The challenge to reduce crude protein contents of wheat-based broiler diets
Peter H. Selle A B * , Shemil P. Macelline A C , Peter V. Chrystal A D and Sonia Yun Liu A C
A B * , Shemil P. Macelline A C , Peter V. Chrystal A D and Sonia Yun Liu A C
A Poultry Research Foundation within The University of Sydney, 425 Werombi Road, Camden, NSW 2570, Australia.
B Sydney School of Veterinary Science, 425 Werombi Road, Camden, NSW 2570, Australia.
C School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, Camperdown, NSW 2006, Australia.
D Complete Feed Solutions, Howick 2145, New Zealand.
Animal Production Science 63(18) 1899-1910 https://doi.org/10.1071/AN22419
Submitted: 14 November 2022 Accepted: 6 January 2023 Published: 2 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The challenge to reduce crude protein (CP) contents of wheat-based broiler diets is both justified and formidable because the performance of broiler chickens offered reduced-CP, wheat-based diets is usually compromised. Moreover, broiler chickens offered wheat-based diets do not accommodate CP reductions as well as do those offered maize-based diets; this appears to stem from the higher protein concentrations and more rapid starch digestion rates of wheat. The higher protein concentrations of wheat than maize result in elevated inclusion levels of non-bound (synthetic, crystalline) amino acids (NBAA). This may be an impediment, because non-bound and protein-bound amino acids are not bioequivalent and intestinal uptakes of NBAA are more rapid than their protein-bound counterparts. This leads to post-enteral amino acid imbalances and the deamination of surplus amino acids, which generates ammonia (NH3). Because NH3 is inherently detrimental, it must be detoxified and eliminated as uric acid, which attracts metabolic costs. Moreover, inadequate NH3 detoxification may seriously compromise broiler growth performance. Also, consideration is given to some intrinsic wheat factors, including soluble non-starch polysaccharides, amylase–trypsin inhibitors and gluten, that may hold relevance. Several strategies are proposed that may enhance the performance of birds offered reduced-CP, wheat-based diets, including capping dietary starch:protein ratios, blending wheat with sorghum, whole-grain feeding in association with phytase, dietary inclusions of L-carnitine and the use of protected or slow-release amino acids. In future research, it should prove instructive to compare different wheats with a wide range of protein contents that, importantly, have been fully characterised for relevant parameters, to ascertain the most appropriate properties. The successful development and adoption of reduced-CP, wheat-based diets would be an enormous advantage for the Australian chicken-meat industry as it would diminish the huge dependence on imported, expensive soybean meal.
Keywords: amino acids, broiler chickens, crude protein, glucose, maize, sorghum, starch, wheat.
Introduction
The reduction of crude protein (CP) concentrations in broiler diets for commercial application in Europe was considered in Garland (2018) and Lemme et al. (2022) and some of the attendant advantages have been clearly demonstrated by Brink et al. (2022) in birds offered wheat-based diets. The objective of successfully developing reduced-CP diets for Australian chicken-meat production is amply justified. The advantages of reduced-CP broiler diets extend to less environmental pollution via attenuated nitrogen (N) and ammonia (NH3) emissions, to bird welfare via improved litter quality with lower incidences of foot-pad lesions and related conditions, and to flock health via diminished flows of undigested protein into the hind-gut that fuel proliferation of potential pathogens (Greenhalgh et al. 2020a; Alfonso-Avila et al. 2022). A reduced-CP diet is one in which dietary protein is reduced by 20 g/kg or more, usually by partially replacing soybean meal with non-bound (synthetic, crystalline) amino acids to meet requirements as non-bound amino acids (NBAA) are effectively an alternative ‘protein’ source to soybean meal (Selle et al. 2020). This substitution increases the feed grain component and, axiomatically, starch concentrations in the diet, which affects starch:protein digestive dynamics in birds offered reduced-CP diets (Liu and Selle 2017). The partial substitution of soybean meal with non-bound amino acids and/or alternative protein feedstuffs is an enormous potential advantage for reduced-CP diets, given the huge dependence of the Australian chicken-meat industry on imported, expensive soybean meal. The objective of this review is to identify the challenges to reducing CP in wheat-based diets and to suggest strategies that may be able to overcome them.
Background
The outcomes of seven evaluations of reduced-CP, wheat-based diets, involving 11 observations, completed in New South Wales, have not been entirely promising. Dietary CP concentrations were reduced by an average of 32 g/kg (174 vs 206 g/kg) in these evaluations, as shown in Table 1. This approach reduced average soybean meal inclusions from 230 to 98 g/kg, or 57.4%, accompanied by increases in NBAA from 7.13 to 24.34 g/kg and average wheat inclusions increased from 553 to 686 g/kg. However, the 32 g/kg reduction in dietary CP compromised growth performance as average weight gains were depressed from 1916 to 1638 g/bird and feed conversion ratio (FCR) was compromised from 1.524 to 1.694 from 13.4 to 35 days post-hatch, as shown in Table 2. The magnitude of the range of these responses is remarkable as evidenced by the tabulated coefficients of variation and outcomes varied from promising in Yin et al. (2020) to profoundly compromised weight gain and FCR in Greenhalgh et al. (2020b) and Chrystal et al. (2021). Clearly, the factors contributing to these profound variations when CP concentrations of wheat-based diets are reduced demand identification, if at all possible.

|
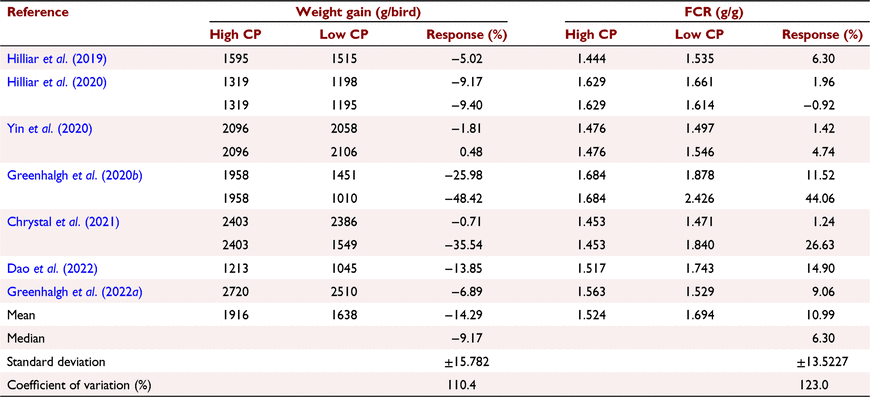
|
Maize is the principal feed grain in global chicken-meat production, but wheat is dominant in Australia with sorghum second. However, wheat-based diets are inferior to maize in two direct comparisons (Chrystal et al. 2021; Greenhalgh et al. 2022a) as shown in Table 3. In both studies, wheat-based diets supported similar or slightly better growth performance with standard-CP diets, but these advantages were eroded in reduced-CP diets. This pattern was more pronounced in Chrystal et al. (2021) than in Greenhalgh et al. (2022a), as were the reductions in dietary CP and, paradoxically, increases in relative fat-pad weights were more evident in birds offered maize-based diets. Collectively, these outcomes epitomise the challenge to reduce CP contents of wheat-based broiler diets.

|
Starch and protein in feed grains
Attempts have been made to identify the shortfalls of wheat in this context (Selle et al. 2022a) because of the inconsistent responses in broilers to reduced-CP diets, but it is a complex issue. Feed grain properties and their starch characteristics assume more importance in reduced-CP diets because of their higher inclusion rates. In addition, protein contents and amino acid profiles in a given feed grain affect the extent and pattern of NBAA inclusions in reduced-CP diets.
The relevance of starch:protein disappearance rate ratios from the small intestine to broiler growth performance was demonstrated in Sydenham et al. (2017). In this study, maximum weight gain was supported by a starch:protein disappearance rate ratio of 3.59 and minimum FCR by a ratio of 3.88, as predicted by the quadratic relationships detected for weight gain (r = 0.849; P < 0.001) and FCR (r = 0.838; P < 0.001). The fundamental concept is that there should be an appropriate balance of amino acids and energy, principally provided as glucose, at sites of protein synthesis to drive efficient skeletal muscle deposition and growth (Liu and Selle 2015).
In reduced-CP diets, dietary starch:protein ratios are axiomatically expanded and this expansion translates to increased starch:protein disappearance-rate ratios in birds. For example, Chrystal et al. (2020) evaluated maize-based diets with CP contents of 200, 188, 172 and 156 g/kg in which analysed dietary starch:protein ratios expanded from 1.48 to 1.76, 2.05 and 2.54 respectively. In birds, distal jejunal starch:protein disappearance-rate ratios linearly increased from 2.08 to 3.17, with a similar increase from 2.01 to 3.06 in the distal ileum. However, distal jejunal ratios were negatively correlated to weight gain (r = −0.422; P = 0.025) and positively correlated to FCR (r = 0.835; P < 0.001), which deteriorated from 1.495 to 1.500, 1.522 and 1.629 with dietary CP reductions for an overall 8.96% decline in efficiency of feed conversion. Also, distal ileal ratios were positively correlated with FCR (r = 0.767; P < 0.001). Relative abdominal fat-pad weights increased by 62.7% (12.40 vs 7.62 g/kg) as dietary CP was reduced from 200 to 156 g/kg and FCR increased quadratically (r = 0.752; P < 0.001) in relation to increased fat deposition. This, and similar outcomes, led to the approach of ‘capping’ dietary statch:protein ratios, as discussed later in this review.
Starch and glucose
The digestion-rate constant of wheat starch (0.035) is more rapid than those of maize (0.017) and sorghum (0.018) under in vitro conditions (Giuberti et al. 2012) and similar patterns have been reported in broiler chickens (Selle et al. 2021a), where the value for wheat starch (0.117) was again more rapid than maize (0.086) and sorghum (0.075). The provision of some slowly digestible starch in broiler diets has been shown to be advantageous in broilers offered atypical diets containing either (rapidly digestible) wheat starch or (slowly digestible) pea starch by Herwig et al. (2019). A similar evaluation with reduced-CP diets could prove instructive. It is also possible that slowly digestible starch spares amino acids from catabolism in the gut mucosa for the provision of energy to drive digestive processes (Enting et al. 2005). The mean proportion of wheat starch digested in the proximal jejunum (by definition rapidly digestible starch), as opposed to the entire small intestine, was 38.2% in broilers offered diets based on six classes of Canadian wheats in Karunaratne et al. (2018). However, the proportion of rapidly digestible starch ranged from 25.0% to 51.4% and this variation may be contributing to the inconsistent responses of broiler chickens following CP reductions in wheat-based diets.
Protein and amino acids
The protein contents and amino acid profiles of wheat and maize are different. In one Australian survey (Bryden et al. 2009), the mean protein concentration of 27 wheat samples was 115.5 g/kg ± 22.32, as opposed to 80.0 g/kg ± 3.56 in seven maize samples. Thus, the protein contents of wheat are both higher and more variable than maize. The amino acid profiles of the two feed grains from the same survey are shown in Table 4. There are considerably higher concentrations of glutamic acid in wheat than maize, but the reverse applies to leucine, methionine, threonine, alanine and aspartic acid. Importantly, protein contents and amino acid concentrations in a given feed grain will influence the extents of NBAA inclusions and the balance of protein-bound to non-bound amino acids in reduced-CP diets. The higher protein content of wheat dictates that there will be higher NBAA inclusions in wheat-based, reduced-CP diets than in corresponding maize-based diets. This is illustrated in Table 5, where 165 g/kg CP, maize-based diets contained 38.49 g/kg NBAA, but the corresponding wheat-based diets contained 49.39 g/kg NBAA. However, this is probably disadvantageous, simply because it is unlikely that non-bound and protein-bound amino acids are bioequivalent, which probably results in post-enteral amino acid imbalances (Selle et al. 2022b).
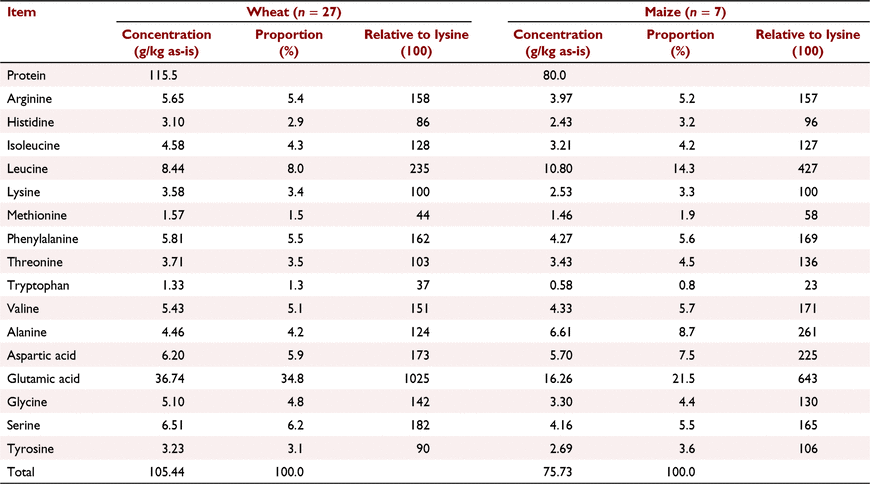
|

|
Protein and starch digestion and intestinal uptakes of amino acids and glucose
Intestinal uptake rates of nutrients, including amino acids and glucose, are pivotal to the growth performance of broiler chickens (Croom et al. 1999). Reduced-CP diets contain higher starch concentrations than do standard broiler diets; however, there is evidence to suggest that glucose may impede intestinal uptakes of amino acids (Murer et al. 1975; Alvarado and Robinson 1979; Stevens et al. 1984; Vinardell 1990). To some extent, these findings may reflect the more general impact of starch–protein interactions, which are complex (Chao et al. 2018).
However, dietary CP of maize-based broiler diets was reduced from 213 to 183 g/kg mainly by increasing maize starch (0–464 g/kg) at the expense of maize grain (465–114 g/kg) in Moss et al. (2018). NBAA inclusions were increased from 3.54 to 17.82 g/kg and soybean meal inclusions were slightly reduced from 370 to 340 g/kg. Interestingly, apparent starch digestibility coefficients were negatively correlated with digestibility coefficients of four amino acids in proximal jejunum, nine in distal jejunum, 12 in proximal ileum and 11 amino acids in distal ileum to significant extents. In the proximal ileum, 8 of the 12 relevant amino acids (arginine, isoleucine, lysine, methionine, threonine, valine, glycine, serine) were included in the 183 g/kg CP diet as both non-bound and protein-bound entities. However, the balance of four amino acids (histidine, leucine, phenylalanine, glutamic acid) was present only as protein-bound entities. Thus, it appears that glucose was impeding intestinal uptakes of both protein-bound and non-bound amino acids in Moss et al. (2018). Non-bound or monomeric amino acids are absorbed via an array of some 23 Na+-dependent and Na+-independent transporters with overlapping amino acid affinities (Hyde et al. 2003). Some monomeric amino acids and glucose are co-absorbed via the Na+-dependent transporter, SGLT-1 (Wright 1993; Shibata et al. 2019). Alternatively, di- and tri-peptides derived from intact protein digestion are mainly absorbed via the peptide transporter, PepT-1 (Zwarycz and Wong 2013; Wang et al. 2021). Importantly, intestinal uptakes of amino acids as di- and tri-peptides, or oligopeptides, are dominant and energetically more efficient than are intestinal uptakes of monomeric amino acids (Daniel 2004; Gilbert et al. 2008). Inclusions of NBAA were increased from 3.54 to 17.82 g/kg with the dietary CP reduction from 213 to 183 g/kg in the Moss et al. (2018) study. Also, the mean proportion of dietary NBAA inclusions of total analysed amino acid concentrations ranged from 3.65% (arginine) to 72.3% (methionine) across nine amino acids. This raises the possibility that NBAA were competing for co-absorption with Na via SGLT-1 to the detriment of amino acid digestibilities. The other possibility is that starch may have impeded the digestive conversion of intact protein to oligopeptides and their intestinal uptakes via PepT-1, again to disadvantage amino acid digestibilities in the Moss et al. (2018) study.
Apparent jejunal and ileal amino acid digestibility coefficients in broilers offered maize- or wheat-based diets were directly compared by Greenhalgh et al. (2022a). Wheat supported inferior average digestibility coefficients of 16 amino acids in distal jejunum by 5.24% (0.741 vs 0.782) in 220 g/kg CP diets but this increased to a discrepancy of 9.94% (0.734 vs 0.811) in 180 g/kg CP diets. A similar pattern was observed in the distal ileum where the shortfall of 2.38% (0.862 vs 0.883) in 220 g/kg CP diets expanded to 6.67% (0.840 vs 0.900) in 180 g/kg CP diets. Soluble non-starch polysaccharides (NSP) are one possible cause of the relative inferiority of wheat. Wheat contains higher concentrations of soluble NSP than does maize by 25 vs 9 g/kg on a dry-matter basis (Knudsen 1997). While all diets contained NSP- and phytate-degrading feed enzymes in the Greenhalgh et al. (2022a) study, Munyaka et al. (2016) reported that wheat generated higher average gut viscosities in broiler chickens than did maize by 61.3% (3.08 vs 1.91 MPa), irrespective of inclusions of dietary NSP-degrading enzymes. Also, Choct et al. (1996) found that NSP-enriched diets promoted small intestinal fermentation and considered this to contribute to the anti-nutritive effects of soluble NSP. Xylanase inclusions in either wheat- or maize-based broiler diets had differing impacts on the composition of caecal microbiota in Wang et al. (2021). Thus, it is possible that (1) higher gut viscosities in birds offered wheat-based diets impeded protein digestion and amino acid absorption and (2) increased microbial fermentation along the small intestine depressed apparent amino acid digestibilities by increasing concentrations of small intestinal microbial amino acids in Greenhalgh et al. (2022a).
Support for this is provided by the relative proportions of dietary, endogenous and microbial amino acids in distal ileal digesta estimated by the Duvaux et al. (1990) model in the Greenhalgh et al. (2022a) study. The transition from 220 to 180 g/kg CP diets modestly increased the proportion of microbial amino acids from 22.1% to 24.4% in birds offered maize-based diets. However, the same transition increased the proportion of microbial amino acids in wheat-based diets from 29.2% to 38.6%. Moreover, estimated microbial amino acid proportions in distal ileal digesta were negatively correlated with apparent digestibility coefficients of all 16 amino acids assessed, to highly significant extents across the entire study.
A second possible factor is amylase–trypsin inhibitors (ATI), which are abundant in the soluble albumin fraction of wheat (Geisslitz et al. (2022). Potentially, wheat ATI could increase pancreatic secretions of amylase and trypsin to counteract the inhibition, which would increase endogenous flows of relevant amino acids and could even compromise starch and protein digestibilities. However, whether wheat ATI are potent anti-nutritive factors in broiler chickens is problematic. Macri et al. (1977) reported that albumin amylase inhibitors from wheat depressed broiler growth performance, but only when administered as cellulose-coated microgranules resistant to pepsin activity. Unprotected wheat albumins did not compromise growth performance, which implies that wheat ATI may be vulnerable to pepsin activity and/or low pH in the gizzard. Nevertheless, further investigations into wheat ATI in broilers are probably justified, given their potential to affect starch and protein digestion.
A third possible factor is the gluten content of wheat, which is an insoluble storage protein comprising ~80–85% of the wheat proteome and is subdivided into gliadins and glutenins (Van der Borght et al. 2005). Gliadin has the capacity to compromise the integrity of small intestinal tight junctions by inducing inflammation and increasing intestinal permeability (De Punder and Pruimboom 2013). Moreover, dietary additions of gluten have been reported to depress broiler growth performance (Afshar and Moslehi 2006; Fang et al. 2017; Kang et al. 2019). A typical 120 g/kg protein wheat would contain in the order of 100 g/kg gluten and the increase in wheat inclusion level of 133 g/kg, from 553 to 686 g/kg (Table 1), would increase gluten by about 13.3 g/kg in the reduced-CP diet. Given that Fang et al. (2017) found that the addition of 20 g/kg gluten to broiler diets depressed weight gain (8.37%), feed intake (6.21%) and FCR (2.65%), then the increased gluten concentrations in reduced-CP, wheat-based diets could assume importance.
Intestinal uptake rates of protein-bound versus non-bound amino acids
It is improbable that protein-bound and non-bound amino acids are bioequivalent in broiler chickens (Selle et al. 2022b) and elevated inclusions of NBAA increase the pertinence of this issue in reduced-CP, wheat-based diets. From first principles (Wu 2009), NBAA are more rapidly absorbed along the small intestine. Synthetic d,l-methionine was made available for animals in 1950s; however, Canolty and Nasset (1975) subsequently found that cumulative plasma methionine concentrations in rats offered synthetic methionine at 15, 30, 60 and 120 min post-administration were 2.75 time higher (858 vs 312 μmol/L) than in rats receiving methionine only from intact protein. Of direct relevance is that intestinal uptakes of NBAA are more rapid than are their protein-bound counterparts in broiler chickens. This was unequivocally demonstrated by Liu et al. (2013), where standard CP, sorghum-based diets contained 3.7 g/kg lysine (as lysine HCl), 3.4 g/kg d,l-methionine and 1.3 g/kg threonine or a total of 8.4 g/kg NBAA. It may be deduced from this study that the mean digestion constant of 13 protein-bound amino acids was 2.35 × 10−2 min−1 as opposed to 8.78 × 10−2 min−1 for non-bound lysine and 8.49 × 10−2 min−1 for non-bound methionine. Therefore, intestinal uptakes of NBAA were nearly four times more rapid than were those of protein-bound amino acids. More recently, Zamani et al. (2021) reported that 60-min, post-prandial methionine concentrations in plasma from broiler chickens were higher in diets supplemented with either a methionine dipeptide (163 ng/μL) or d,l-methionine (155 ng/μL) by more than a four-fold, factor than were those in the control, maize-soy diet (36 ng/μL), which did not contain any non-bound methionine. Thus, bioequivalency is effectively precluded by these differences in intestinal uptake rates between protein-bound and non-bound amino acids and this disparity has post-enteral consequences.
Hepatic deamination of amino acids and ammonia
The negative impacts of NH3 generated systemically by hepatic deamination of amino acids and by microbial fermentation along the digestive tract merit attention. Importantly, the intravenous LD50 of ammonium acetate in broilers (2.72 mmol/kg) was half that in mice (5.64 mmol/kg); thus, NH3 is noticeably more toxic in poultry than in mice (Wilson et al. 1968) and, presumably, other mammalian species. The negative impacts of atmospheric NH3 in poultry are well established (Han et al. 2021), but are essentially irrelevant to this review.
Protein turnover is a dynamic process involving both protein deposition and protein degradation taking place mainly in skeletal muscles and a positive balance represents protein accretion, or net protein synthesis and growth (Swick 1982; Tesseraud et al. 2000). Nevertheless, protein degradation is a continuously ongoing process generating endogenous NH3; however, post-enteral imbalances between non-bound and protein-bound amino acids and the deamination of surplus NBAA are likely to trigger additional NH3 concentrations in broiler chickens offered reduced-CP diets (Selle et al. 2022b). Amino acids exceeding requirements for protein synthesis are rapidly catabolised (Brosnan 2003); therefore, the likelihood is that NBAA will undergo catabolism, or post-prandial oxidation (Schreurs et al. 1997) to greater extents than do protein-bound amino acids because NBAA are more rapidly absorbed. The post-prandial catabolism of non-bound leucine was shown to be greater than that of leucine bound in albumen in rats by Nolles et al. (2009). The post-prandial oxidation of egg white protein versus a corresponding blend of NBAA was determined via 13CO2 breath tests in this study. Post-prandial oxidative losses of non-bound leucine were significantly higher than those of protein-bound leucine by an approximate factor of 1.52 (24.8 vs 16.3%) after a short adaptation period. In a similar earlier study, Bujko et al. (2007) found that cumulative oxidative losses of non-bound leucine (26.9%) exceeded those of protein-bound leucine (16.0%) over a post-prandial period of 300 min in rats. Thus, NBAA are more likely to be lost to post-prandial oxidation from post-enteral amino acid imbalances triggered by their more rapid intestinal uptakes than are protein-bound amino acids.
In an unpublished study completed on the Camden Campus, CP concentrations of broiler diets were reduced from 210 to 190 and 170 g/kg, with corresponding increases in NBAA from 15.1 to 39.0 and 55.4 g/kg. Reducing dietary CP linearly increased (r = 0.528; P = 0.024) plasma NH3 concentrations by 17.1%, from 1.87 to 2.19 μg/g. This outcome accords with the proposal that NBAA are more likely to generate NH3 following their post-enteral deamination.
In addition, NH3 is generated along the digestive tract by microbial fermentation and is readily absorbed in poultry (Yutaka and Chitose 1986). Concentrations of NH3 in the caeca (6–10 mM) of poultry exceeded those in the small intestine (1.2–1.9 mM) in poultry in Prior et al. (1974) and Isshiki (1980) found that caecal concentrations of NH3-N (11.58 mg/kg) were substantially higher than those in the distal ileum (1.93 mg/kg) of broiler chickens. These differences are largely caused by urinary uric acid entering the coprodeum of the large intestine and entering the caeca via reverse peristalsis, to be degraded to NH3 by microbial fermentation (Gilbert et al. 2018). Interestingly, Visek (1978) proposed that the suppression of NH3 concentrations derived from microbial fermentation may be the principal mode of action of the so-called antibiotic growth promotants.
In an instructive study, Okumura and Tasaki (1969) determined the effect of dietary protein concentration on NH3 and uric acid plasma concentrations in chickens for up to 360 min post-prandially. Dietary protein concentrations were increased by casein inclusions ranging from 0 to 400 g/kg in a stepwise manner. Increasing dietary protein increased average plasma NH3 concentrations from 0.34 up to 0.49 NH3-N mg/100 mL and plasma uric acid concentrations from 5.6 up to 15.0 mg/100 mL. However, it may be deduced from the Okumura and Tasaki (1969) study that NH3 to uric acid plasma concentration ratios increased quadratically (r = 0.899; P = 0.037) as protein contents were reduced. In a subsequent study, Karasawa (1986) infused 15N-ammonia into chickens offered either 50 or 200 g/kg CP diets. The infused NH3 was almost entirely excreted as uric acid in birds offered 200 g/kg CP diets. In contrast, infused NH3 was excreted as both NH3 and uric acid in birds offered 50 g/kg CP diets. These outcomes suggest that low dietary CP concentrations limit the capacity of birds to detoxify NH3, which is consistent with the proposal that one molecule of glycine and aspartic acid and two molecules of glutamine are required for the conversion of four NH3 molecules into uric acid, as advanced by Mapes and Krebs (1978).
Ammonia detoxification
As NH3 is inherently noxious, detoxification is demanded (Stern and Mozdziak 2019). Briefly, this is achieved first by the condensation of NH3 with glutamic acid to generate glutamine in a reaction catalysed by glutamine synthetase (Hakvoort et al. 2017). Second, glutamine enters the Krebs uric acid cycle where it is converted to uric acid, which requires an input of one mole of glycine for every mole of uric acid excreted (Salway 2018). An average of 49.2% of dietary glycine, ranging from 25.0% to 80.9%, was partitioned into the Krebs uric acid cycle in the Chrystal et al. (2021) study. This was from a retrospective estimate that does not make an allowance for any biosynthesis of glycine (Selle et al. 2021b). In addition, NH3 detoxification attracts metabolic costs in terms of energy inputs. The synthesis and excretion of uric acid to void NH3-N in urine generates a minimal energy cost of 64.7 kJ/g N excreted as uric acid in poultry (van Milgen 2021). Uric acid concentrations in broiler excreta were determined in Selle et al. (2021b) during the total collection period, to determine nutrient utilisation. It may be deduced from these data that the proportion of dietary energy intake partitioned to uric acid synthesis and excretion amounted to 2.26% of gross energy (17.21 MJ/kg GE) or 2.98% of metabolisable energy (13.06 MJ/kg AME).
Detoxification of NH3 is usually adequate as it is converted into and excreted as uric acid; however, increasing plasma NH3 concentrations have been associated with compromised performance in three broiler studies (Namroud et al. 2008; Ospina-Rojas et al. 2013, 2014), which suggests that NH3 was not being adequately detoxified and excessive systemic NH3 concentrations were depressing growth performance. In addition, excreta NH3 concentrations from birds offered maize–soy diets containing 163, 147 and 132 g/kg CP with four levels of glycine equivalents from 7 to 21 days post-hatch were determined by Hofmann et al. (2019). Dietary NBAA inclusions ranged from 17.5 to 67.3 g/kg across 12 dietary treatments. Excreta NH3 concentrations were indicative as they were negatively correlated with weight gain (r = −0.761; P = 0.004), feed intake (r = −0.754; P = 0.005) and gain to feed (r = −0.753; P = 0.005). Moreover, a quadratic relationship (r = 0.978; P < 0.0001) may be derived between dietary NBAA inclusions and excreta NH3 concentrations, as shown in Fig. 1. The regression equation predicts that when NBAAA inclusions exceed 31.1 g/kg, excreta NH3 concentrations increase in a quadratic manner. Reductions in dietary CP were investigated by Brink et al. (2022), in which finisher broiler diets were formulated to 195, 180 and 165.6 g/kg CP, which contained 5.4, 10.1 and 16.9 g/kg NBAA respectively. The transition from 195 to 165.6 g/kg CP significantly reduced excreta N concentrations by 20.3% (255 vs 320 g/kg) and uric acid by 36.7% (50.1 vs 79.2 g/kg) at 39 days post-hatch. However, total NH3-N in excreta increased numerically by 9.05% (2.29 vs 2.10) in absolute terms or by a factor of 1.36 from 0.66% to 0.90% as a proportion of total excreta N. Collectively, these outcomes indicate that NBAA inclusions may become excessive and contribute to inadequate detoxification, and the resultant NH3 overload compromises growth performance.
|
|
Investigations into NH3 overload should include simultaneous determinations of NH3 and uric acid concentrations in systemic plasma, digesta from segments of the small and large intestine, excreta and hepatic tissue in broiler chickens offered diets with different CP contents. Both NH3 and uric acid liver concentrations were determined in Okumura and Tasaki (1969). Also, allied determinations of orotic acid in excreta may be instructive because urinary excretion of orotic acid becomes elevated when NH3 concentrations exceed hepatic detoxification capacity of the ureotelic species (Visek 1984). Thus, orotic acid may be a valuable biomarker in NH3 overload investigations in broiler chickens if it is similarly indicative in avian species.
Future directions
It should prove instructive to compare a range of fully characterised wheats to ascertain the most appropriate properties in the context of reduced-CP diets. Valuable data could include determinations of in vitro starch digestion rates and starch pasting profiles by rapid visco-analysis (RVA). There would appear to be a ceiling on the extent to which starch concentrations and NBAA inclusions may be increased in reduced-CP, wheat-based diets before growth performance is compromised. Thus, future research should identify alternative approaches to reduce CP in wheat-based diets.
Capping dietary starch:protein ratios
Capping, or condensing, dietary starch:protein ratios in reduced-CP diets has shown some advantages in both maize-based (Greenhalgh et al. 2022b) and wheat-based (Greenhalgh et al. 2020b) diets. Capping dietary starch:protein ratios in 197.5 g/kg CP, wheat-based diets significantly increased weight gain by 10.4% (2161 vs 1958 g/bird), numerically improved FCR by 4.04% (1.616 vs 1.684) from 7 to 35 days post-hatch and fractionally reduced fat-pad weights by 2.70% (8.15 vs 8.37 g/kg). In both studies, capping dietary starch:protein ratios was achieved by substituting soybean meal (505 g/kg CP) with ‘full-fat’ soy (362 g/kg CP) procured from the same provider. Given the Australian situation, substituting soybean meal with canola meal rather than ‘full-fat’ soy would be a preferable approach and certainly merits evaluation.
Blended feed grain diets
Reduced-CP diets based on an equal blend of wheat and maize were evaluated in straight-run Cobb 500 birds kept in floor-pens from 1 to 41 days post-hatch by Maynard et al. (2021). The dietary treatments were formulated to contain 215, 190 and 165 g/kg CP. No significant differences were observed in weight gain (3186 vs 3145 g/bird) or feed intake (4873 vs 5009 g/bird) between the high- and low-CP diets, but FCR was significantly compromised by 3.43% (1.597 vs 1.544) following the transition from 215 to 165 g/kg CP diets. Nevertheless, soybean meal consumption was reduced by 62.7% (500 vs 1339 g/bird) by the same transition. Also, the transition from 215 to 190 CP diets did not statistically influence growth performance but did reduce soybean meal consumption by 30.9% (925 vs 1339 g/bird). Compared with ‘wheat only’, an equal wheat and maize blend is probably advantaged by a lower protein content, which leads to decreased NBAA inclusions, coupled with slower starch digestion rates. Maize is not a practical option in Australia, but sorghum is similar in respect of protein content, amino acid profile and starch digestion rate. Moreover, an equal wheat–sorghum blend has shown some promise relative to wheat in a branched-chain amino acid feeding study (Greenhalgh et al. 2022a).
Whole-grain feeding
With wheat-based diets, the inclusion of some whole grain, either pre- or post-pelleting, has become the standard practice in Australia. Whole-grain feeding (WGF) has been evaluated in the context of reduced-CP diets by Yin et al. (2020), in which WGF had no impact on growth performance but WGF did generate significantly lighter relative abdominal fat-pad weights. In this study, dietary inclusions of 150 and 250 g/kg whole wheat increased relative gizzard weights by 36.5% and 53.8% respectively, which is a hallmark WGF response; however, the capacity of birds to accommodate dietary CP reductions was not enhanced. Nevertheless, further investigations into WGF in association with reduced-CP, wheat-based diets do appear justified on the basis of the lighter relative abdominal fat-pad weights reported by Yin et al. (2020). In addition, Moss et al. (2019) concluded that the combination of phytase inclusions and ‘pre-pellet’ cracked maize advantaged the performance of broilers offered reduced-CP diets. The dietary inclusion of some cracked maize (150 and 300 g/kg) prior to steam-pelleting is somewhat analogous to whole grain feeding to stimulate gizzard function and should, in turn, enhance phytase efficacy as the gizzard is the principal site of enzymatic phytate degradation (Truong et al. 2016).
L-carnitine
L-carnitine is a quaternary amino compound that is present in relevant feedstuffs and may be biosynthesised from its amino acid precursors, lysine and methionine, which involves methylation of only protein-bound lysine (Rebouche 2004). Of relevance is that L-carnitine promotes fat mobilisation via the so-called ‘carnitine shuttle’, which transfers long-chain fatty acids across the barrier of inner mitochondria membranes to undergo β-oxidation and fat is partitioned to energy production (Eder 2009). L-carnitine has displayed promise in limiting lipid deposition in some feeding studies (Rabie et al. 1997a, 1997b; Rabie and Szilágyi 1998; Xu et al. 2003), although responses of broiler chickens to dietary L-carnitine inclusions are not consistent. However, Rabie et al. (1997a) included 50 mg/kg L-carnitine in 180 g/kg CP diets, which reduced relative abdominal fat-pad weights by 20.5% (22.9 vs 28.8 g/kg), with an associated improvement in FCR by 5.48% (2.07 vs 2.19), from 18 to 53 days post-hatch. Also, 50 mg/kg L-carnitine reduced relative abdominal fat-pad weights by 16.2% (9.8 vs 11.7 g/kg) in broiler chickens at 49 days post-hatch in Xu et al. (2003). The inconsistency of L-carnitine responses may stem from variations in its concentrations in relevant feedstuffs, but its potential to depress lipid deposition certainly holds relevance in reduced-CP diets.
Moreover, L-carnitine is protective against NH3 toxicity (Kloiber et al. 1988). In Greenhalgh et al. (2022c), broiler chickens were offered sorghum-based diets with CP concentrations of 220, 190 and 160 g/kg from 7 to 33 days post-hatch. The transition from 220 to 160 g/kg CP diets severely depressed weight gain (38.2%), feed intake (25.8%) and FCR (20.3%). However, the 75 mg/kg L-carnitine inclusion in 160 g/kg CP diets increased weight gain by 15.0% (1580 vs 1374 g/bird; P < 0.001), feed intake by 8.34% (2403 vs 2218 g/kg; P < 0.001) and improved FCR by 5.82% (1.521 vs 1.615; P = 0.003), but similar L-carnitine responses were not observed in 190 and 220 g/kg CP diets. These outcomes are consistent with the possibility that L-carnitine was counteracting the impacts of NH3 intoxication in birds offered 160 g/kg CP diets loaded with 51.02 g/kg NBAA.
‘Slow-release’ NBAA
Finally, the availability of protected or slow-release NBAA with intestinal uptake rates more akin to protein-bound amino acids would almost certainly be advantageous for the acceptance of reduced-CP diets. The obvious caveat is the economic feasibility of slow-release NBAA. However, it has been reported that lipid-encapsulated lysine and methionine were both more effectively utilised in broiler chickens than were non-bound entities (Sun et al. 2020).
Conclusions
Clearly, the challenge to reduce CP contents of wheat-based broiler diets is formidable. However, it should prove instructive to compare different wheats with a wide range of protein contents that, importantly, have been fully characterised in respect of amino acid profiles, concentrations of soluble NSP, ATI and gluten. In addition, RVA starch pasting profiles and in vitro starch digestion rates may prove valuable indicators. The potential advantages that the successful development and adoption of reduced-CP, wheat-based diets hold for the Australian chicken-meat industry certainly merit well directed and well funded research to achieve this objective.
Data availability
The data that support this review are based on published research articles and reviews published in peer-reviewed, scientific journals; therefore, they are readily accessible.
Conflicts of interest
The authors declare that there are not any conflicts of interest.
Declaration of funding
This review did not receive any direct funding.
Acknowledgements
The authors acknowledge all the various organisations that have supported our research into reduced-CP broiler diets in recent years and our colleagues within the Poultry Research Foundation for their ongoing encouragement and assistance.
References
Afshar M, Moslehi H (2006) Investigation in the effect of using wheat gluten meal on broiler performance. In ‘XII European poultry conference’, Verona, Italy. (World’s Poultry Science Association)Alfonso-Avila AR, Cirot O, Lambert W, Létourneau-Montminy MP (2022) Effect of low-protein corn and soybean meal-based diets on nitrogen utilization, litter quality, and water consumption in broiler chicken production: insight from meta-analysis. Animal 16,
| Effect of low-protein corn and soybean meal-based diets on nitrogen utilization, litter quality, and water consumption in broiler chicken production: insight from meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Alvarado F, Robinson JW (1979) A kinetic study of the interactions between amino acids and monosaccharides at the intestinal brush-border membrane. The Journal of Physiology 295, 457–475.
| A kinetic study of the interactions between amino acids and monosaccharides at the intestinal brush-border membrane.Crossref | GoogleScholarGoogle Scholar |
Brink M, Janssens GPJ, Demeyer P, Bagci Ö, Delezie E (2022) Reduction of dietary crude protein and feed form: impact on broiler litter quality, ammonia concentrations, excreta composition, performance, welfare, and meat quality. Animal Nutrition 9, 291–303.
| Reduction of dietary crude protein and feed form: impact on broiler litter quality, ammonia concentrations, excreta composition, performance, welfare, and meat quality.Crossref | GoogleScholarGoogle Scholar |
Brosnan JT (2003) Interorgan amino acid transport and its regulation. The Journal of Nutrition 133, 2068S–2072S.
| Interorgan amino acid transport and its regulation.Crossref | GoogleScholarGoogle Scholar |
Bryden WL, Li X; Ravindran G; Hew LI; Ravindran V (2009) ‘Ileal digestible amino acid values in feedstuffs for poultry.’ RIRDC Publication No 09/071. (Rural Industries Research and Development Corporation: Canberra, ACT, Australia)
Bujko J, Schreurs VVAM, Nolles JA, Verreijen AM, Koopmanschap RE, Verstegen MWA (2007) Application of a [13CO2] breath test to study short-term amino acid catabolism during the postprandial phase of a meal. British Journal of Nutrition 97, 891–897.
| Application of a [13CO2] breath test to study short-term amino acid catabolism during the postprandial phase of a meal.Crossref | GoogleScholarGoogle Scholar |
Canolty NL, Nasset ES (1975) Intestinal absorption of free and protein-bound dietary methionine in the rat. The Journal of Nutrition 105, 867–877.
| Intestinal absorption of free and protein-bound dietary methionine in the rat.Crossref | GoogleScholarGoogle Scholar |
Chao C, Cai J, Yu J, Copeland L, Wang S, Wang S (2018) Toward a better understanding of starch−monoglyceride−protein interactions. Journal of Agricultural and Food Chemistry 66, 13253–13259.
| Toward a better understanding of starch−monoglyceride−protein interactions.Crossref | GoogleScholarGoogle Scholar |
Choct M, Hughes RJ, Wang J, Bedford MR, Morgan AJ, Annison G (1996) Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. British Poultry Science 37, 609–621.
| Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens.Crossref | GoogleScholarGoogle Scholar |
Chrystal PV, Moss AF, Khoddami A, Naranjo VD, Selle PH, Liu SY (2020) Effects of reduced crude protein levels, dietary electrolyte balance, and energy density on the performance of broiler chickens offered maize-based diets with evaluations of starch, protein, and amino acid metabolism. Poultry Science 99, 1421–1431.
| Effects of reduced crude protein levels, dietary electrolyte balance, and energy density on the performance of broiler chickens offered maize-based diets with evaluations of starch, protein, and amino acid metabolism.Crossref | GoogleScholarGoogle Scholar |
Chrystal PV, Greenhalgh S, McInerney BV, McQuade LR, Akter Y, de Paula Dorigam JC, Selle PH, Liu SY (2021) Maize-based diets are more conducive to crude protein reductions than wheat-based diets for broiler chickens. Animal Feed Science and Technology 275,
| Maize-based diets are more conducive to crude protein reductions than wheat-based diets for broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Croom WJ, Brake J, Coles BA, Havenstein GB, Christensen VL, McBride BW, Peebles ED, Taylor IL (1999) Is intestinal absorption capacity rate-limiting for performance in poultry? Journal of Applied Poultry Research 8, 242–252.
| Is intestinal absorption capacity rate-limiting for performance in poultry?Crossref | GoogleScholarGoogle Scholar |
Daniel H (2004) Molecular and integrative physiology of intestinal peptide transport. Annual Review of Physiology 66, 361–384.
| Molecular and integrative physiology of intestinal peptide transport.Crossref | GoogleScholarGoogle Scholar |
Dao HT, Clay JW, Sharma NK, Bradbury EJ, Swick RA (2022) Effects of l-arginine and l-citrulline supplementation in reduced protein diets on cecal fermentation metabolites of broilers under normal, cyclic warm temperature and necrotic enteritis challenge. Livestock Science 257,
| Effects of l-arginine and l-citrulline supplementation in reduced protein diets on cecal fermentation metabolites of broilers under normal, cyclic warm temperature and necrotic enteritis challenge.Crossref | GoogleScholarGoogle Scholar |
De Punder K, Pruimboom L (2013) The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 5, 771–787.
| The dietary intake of wheat and other cereal grains and their role in inflammation.Crossref | GoogleScholarGoogle Scholar |
Duvaux C, Guilloteau P, Toullec R, Sissons JW (1990) A new method of estimating the proportions of different proteins in a mixture using amino acid profiles: application to undigested proteins in the preruminant calf. Annales de Zootechnie 39, 9–18.
| A new method of estimating the proportions of different proteins in a mixture using amino acid profiles: application to undigested proteins in the preruminant calf.Crossref | GoogleScholarGoogle Scholar |
Eder K (2009) Influence of L-carnitine on metabolism and performance of sows. British Journal of Nutrition 102, 645–654.
| Influence of L-carnitine on metabolism and performance of sows.Crossref | GoogleScholarGoogle Scholar |
Enting H, Pos J, Weurding RE, Veldman A (2005) Starch digestion rate affects broiler performance. In ‘Proceedings of the 17th Australian Poultry Science Symposium’, pp. 17–20. (Poultry Research Foundation)
Fang J, Martínez Y, Deng C, Zhu D, Peng H, Jiang H, Li A (2017) Effects of dietary enzymolysis products of wheat gluten on the growth performance, serum biochemical, immune, and antioxidant status of broilers. Food and Agricultural Immunology 28, 1155–1167.
| Effects of dietary enzymolysis products of wheat gluten on the growth performance, serum biochemical, immune, and antioxidant status of broilers.Crossref | GoogleScholarGoogle Scholar |
Garland PW (2018) The challenges confronting chicken meat producers in Great Britain in relation to low protein diets. Proceedings, Australian Poultry Science Symposium 29, 1–7.
Geisslitz S, Weegels P, Shewry P, Zevallos V, Masci S, Sorrells M, Gregorini A, Colomba M, Jonkers D, Huang X, de Giorgio R, Caio GP, D’Amico S, Larre C, Brouns F (2022) Wheat amylase/trypsin inhibitors (ATIs): occurrence, function and health aspects. European Journal of Nutrition 61, 2873–2880.
| Wheat amylase/trypsin inhibitors (ATIs): occurrence, function and health aspects.Crossref | GoogleScholarGoogle Scholar |
Gilbert ER, Wong EA, Webb KE (2008) BOARD-INVITED REVIEW: Peptide absorption and utilization: implications for animal nutrition and health. Journal of Animal Science 86, 2135–2155.
| BOARD-INVITED REVIEW: Peptide absorption and utilization: implications for animal nutrition and health.Crossref | GoogleScholarGoogle Scholar |
Gilbert MS, Ijssennagger N, Kies AK, van Mil SWC (2018) Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. American Journal of Physiology: Gastrointestinal and Liver Physiology 315, G159–G170.
| Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry.Crossref | GoogleScholarGoogle Scholar |
Giuberti G, Gallo A, Cerioli C, Masoero F (2012) In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition. Animal Feed Science and Technology 174, 163–173.
| In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, Chrystal PV, Selle PH, Liu SY (2020a) Reduced-crude protein diets in chicken-meat production: justification for an imperative. World’s Poultry Science Journal 76, 537–548.
| Reduced-crude protein diets in chicken-meat production: justification for an imperative.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, McInerney BV, McQuade LR, Chrystal PV, Khoddami A, Zhuang MAM, Liu SY, Selle PH (2020b) Capping dietary starch:protein ratios in moderately reduced crude protein, wheat-based diets showed promise but further reductions generated inferior growth performance in broiler chickens. Animal Nutrition 6, 168–178.
| Capping dietary starch:protein ratios in moderately reduced crude protein, wheat-based diets showed promise but further reductions generated inferior growth performance in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, Lemme A, Dorigam JCdeP, Chrystal PV, Macelline SP, Liu SY, Selle PH (2022a) Dietary crude protein concentrations, feed grains, and whey protein interactively influence apparent digestibility coefficients of amino acids, protein, starch, and performance of broiler chickens. Poultry Science 101,
| Dietary crude protein concentrations, feed grains, and whey protein interactively influence apparent digestibility coefficients of amino acids, protein, starch, and performance of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, Chrystal PV, Lemme A, Dorigam JCdeP, Macelline SP, Liu SY, Selle PH (2022b) Capping dietary starch:protein ratios enhances performance of broiler chickens offered reduced-crude protein, maize-based diets. Animal Feed Science and Technology 290,
| Capping dietary starch:protein ratios enhances performance of broiler chickens offered reduced-crude protein, maize-based diets.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh S, Hamilton EJ, Macelline SP, Toghyani M, Chrystal PV, Liu SY, Selle PH (2022c) Dietary crude protein concentrations and L-carnitine inclusions interactively influence performance parameters of grower broiler chickens offered sorghum-based diets. Animal Feed Science and Technology 291,
| Dietary crude protein concentrations and L-carnitine inclusions interactively influence performance parameters of grower broiler chickens offered sorghum-based diets.Crossref | GoogleScholarGoogle Scholar |
Hakvoort TBM, He Y, Kulik W, Vermeulen JLM, Duijst S, Ruijter JM, Runge JH, Deutz NEP, Koehler SE, Lamers WH (2017) Pivotal role of glutamine synthetase in ammonia detoxification. Hepatology 65, 281–293.
| Pivotal role of glutamine synthetase in ammonia detoxification.Crossref | GoogleScholarGoogle Scholar |
Han H, Zhou Y, Liu Q, Wang G, Feng J, Zhang M (2021) Effects of ammonia on gut microbiota and growth performance of broiler chickens. Animals 11,
| Effects of ammonia on gut microbiota and growth performance of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Herwig E, Abbott D, Schwean-Lardner KV, Classen HL (2019) Effect of rate and extent of starch digestion on broiler chicken performance. Poultry Science 98, 3676–3684.
| Effect of rate and extent of starch digestion on broiler chicken performance.Crossref | GoogleScholarGoogle Scholar |
Hilliar M, Huyen N, Girish CK, Barekatain R, Wu S, Swick RA (2019) Supplementing glycine, serine, and threonine in low protein diets for meat type chickens. Poultry Science 98, 6857–6865.
| Supplementing glycine, serine, and threonine in low protein diets for meat type chickens.Crossref | GoogleScholarGoogle Scholar |
Hilliar M, Hargreave G, Girish CK, Barekatain R, Wu S-B, Swick RA (2020) Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets. Poultry Science 99, 1551–1563.
| Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets.Crossref | GoogleScholarGoogle Scholar |
Hofmann P, Siegert W, Kenéz Á, Naranjo VD, Rodehutscord M (2019) Very low crude protein and varying glycine concentrations in the diet affect growth performance, characteristics of nitrogen excretion, and the blood metabolome of broiler chickens. The Journal of Nutrition 149, 1122–1132.
| Very low crude protein and varying glycine concentrations in the diet affect growth performance, characteristics of nitrogen excretion, and the blood metabolome of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Hyde R, Taylor PM, Hundal HS (2003) Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochemical Journal 373, 1–18.
| Amino acid transporters: roles in amino acid sensing and signalling in animal cells.Crossref | GoogleScholarGoogle Scholar |
Isshiki Y (1980) Nitrogenous components of cecal contents in fasted chickens. Japanese Journal of Zootechnical Science 51, 12–16.
Kang DR, Belal SA, Tian W, Park BY, Choe HS, Shim KS (2019) Effect of dietary gluten content on small intestinal inflammatory response of broilers. European Poultry Science 83
| Effect of dietary gluten content on small intestinal inflammatory response of broilers.Crossref | GoogleScholarGoogle Scholar |
Karasawa Y (1986) Ammonia production and its contribution to urinary nitrogenous compounds in chickens fed low or high protein diet. The Journal of Nutrition 116, 2378–2386.
| Ammonia production and its contribution to urinary nitrogenous compounds in chickens fed low or high protein diet.Crossref | GoogleScholarGoogle Scholar |
Karunaratne ND, Abbott DA, Hucl PJ, Chibbar RN, Pozniak CJ, Classen HL (2018) Starch digestibility and apparent metabolizable energy of western Canadian wheat market classes in broiler chickens. Poultry Science 97, 2818–2828.
| Starch digestibility and apparent metabolizable energy of western Canadian wheat market classes in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Kloiber O, Banjac B, Drewes LR (1988) Protection against acute hyperammonemia: the role of quaternary amines. Toxicology 49, 83–90.
| Protection against acute hyperammonemia: the role of quaternary amines.Crossref | GoogleScholarGoogle Scholar |
Knudsen KEB (1997) Carbohydrate and lignin contents of plant materials used in animal feeding. Animal Feed Science and Technology 67, 319–338.
| Carbohydrate and lignin contents of plant materials used in animal feeding.Crossref | GoogleScholarGoogle Scholar |
Lemme A, Fenske K, Westendarp H, Guhe M, Rother E (2022) Reduction of protein levels in broiler feed for commercial application – a German case. Proceedings, Arkansas Nutrition Conference 2022,
Liu SY, Selle PH (2015) A consideration of starch and protein digestive dynamics in chicken-meat production. World’s Poultry Science Journal 71, 297–310.
| A consideration of starch and protein digestive dynamics in chicken-meat production.Crossref | GoogleScholarGoogle Scholar |
Liu SY, Selle PH (2017) Starch and protein digestive dynamics in low-protein diets supplemented with crystalline amino acids. Animal Production Science 57, 2250–2256.
| Starch and protein digestive dynamics in low-protein diets supplemented with crystalline amino acids.Crossref | GoogleScholarGoogle Scholar |
Liu SY, Selle PH, Court SG, Cowieson AJ (2013) Protease supplementation of sorghum-based broiler diets enhances amino acid digestibility coefficients in four small intestinal sites and accelerates their rates of digestion. Animal Feed Science and Technology 183, 175–183.
| Protease supplementation of sorghum-based broiler diets enhances amino acid digestibility coefficients in four small intestinal sites and accelerates their rates of digestion.Crossref | GoogleScholarGoogle Scholar |
Macri A, Parlamenti R, Silano V, Valfre F (1977) Adaptation of the domestic chicken, Gallus Domesticus, to continuous feeding of albumin amylase inhibitors from wheat flour as gastro-resistant microgranules. Poultry Science 56, 434–441.
| Adaptation of the domestic chicken, Gallus Domesticus, to continuous feeding of albumin amylase inhibitors from wheat flour as gastro-resistant microgranules.Crossref | GoogleScholarGoogle Scholar |
Mapes JP, Krebs HA (1978) Rate-limiting factors in urate synthesis and gluconeogenesis in avian liver. Biochemical Journal 172, 193–203.
| Rate-limiting factors in urate synthesis and gluconeogenesis in avian liver.Crossref | GoogleScholarGoogle Scholar |
Maynard CW, Ghane A, Chrystal PV, Selle PH, Liu SY (2021) Sustaining live performance in broilers offered reduced crude protein diets based on corn and wheat blend. Animal Feed Science and Technology 276,
| Sustaining live performance in broilers offered reduced crude protein diets based on corn and wheat blend.Crossref | GoogleScholarGoogle Scholar |
Moss AF, Sydenham CJ, Khoddami A, Naranjo VD, Liu SY, Selle PH (2018) Dietary starch influences growth performance, nutrient utilisation and digestive dynamics of protein and amino acids in broiler chickens offered low-protein diets. Animal Feed Science and Technology 237, 55–67.
| Dietary starch influences growth performance, nutrient utilisation and digestive dynamics of protein and amino acids in broiler chickens offered low-protein diets.Crossref | GoogleScholarGoogle Scholar |
Moss AF, Chrystal PV, Dersjant-Li Y, Selle PH, Liu SY (2019) The influence of phytase, pre-pellet cracked maize and dietary crude protein level on broiler performance via response surface methodology. Journal of Animal Science and Biotechnology 10,
| The influence of phytase, pre-pellet cracked maize and dietary crude protein level on broiler performance via response surface methodology.Crossref | GoogleScholarGoogle Scholar |
Munyaka PM, Nandha NK, Kiarie E, Nyachoti CM, Khafipour E (2016) Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poultry Science 95, 528–540.
| Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets.Crossref | GoogleScholarGoogle Scholar |
Murer H, Sigrist-Nelson K, Hopfer U (1975) On the mechanism of sugar and amino acid interaction in intestinal transport. Journal of Biological Chemistry 250, 7392–7396.
| On the mechanism of sugar and amino acid interaction in intestinal transport.Crossref | GoogleScholarGoogle Scholar |
Namroud NF, Shivazad M, Zaghari M (2008) Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poultry Science 87, 2250–2258.
| Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks.Crossref | GoogleScholarGoogle Scholar |
Nolles JA, Verreijen AM, Koopmanschap RE, Verstegen MWA, Schreurs VVAM (2009) Postprandial oxidative losses of free and protein-bound amino acids in the diet: interactions and adaptation. Journal of Animal Physiology and Animal Nutrition 93, 431–438.
| Postprandial oxidative losses of free and protein-bound amino acids in the diet: interactions and adaptation.Crossref | GoogleScholarGoogle Scholar |
Okumura J-I, Tasaki I (1969) Effect of fasting, refeeding and dietary protein level on uric acid and ammonia content of blood, liver and kidney in chickens. The Journal of Nutrition 97, 316–320.
| Effect of fasting, refeeding and dietary protein level on uric acid and ammonia content of blood, liver and kidney in chickens.Crossref | GoogleScholarGoogle Scholar |
Ospina-Rojas IC, Murakami AE, Moreira I, Picoli KP, Rodrigueiro RJB, Furlan AC (2013) Dietary glycine+serine responses of male broilers given low-protein diets with different concentrations of threonine. British Poultry Science 54, 486–493.
| Dietary glycine+serine responses of male broilers given low-protein diets with different concentrations of threonine.Crossref | GoogleScholarGoogle Scholar |
Ospina-Rojas IC, Murakami AE, Duarte CRA, Eyng C, Oliveira CAL, Janeiro V (2014) Valine, isoleucine, arginine and glycine supplementation of low-protein diets for broiler chickens during the starter and grower phases. British Poultry Science 55, 766–773.
| Valine, isoleucine, arginine and glycine supplementation of low-protein diets for broiler chickens during the starter and grower phases.Crossref | GoogleScholarGoogle Scholar |
Prior RL, Topping DC, Visek WJ (1974) Metabolism of isolated chick small intestinal cells. Effects of ammonia and various salts. Biochemistry 13, 178–183.
| Metabolism of isolated chick small intestinal cells. Effects of ammonia and various salts.Crossref | GoogleScholarGoogle Scholar |
Rabie MH, Szilágyi M (1998) Effects of L-carnitine supplementation of diets differing in energy levels on performance, abdominal fat content, and yield and composition of edible meat of broilers. British Journal of Nutrition 80, 391–400.
| Effects of L-carnitine supplementation of diets differing in energy levels on performance, abdominal fat content, and yield and composition of edible meat of broilers.Crossref | GoogleScholarGoogle Scholar |
Rabie MH, Szilágyi M, Gippert T (1997a) Effects of dietary L-carnitine supplementation and protein level on performance and degree of meatness and fatness of broilers. Acta Biologica Hungarica 48, 221–239.
| Effects of dietary L-carnitine supplementation and protein level on performance and degree of meatness and fatness of broilers.Crossref | GoogleScholarGoogle Scholar |
Rabie MH, Szilágyi M, Gippert T, Votisky E, Gerendai D (1997b) Influence of dietary L-carnitine on performance and carcass quality of broiler chickens. Acta Biologica Hungarica 48, 241–252.
| Influence of dietary L-carnitine on performance and carcass quality of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Rebouche CJ (2004) Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism. Annals of the New York Academy of Sciences 1033, 30–41.
| Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism.Crossref | GoogleScholarGoogle Scholar |
Salway JG (2018) The Krebs uric acid cycle: a forgotten Krebs cycle. Trends in Biochemical Sciences 43, 847–849.
| The Krebs uric acid cycle: a forgotten Krebs cycle.Crossref | GoogleScholarGoogle Scholar |
Schreurs VVAM, Koopmanschap RE, Boekholt HA (1997) Short-term dynamics in protein and amino acid metabolism. Zeitschrift für Ernährungswissenschaft 36, 336–339.
| Short-term dynamics in protein and amino acid metabolism.Crossref | GoogleScholarGoogle Scholar |
Selle PH, de Paula Dorigam JC, Lemme A, Chrystal PV, Liu SY (2020) Synthetic and crystalline amino acids: alternatives to soybean meal in chicken-meat production. Animals 10,
| Synthetic and crystalline amino acids: alternatives to soybean meal in chicken-meat production.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Moss AF, Khoddami A, Chrystal PV, Liu SY (2021a) Starch digestion rates in multiple samples of commonly used feed grains in diets for broiler chickens. Animal Nutrition 7, 450–459.
| Starch digestion rates in multiple samples of commonly used feed grains in diets for broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Cantor DI, McQuade LR, McInerney BV, de Paula Dorigam JC, Macelline SP, Chrystal PV, Liu SY (2021b) Implications of excreta uric acid concentrations in broilers offered reduced crude protein diets and dietary glycine requirements for uric acid synthesis. Animal Nutrition 7, 939–946.
| Implications of excreta uric acid concentrations in broilers offered reduced crude protein diets and dietary glycine requirements for uric acid synthesis.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Macelline SP, Greenhalgh S, Chrystal PV, Liu SY (2022a) Identifying the shortfalls of crude protein-reduced, wheat-based broiler diets. Animal Nutrition 11, 181–189.
| Identifying the shortfalls of crude protein-reduced, wheat-based broiler diets.Crossref | GoogleScholarGoogle Scholar |
Selle PH, Macelline SP, Chrystal PV, Liu SY (2022b) The impact of digestive dynamics on the bioequivalence of amino acids in broiler chickens. Frontiers in Bioscience – Landmark 27,
| The impact of digestive dynamics on the bioequivalence of amino acids in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Shibata M, Takahashi T, Kozakai T, Kakudo M, Kasuga S, Azuma Y, Kurose Y (2019) Active transport of glucose across the jejunal epithelium decreases with age in broiler chickens. Poultry Science 98, 2570–2576.
| Active transport of glucose across the jejunal epithelium decreases with age in broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Stern RA, Mozdziak PE (2019) Differential ammonia metabolism and toxicity between avian and mammalian species, and effect of ammonia on skeletal muscle: a comparative review. Journal of Animal Physiology and Animal Nutrition 103, 774–785.
| Differential ammonia metabolism and toxicity between avian and mammalian species, and effect of ammonia on skeletal muscle: a comparative review.Crossref | GoogleScholarGoogle Scholar |
Stevens BR, Kaunitz JD, Wright EM (1984) Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annual Review of Physiology 46, 417–433.
| Intestinal transport of amino acids and sugars: advances using membrane vesicles.Crossref | GoogleScholarGoogle Scholar |
Sun M, Jiao H, Wang X, Uyanga VA, Zhao J, Lin H (2020) Encapsulated crystalline lysine and DL-methionine have higher efficiency than the crystalline form in broilers. Poultry Science 99, 6914–6924.
| Encapsulated crystalline lysine and DL-methionine have higher efficiency than the crystalline form in broilers.Crossref | GoogleScholarGoogle Scholar |
Swick RW (1982) Growth and protein turnover in animals. CRC Critical Reviews in Food Science and Nutrition 16, 117–126.
| Growth and protein turnover in animals.Crossref | GoogleScholarGoogle Scholar |
Sydenham CJ, Truong HH, Moss AF, Selle PH, Liu SY (2017) Fishmeal and maize starch inclusions in sorghum-soybean meal diets generate different responses in growth performance, nutrient utilisation, starch and protein digestive dynamics of broiler chickens. Animal Feed Science and Technology 227, 32–41.
| Fishmeal and maize starch inclusions in sorghum-soybean meal diets generate different responses in growth performance, nutrient utilisation, starch and protein digestive dynamics of broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Tesseraud S, Chagneau AM, Grizard J (2000) Muscle protein turnover during early development in chickens divergently selected for growth rate. Poultry Science 79, 1465–1471.
| Muscle protein turnover during early development in chickens divergently selected for growth rate.Crossref | GoogleScholarGoogle Scholar |
Truong HH, Yu S, Moss AF, Liu SY, Selle PH (2016) Phytate degradation in the gizzard is pivotal to phytase responses in broiler chickens. Proceedings, Australian Poultry Science Symposium 27, 174–177.
Van der Borght A, Goesaert H, Veraverbeke WS, Delcour JA (2005) Fractionation of wheat and wheat flour into starch and gluten: overview of the main processes and the factors involved. Journal of Cereal Science 41, 221–237.
| Fractionation of wheat and wheat flour into starch and gluten: overview of the main processes and the factors involved.Crossref | GoogleScholarGoogle Scholar |
van Milgen J (2021) The role of energy, serine, glycine, and 1-carbon units in the cost of nitrogen excretion in mammals and birds. Animal 15,
| The role of energy, serine, glycine, and 1-carbon units in the cost of nitrogen excretion in mammals and birds.Crossref | GoogleScholarGoogle Scholar |
Vinardell MP (1990) Mutual inhibition of sugars and amino acid intestinal absorption. Comparative Biochemistry and Physiology Part A: Physiology 95, 17–21.
| Mutual inhibition of sugars and amino acid intestinal absorption.Crossref | GoogleScholarGoogle Scholar |
Visek WJ (1978) The mode of growth promotion by antibiotics. Journal of Animal Science 46, 1447–1469.
| The mode of growth promotion by antibiotics.Crossref | GoogleScholarGoogle Scholar |
Visek WJ (1984) Ammonia: its effects on biological systems, metabolic hormones, and reproduction. Journal of Dairy Science 67, 481–498.
| Ammonia: its effects on biological systems, metabolic hormones, and reproduction.Crossref | GoogleScholarGoogle Scholar |
Wang J, Cao H, Bao C, Liu Y, Dong B, Wang C, Shang Z, Cao Y, Liu S (2021) Effects of xylanase in corn- or wheat-based diets on cecal microbiota of broilers. Frontiers in Microbiology 12,
| Effects of xylanase in corn- or wheat-based diets on cecal microbiota of broilers.Crossref | GoogleScholarGoogle Scholar |
Wilson RP, Muhrer ME, Bloomfield RA (1968) Comparative ammonia toxicity. Comparative Biochemistry and Physiology 25, 295–301.
| Comparative ammonia toxicity.Crossref | GoogleScholarGoogle Scholar |
Wright EM (1993) The intestinal Na+/glucose cotransporter. Annual Review of Physiology 55, 575–589.
| The intestinal Na+/glucose cotransporter.Crossref | GoogleScholarGoogle Scholar |
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17.
| Amino acids: metabolism, functions, and nutrition.Crossref | GoogleScholarGoogle Scholar |
Xu ZR, Wang MQ, Mao HX, Zhan XA, Hu CH (2003) Effects of L-carnitine on growth performance, carcass composition, and metabolism of lipids in male broilers. Poultry Science 82, 408–413.
| Effects of L-carnitine on growth performance, carcass composition, and metabolism of lipids in male broilers.Crossref | GoogleScholarGoogle Scholar |
Yin D, Chrystal PV, Moss AF, Yun Liu S, Yuan J, Selle PH (2020) Effects of reducing dietary crude protein and whole grain feeding on performance and amino acid metabolism in broiler chickens offered wheat-based diets. Animal Feed Science and Technology 260,
| Effects of reducing dietary crude protein and whole grain feeding on performance and amino acid metabolism in broiler chickens offered wheat-based diets.Crossref | GoogleScholarGoogle Scholar |
Yutaka K, Chitose N (1986) Ammonia absorption from different parts of chicken intestine and its quantitative evaluation in situ. Comparative Biochemistry and Physiology Part A: Physiology 84, 747–750.
| Ammonia absorption from different parts of chicken intestine and its quantitative evaluation in situ.Crossref | GoogleScholarGoogle Scholar |
Zamani M, Zaghari M, Ghaziani F (2021) Comparison of absorption kinetics and utilisation of DL-methionine (DL-Met), Met-Met product (AQUAVI® Met-Met), and protein-bound methionine (PB-Met) by female broiler chickens. British Poultry Science 62, 539–551.
| Comparison of absorption kinetics and utilisation of DL-methionine (DL-Met), Met-Met product (AQUAVI® Met-Met), and protein-bound methionine (PB-Met) by female broiler chickens.Crossref | GoogleScholarGoogle Scholar |
Zwarycz B, Wong EA (2013) Expression of the peptide transporters PepT1, PepT2, and PHT1 in the embryonic and posthatch chick. Poultry Science 92, 1314–1321.
| Expression of the peptide transporters PepT1, PepT2, and PHT1 in the embryonic and posthatch chick.Crossref | GoogleScholarGoogle Scholar |


