120 treatments would suggest that it has a low requirement for P.
Shoot P and N concentrations
Critical internal P requirements were relatively low across the tropical pasture species, which suggests that they may require less available P than temperate pasture species to be productive. Though this is well known for Stylosanthes spp., our findings now include a more diverse range of tropical grasses and legumes. Low critical internal P requirements indicate that the tropical pasture species are suited to relatively P-deficient soils, the trade-off is that low tissue P concentrations can be below the levels required for grazing livestock (Dixon et al. 2020). The critical internal P requirements could be used to inform the need for, and management of, fertiliser application in tropical pasture swards. This is because previous work has suggested that the critical internal P requirements of tropical pasture species (specifically Premier Digit and JCU 9 Desmanthus) are relatively stable across a range of soil pH and PBI conditions (McLachlan et al. 2024a). We also observed significant differences in shoot N:P ratio of the species, with the legumes having a higher N:P ratio, even though they had not been inoculated with Rhizobia. This result highlights the importance of the legume component for improving the quality of C4 grass-dominant pasture swards.
Root traits
Random forest modelling was used to identify the most important traits for P acquisition among the 12 tropical pasture species. The species were divided into grasses and legumes because it was expected that these pasture components would differ in their dependence on the various root traits assessed in our study. The most important trait for P acquisition among the tropical pasture grasses was the surface area of the root hair cylinder. This trait combines average root diameter, root length and root hair length to determine the total surface area developed by the plants for P acquisition, as described by Haling et al. (2016b). In contrast, the most important trait for the tropical pasture legumes was root dry mass, which suggests that traits such as average root diameter and root hair length are not as important for P acquisition. These contrasting results suggest that tropical pasture legumes may rely on other factors such as mycorrhizal fungi or organic exudates when acquiring P from relatively low-P soil. Indeed, mycorrhizal fungi colonisation was the third-most important trait for the legumes, whereas root exudates were not considered in the present work due to the complexity of analysis. Previous work has shown that organic acid exudates are important for other pasture species (Kidd et al. 2016, 2018); so, it is plausible that organic exudates were also important for the tropical legumes examined in our study. Nevertheless, for both pasture components, the second-most important trait was root length. Previous work by McLachlan et al. (2021) identified root length as the primary determinant of P acquisition among nine Desmanthus spp. genotypes. This result suggests that, although there may be differences in root morphology between grasses and legumes, and even among different grass and legume species, the key driver of P acquisition is the development of long roots that effectively forage the soil for P.
The importance of root length for P acquisition is well documented (Lynch 1995; Lynch and Brown 2001), and similarly we observed moderate to strong correlations between root length and shoot P content across most of the P treatments when the species were separated into grasses and legumes. Yet, there was some variation in the relationship between root length and shoot P content, which may be due to a species reliance on other traits that influence P acquisition, or because of differences in root architecture through the development of root length that influences the effectiveness of root placement for P acquisition. Specifically, there are two main ways that root length is developed. Root proliferation is a localised response whereby root branching leads to lateral root production that maximises root surface area within a relatively small area (e.g. in response to a patch of P). In contrast, root elongation occurs when roots explore the soil profile for more mobile nutrients and water, thus generally increasing the extent of the root system (Hodge 2004, 2006; Lynch and Wojciechowski 2015). These different, yet complimentary, strategies are important for the productivity and persistence of pasture species because of the varying location of nutrients and water, particularly in nutrient-deficient soils that are often dry, and to the generally patchiness of relatively immobile nutrients such as P. The importance of root length for P acquisition has the following two implications:
Constraints to the development of root length are likely to reduce P acquisition and, consequently, affect the productivity of tropical pasture species. This is particularly relevant in the relatively hostile soils of northern Australia that are not only low in P but are also low in organic matter, and can be high in exchangeable sodium and therefore prone to surface crusting (Shaw et al. 1994; McIvor et al. 2011). Where possible, management of soil condition should prioritise the development of root length (e.g. through the amelioration of adverse soil conditions or the application of starter fertiliser). For example, previous work by McLachlan et al. (2023b) demonstrated that the ability of four Desmanthus species to emerge and grow in three alkaline clay soils varied significantly. In general, the application of gypsum increased seedling emergence, but was not as beneficial as the application of starter fertiliser, which increased early yield.
Selection for longer roots in tropical pasture species might be a beneficial breeding objective. Because root length was relatively important for the grasses and legumes, this is perhaps a key trait that would have significant contributions to growth and persistence in the development of pasture varieties for P efficiency or when selecting for more P-efficient pasture species.
Root hair coverage varied significantly among the tropical pasture species, and the proportion of root length covered with root hairs was generally relatively low. Similar observations have been made among both temperate (McLachlan et al. 2019) and tropical (Crush 1974; McLachlan et al. 2021) pasture legumes. Because root hair coverage was assessed only at the end of the experiment, it is not known whether root hairs were produced but later lost (turned over), or whether the production of root hairs was generally patchy. Indeed, among some of the legumes, such as the two Desmanthus spp. and two Stylosanthes spp., the proportion of root length covered in root hairs was <13%. This is likely to have significantly reduced their root surface area for P acquisition. Because root hairs are known to improve P acquisition at a relatively limited metabolic cost (Richardson et al. 2011), they might be a reasonable target for improving P-acquisition efficiency.
Practical implications
There were considerable differences in critical external P requirements among the tropical pasture species, which is something that could be exploited for improved pasture productivity and persistence in P-limited environments. It may be possible to select for species that have relatively low critical P requirements (e.g. Rhodes grass) and thereby improve the productivity of low-input systems, but it is also suggested that the relative efficiency of the different pasture components should be considered because if there is a large difference between grasses and legumes then the grass is likely to dominate in mixed swards over time. Of course, consideration of a single trait such as P efficiency may result in a one-dimensional view and the observed differences in shoot yield and canopy height could likewise lead to the legume component being overwhelmed by the large amount of biomass produced by the highly productive C4 grasses.
Differences in critical external P requirements primarily occurred between the grasses and legumes, whereas there were fewer differences among the legumes. This contrasts with some of our previous work that showed larger differences in critical external P requirement among nine Desmanthus spp. genotypes (McLachlan et al. 2021), including JCU 7 and JCU 9 Desmanthus, which were grown in the present experiment. Further work is therefore required to validate differences in P efficiency among tropical pasture species, particularly for plants grown under field conditions that are influenced by other factors such as moisture stress and grazing pressure.
Differences in P efficiency were associated, to varying degrees, with the development of root length and related traits. Future improvements in P acquisition efficiency could be achieved by selecting and breeding for plants with longer roots that forage the soil for available nutrients. Indeed, the efficient development of root length is likely to be important in northern Australia where soils are nutrient deficient and seasonally dry, meaning that surface-foraging roots for nutrient acquisition and deeper roots for water acquisition could be important in driving productivity and persistence.
Conclusions
There were differences in shoot yields, critical P requirements and root traits associated with P acquisition among the tropical pasture species examined in this study. In general, the grasses were highly productive and produced dry matter more efficiently in terms of P than did the legumes, even though their critical external P requirements were not always substantially lower than those of the legumes. This difference in productivity suggests that tropical legumes are likely to be out-yielded by tropical grasses, even though different varieties of grasses and legumes may require a similar amount of soil P to achieve maximum yield. The relatively low critical P requirements of tropical pasture species require field validation, but suggests that tropical pasture systems could be managed at a lower level of soil P supply than is recommended for temperate pasture systems in southern Australia.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This work was funded by Meat and Livestock Australia (MLA) and the Australian Government through the Meat Donor Company, by an investment in ‘Phosphorus management and requirements of tropical legume pasture swards’ Project P.PSH.1050.
Acknowledgements
The preliminary results of this work are presented in the final report for the MLA Project P.PSH.1050 (McLachlan et al. 2024b). Emma Flavel and Jennifer Bendeich are thanked for technical assistance.
References
Bell A, Sangster N (2022) Research, development and adoption for the north Australian beef cattle breeding industry: an analysis of needs and gaps. Animal Production Science 63(1), 1-40.
| Crossref | Google Scholar |
Boschma SP, Lodge GM, McCormick LH (2010) Recent tropical perennial grass research and their potential role in maintaining production in a variable and changing climate. In ‘Proceedings of the 25th Annual Conference of the Grassland Society of NSW’, 28-29 July 2010, Dubbo, NSW, Australia. pp. 85–92. (The Grassland Society of NSW Inc.)
Bouma TJ, Nielsen KL, Koutstaal B (2000) Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant and Soil 218, 185-196.
| Crossref | Google Scholar |
Burkitt LL, Sale PWG, Gourley CJP (2008) Soil phosphorus buffering measures should not be adjusted for current phosphorus fertility. Australian Journal of Soil Research 46(8), 676-685.
| Crossref | Google Scholar |
Clem RL, Hall TJ (1994) Persistence and productivity of tropical pasture legumes on three cracking clay soils (Vertisols) in north-eastern Queensland. Australian Journal of Experimental Agriculture 34(2), 161-171.
| Crossref | Google Scholar |
Colwell JD (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture and Animal Husbandry 3(10), 190-197.
| Crossref | Google Scholar |
Coombes NE (2006) DiGGer, a design generator. Available at http://www.austatgen.org/files/software/downloads
Crush JR (1974) Plant growth responses to vesicular-arbuscular mycorrhiza VII. Growth and modulation of some herbage legumes. New Phytologist 73(4), 743-749.
| Crossref | Google Scholar |
Dear BS, Virgona JM (1996) Legumes in low-input perennial pastures of southern Australia: historical role and future development. New Zealand Journal of Agricultural Research 39(4), 579-589.
| Crossref | Google Scholar |
Dixon RM, Anderson ST, Kidd LJ, Fletcher MT (2020) Management of phosphorus nutrition of beef cattle grazing seasonally dry rangelands: a review. Animal Production Science 60(7), 863-879.
| Crossref | Google Scholar |
Evans PS (1977) Comparative root morphology of some pasture grasses and clovers. New Zealand Journal of Agricultural Research 20(3), 331-335.
| Crossref | Google Scholar |
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist 84(3), 489-500.
| Crossref | Google Scholar |
Graham TWG, Webb AA, Waring SA (1981) Soil nitrogen status and pasture productivity after clearing of brigalow (Acacia harpophylla). Australian Journal of Experimental Agriculture and Animal Husbandry 21(108), 109-118.
| Crossref | Google Scholar |
Haling RE, Campbell CD, Tighe MK, Guppy CN (2013) Effect of competition from a C4 grass on the phosphorus response of a subtropical legume. Crop & Pasture Science 64(10), 985-992.
| Crossref | Google Scholar |
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016a) Growth and root dry matter allocation by pasture legumes and a grass with contrasting external critical phosphorus requirements. Plant and Soil 407, 67-79.
| Crossref | Google Scholar |
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016b) Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species. Functional Plant Biology 43(9), 815-826.
| Crossref | Google Scholar |
Hall TJ, Walker RW (2005) Pasture legume adaptation to six environments of the seasonally dry tropics of north Queensland. Tropical Grasslands 39, 182-196.
| Google Scholar |
Hill JO, Simpson RJ, Wood JT, Moore AD, Chapman DF (2005) The phosphorus and nitrogen requirements of temperate pasture species and their influence on grassland botanical composition. Australian Journal of Agricultural Research 56(10), 1027-1039.
| Crossref | Google Scholar |
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162(1), 9-24.
| Crossref | Google Scholar |
Hodge A (2006) Plastic plants and patchy soils. Journal of Experimental Botany 57(2), 401-411.
| Crossref | Google Scholar | PubMed |
Hopkinson JM, English BH (2004) Germination and hardseededness in desmanthus. Tropical Grasslands 38, 1-16.
| Google Scholar |
Irving GCJ, McLaughlin MJ (1990) A rapid and simple field test for phosphorus in Olsen and Bray No. 1 extracts of soil. Communications in Soil Science and Plant Analysis 21(19–20), 2245-2255.
| Crossref | Google Scholar |
Jones RK (1974) A study of the phosphorus responses of a wide range of accessions from the genus Stylosanthes. Australian Journal of Agricultural Research 25(6), 847-862.
| Crossref | Google Scholar |
Jones RM, Rees MC (1997) Evaluation of tropical legumes on clay soils at four sites in southern inland Queensland. Tropical Grasslands 31, 95-106.
| Google Scholar |
Jones RM, McDonald CK, Silvey MW (1995) Permanent pastures on a brigalow soil: the effect of nitrogen fertiliser and stocking rate on pastures and liveweight gain. Tropical Grasslands 29, 193-209.
| Google Scholar |
Kidd DR, Ryan MH, Haling RE, Lambers H, Sandral GA, Yang Z, Culvenor RA, Cawthray GR, Stefanski A, Simpson RJ (2016) Rhizosphere carboxylates and morphological root traits in pasture legumes and grasses. Plant and Soil 402, 77-89.
| Crossref | Google Scholar |
Kidd DR, Ryan MH, Hahne D, Haling RE, Lambers H, Sandral GA, Simpson RJ, Cawthray GR (2018) The carboxylate composition of rhizosheath and root exudates from twelve species of grassland and crop legumes with special reference to the occurrence of citramalate. Plant and Soil 424, 389-403.
| Crossref | Google Scholar |
Lenth R (2020) Emmeans: estimated marginal means, aka least-squares means. R package version 1.5.0. Available at https://CRAN.R-project.org/package=emmeans
Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2, 18-22.
| Google Scholar |
Lodge GM, Boschma SP, Harden S (2009) Replacement series studies of competition between tropical perennial and annual grasses and perennial grass mixtures in northern New South Wales. Crop & Pasture Science 60(6), 526-531.
| Crossref | Google Scholar |
Lynch J (1995) Root architecture and plant productivity. Plant Physiology 109(1), 7-13.
| Crossref | Google Scholar | PubMed |
Lynch JP, Brown KM (2001) Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237, 225-237.
| Crossref | Google Scholar |
Lynch JP, Wojciechowski T (2015) Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66(8), 2199-2210.
| Crossref | Google Scholar | PubMed |
Macor JP, Peck G, Newman L, Taylor B, Mclean A (2022) Impact of phosphorus fertiliser on tropical pasture legume production. In ‘Proceedings of the 20th Australian Society of Agronomy Conference’, 18–22 September 2022, Toowoomba, Qld, Australia. (Australian Society of Agronomy Inc.) Available at http://www.agronomyaustraliaproceedings.org/
McIvor JG, Guppy C, Probert ME (2011) Phosphorus requirements of tropical grazing systems: the northern Australian experience. Plant and Soil 349, 55-67.
| Crossref | Google Scholar |
McLachlan JW, Haling RE, Simpson RJ, Li X, Flavel RJ, Guppy CN (2019) Variation in root morphology and P acquisition efficiency among Trifolium subterraneum genotypes. Crop & Pasture Science 70(11), 1015-1032.
| Crossref | Google Scholar |
McLachlan JW, Guppy CN, Flavel RJ (2021) Differences in phosphorus acquisition and critical phosphorus requirements among nine Desmanthus spp. genotypes. Crop & Pasture Science 72, 742-753.
| Crossref | Google Scholar |
McLachlan JW, Staker BJ, Flavel RJ, Guppy CN (2023a) Warm-season pasture species respond to subsurface placement of phosphorus fertiliser. Agronomy 13, 2524.
| Crossref | Google Scholar |
McLachlan JW, Gunadasa SG, Guppy CN (2023b) Emergence and early growth of four Desmanthus species in three alkaline clay soils. Agronomy 13(12), 2996.
| Crossref | Google Scholar |
McLachlan JW, Gunadasa SG, Flavel RJ, Guppy CN (2024a) The effect of pH and PBI on the critical phosphorus requirements of two tropical pasture species. In ‘Proceedings of the 21st Australian Society of Agronomy Conference’, 21–24 October 2024, Albany, WA, Australia. (Australian Society of Agronomy Inc.) Available at http://www.agronomyaustraliaproceedings.org/
Ozanne PG, Howes KMW, Petch A (1976) The comparative phosphate requirements of four annual pastures and two crops. Australian Journal of Agricultural Research 27(4), 479-488.
| Crossref | Google Scholar |
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop & Pasture Science 60(2), 124-143.
| Crossref | Google Scholar |
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349, 121-156.
| Crossref | Google Scholar |
Robertson FA, Myers RJK, Saffigna PG (1997) Nitrogen cycling in brigalow clay soils under pasture and cropping. Australian Journal of Soil Research 35(6), 1323-1340.
| Crossref | Google Scholar |
Sandral GA, Price A, Hildebrand SM, Fuller CG, Haling RE, Stefanksi A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop & Pasture Science 70(12), 1080-1096.
| Crossref | Google Scholar |
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676-682.
| Crossref | Google Scholar | PubMed |
Shaw R, Brebber L, Ahern C, Weinand M (1994) A review of sodicity and sodic soil behaviour in Queensland. Australian Journal of Soil Research 32(2), 143-172.
| Crossref | Google Scholar |
Smith FW, Jackson WA, Berg PJV (1990) Internal phosphorus flows during development of phosphorus stress in Stylosanthes hamata. Australian Journal of Plant Physiology 17(4), 451-464.
| Crossref | Google Scholar |
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64(12), 5004-5007.
| Crossref | Google Scholar | PubMed |


