Roadkill mitigation is paved with good intentions: a critique of Fox et al. (2019)
Graeme Coulson A C and Helena Bender BA School of BioSciences, The University of Melbourne, Vic 3010, Australia.
B School of Ecosystem and Forest Science, The University of Melbourne, Vic 3010, Australia.
C Corresponding author. Email: gcoulson@unimelb.edu.au
Australian Mammalogy 42(1) 122-130 https://doi.org/10.1071/AM19009
Submitted: 14 February 2019 Accepted: 19 April 2019 Published: 27 June 2019
Abstract
In a recent publication, Fox et al. (2019) described a three-year trial of a ‘virtual fence’ installed to reduce wildlife roadkills in north-eastern Tasmania. The authors reported a 50% reduction in total roadkills, concluding that the ‘virtual fence’ had the potential to substantially reduce roadkill rates. The field of roadkill mitigation has a long history of promising techniques that are ultimately found wanting, so we evaluated the conceptual basis of the ‘virtual fence’ and the design and analysis of the trial. Of the two stimuli emitted by the ‘virtual fence’, its lights only partly match the sensory capabilities of the target species, its sound frequency is suitable but the intensity is unknown, and both stimuli are artificial and lack biological significance, so will be prone to habituation once novelty wanes. The trial, conducted in three phases, revealed a total of eight methodological flaws ranging from imprecise measurements, confounding effects of treatments, low statistical power, violation of test assumptions and failure to consider habituation. Greater caution is needed in interpreting the findings of this study, and well designed, long-term trials are required to properly assess the ‘virtual fence’.
Additional keywords: colour vision, natural sound, Notamacropus rufogriseus, Thylogale billardierii, ultrasound, wildlife warning reflector, wildlife warning whistle.
Introduction
Fox et al. (2019) reported the results of a three-year trial of a ‘virtual fence’ installed in northern Tasmania to reduce roadkill of mammals. The authors concluded that the ‘virtual fence’ has ‘enormous potential to substantially reduce roadkill’, and advocated a ‘roll-out of these devices at other identified hotspots in Tasmania’. Unfortunately, the pathway to roadkill mitigation is littered with the remains of promising techniques, each promoted enthusiastically at first. Evaluation of these techniques has too often been hampered by poorly designed studies, but many new techniques have ultimately been found to be ineffective (D’Angelo and van der Ree 2015; Rytwinski et al. 2016; Benten et al. 2018b). Considerable caution is therefore essential when evaluating any new technique such as the ‘virtual fence’ tested in Tasmania.
In our judgement, the trial of the ‘virtual fence’ conducted by Fox et al. (2019) has serious shortcomings: its conceptual basis is unsound, the study design and data analysis are deficient, and the conclusions lack sufficient caution. We consider each of these components to generate a measured assessment of the findings and their implications for roadkill mitigation.
The ‘virtual fence’ concept
According to Fox et al. (2019), ‘virtual fence’ technology was developed in Austria to curb roadkills of ungulates. Following a five-year trial in Austria, which purportedly reduced roadkills by 80–90%, the technology was launched across Europe in 2003. Surprisingly, Fox et al. cite only a personal communication as the source of this background information. Furthermore, we could find no scientific evaluations of this technology 15 years since its European launch, using either the University of Melbourne Library databases or Google Scholar. The only data we could find were summarised in a Masters of Science internship report by de Vries (2015). She presented the results of a study of roadkills of deer on six Austrian roads for 3 years before and 5 years after a ‘virtual fence’ trial began; the average reduction in roadkills was 93.6%, but neither the deer species, nor the location and lengths of the roads, were specified. She attributed these data to Moser (2007), whose work was published in German in OÖ Jäger, the magazine of a provincial Austrian hunting association, rather than a peer-reviewed scientific journal. This lack of robust, independent and accessible evaluation of a new technology is unusual and concerning. In the absence of such data, we examine what is known about ‘virtual fence’ technology and draw on the sensory and behavioural biology of the target species to predict the effectiveness of this technology for roadkill mitigation.
The ‘virtual fence’ is described as an electronic system that generates sound and light stimuli when activated by the headlights of approaching vehicles. According to Fox et al. (2019), the ‘virtual fence’ unit had strobing LEDs, which emit blue and amber light, and two sound settings, one for rural areas and a higher frequency for residential areas. In the sections that follow, we examine what scientific trials have been completed for each sensory modality in turn, and then in combination. We synthesise what is known for deer (Cervidae), which have been more widely studied, then follow with what is known for marsupials.
Effectiveness of light stimuli
The ‘virtual fence’, as tested by Fox et al. (2019), consists of an array of units arranged alternately along both sides of the road. Each unit is activated in turn by the lights of approaching vehicles; the units are aligned so that the light they emit is directed away from the road surface and towards the roadside verge (Fox et al. 2019). As such, the ‘virtual fence’ has many similarities to wildlife warning reflectors, which were first developed in the 1950s to deter animals from entering roads (Brieger et al. 2016). Wildlife warning reflectors are now available in a range of designs produced by several manufacturers (Benten et al. 2018a). In the absence of formal tests of the ‘virtual fence’, we first draw on studies of these reflectors.
Two recent, comprehensive reviews of wildlife warning reflectors concluded that reflectors were not effective in reducing roadkill (Brieger et al. 2016; Benten et al. 2018a, 2018b). This conclusion was based on 53 and 76 studies (respectively) of a variety of reflectors, predominantly from Europe and North America, but including some in Australia. Both reviews drew attention to weak experimental design, noting that few studies used a Before–After–Control–Impact (BACI) design to allow for both temporal and spatial variation in roadkills.
The ineffectiveness of reflectors can be attributed to three phenomena: the target species may be unable to perceive the stimulus, may perceive it but respond inappropriately, or may perceive it but then habituate to it (D’Angelo and van der Ree 2015). All mammals have two classes of visual receptors: rods, which function in dim light, and cones, which operate in daylight and provide the potential for colour vision (Hunt et al. 2009). The rods of deer have a peak sensitivity of 497 nm (Jacobs et al. 1994; VerCauteren and Pipas 2003), equivalent to a blue-green colour. Daylight vision in deer is dichromatic, with short-wavelength cone receptors of peak sensitivity at ~455 nm and medium/long-wavelength cones peaking at ~540 nm (Jacobs et al. 1994, VerCauteren and Pipas 2003; Cohen et al. 2014), corresponding to blue and green light respectively. Colour sensitivity thus provides an explanation for the ineffectiveness of wildlife warning reflectors that reflect red light, such as Swareflex (e.g. Waring et al. 1991; Reeve and Anderson 1993): deer simply cannot detect long-wavelength light. However, devices that reflected other wavelengths, including blue-green and white (full spectrum), were no more effective than red reflectors in changing the behaviour of white-tailed deer (Odocoileus virginianus) on roadsides; instead, the presence of reflectors of any colour increased the level of inappropriate responses, such as movement towards the road, and blue-green reflectors generated the most inappropriate responses (D’Angelo et al. 2006).
The absence of any response, or the occurrence of an inappropriate response, suggests that the artificial stimuli produced by wildlife warning reflectors lack biological significance, because they are not intrinsically associated with any threat to deer (Schakner and Blumstein 2013). For example, white-tailed deer perceived powerful green and blue lasers, but were merely curious about the lights played on vegetation and on their heads, and did not flee the trial area (VerCauteren et al. 2006). Another common problem in applied behaviour is habituation: deterrents soon become ineffective because animals learn to ignore artificial stimuli that are not associated with positive or negative reinforcement (Shivik et al. 2003; Blumstein 2016). As would be expected, deer habituate to wildlife warning reflectors following repeated exposure. For example, fallow deer (Dama dama) initially gave alarm and flight responses when exposed to reflectors, but showed increasing indifference to the stimulus over subsequent nights (Uvjári et al. 1998). Further illustrating the power of novelty, experimental white canvas covers placed over reflectors, which had been on the roadside for up to six years, evoked stronger responses by white-tailed deer than did uncovered reflectors (Riginos et al. 2018).
Less is known about the visual system of marsupials, or their response to wildlife warning reflectors. Studies of the tammar wallaby (Notamacropus eugenii) have shown that this macropodid marsupial has typical mammalian rods with peak sensitivity at 501 nm, corresponding to blue-green light (Hemmi et al. 2000). The tammar wallaby also has dichromatic vision, with cones sensitive to only short and medium wavelengths, corresponding to blue and green colours, respectively (Hemmi 1999; Hemmi et al. 2000; Deeb et al. 2003). This species shows a corresponding surge in melatonin production when exposed to short-wavelength light, but no physiological response to amber (Dimovski and Robert 2018). Unexpectedly, there is evidence of trichromatic vision in another macropodid, the quokka (Setonix brachyurus), which has a third set of cones sensitive to longer wavelengths (~540 nm) in the green band (Arrese et al. 2005). Trichromacy has also been confirmed in three other marsupial taxa: a peramelid (Isoodon obesulus), a dasyurid (Sminthopsis crassicaudata), and the honey possum (Tarsipes rostratus) (Hunt et al. 2009; Ebeling et al. 2010). However, none of these diverse taxa have rod or cone pigments in the red band (Beazeley et al. 2010) so marsupials are similar to deer in that respect.
Insensitivity to red light would account for the lack of any effect of red Swareflex reflectors on roadkills of marsupials when tested in a BACI study at four sites in Victoria (Aspinall 1994). The species involved in that study were predominantly the eastern grey kangaroo (Macropus giganteus), the swamp wallaby (Wallabia bicolor), the common wombat (Vombatus ursinus) and the koala (Phascolarctos cinereus). Similarly, captive red kangaroos (Osphranter rufus) and red-necked wallabies (Notamacropus rufogriseus) showed little or no change in vigilance, or flight, in response to either red Streiter-Lite or Swareflex reflectors along a simulated roadway (Ramp and Croft 2006). However, there was also no appropriate response by either species to white versions of these reflectors (Ramp and Croft 2006).
There have been no equivalent studies of spectral sensitivity in any species of Tasmanian marsupial. Nocturnal marsupials typically have retinas that are dominated by rods, with peak sensitivity of 502–509 nm (Beazeley et al. 2010). Predicting the sensitivity of cones is less certain because of the complex phylogenetic distribution of dichromatic and trichomatic vision (Hunt et al. 2009). Nonetheless, none of the marsupial species known to be trichromatic is sensitive to wavelengths above 557 nm, which is still in the green band (Arrese et al. 2005). The wavelength of the amber light (591 nm) produced by the ‘virtual fence’ (Fox et al. 2019) is well above this sensitivity peak, so it is unlikely that any marsupial could detect amber. In contrast, the royal blue light (470 nm) produced by the ‘virtual fence’ (Fox et al. 2019) is a reasonable match to the peak sensitivity of short-wavelength cones in both dichromatic and trichromatic marsupials, suggesting that all the Tasmanian species would be able to detect this light if it were bright enough to activate their cones.
One feature of the ‘virtual fence’ is that it actively emits light in reponse to a vehicle’s lights, rather than reflecting the vehicles’ light as wildlife warning reflectors do. Typical wildlife warning reflectors reflect <0.1 lx at a distance of 2 m when illuminated by vehicle headlights, which is less than the intensity of a full moon on a clear night (Sielecki 2001). The ‘virtual fence’ has the potential to generate a brighter light stimulus than do wildlife warning reflectors, so perhaps elicit a stronger response. Unfortunately, Fox et al. (2019) did not give specifications for the output intensity of the ‘virtual fence’ LEDs, or for other components of the stimulus such as strobe frequency, duration and interval. However, given the findings for deer worldwide and marsupials on the Australian mainland, it remains unclear why the target species might respond to the blue light emitted by the ‘virtual fence’ in a way that is both appropriate and not prone to habituation. Studies of road-crossing behaviour, using thermal imaging (e.g. D’Angelo et al. 2006; Ramp and Croft 2006; Riginos et al. 2018), would be needed to determine the character and persistence of responses to the ‘virtual fence’.
Effectiveness of acoustic stimuli
The use of sound has a long history in efforts to deter wildlife from roadways and other places (e.g. crops), with many parallels to wildlife warning reflectors. Reviews of acoustic deterrents have concluded that studies of such devices typically have poor experimental design, and there is little evidence of effectiveness (Bomford and O’Brien 1990; D’Angelo and van der Ree 2015). As with wildlife warning reflectors, acoustic stimuli may be ineffective for three reasons: target species may not be able to perceive the stimulus, may perceive it but not respond appropriately, or may habituate to the stimulus (D’Angelo and van der Ree 2015).
Much of the research on acoustic deterrence from roads has been conducted on deer, and much of that has been on several brands of wildlife warning whistles. Wildlife warning whistles were first invented in Austria in the 1970s, and have been marketed (under several brands) to deter deer from roads in Europe and North America (Romin and Dalton 1992). The sound output of whistles is purported to be in the ultrasonic range (>20 kHz), undetectable by the human ear (Scheifele et al. 2003). These claims led to tests of the hearing range of deer. For example, the hearing of white-tailed deer was found to be most sensitive from 4 kHz to 8 kHz, well below ultrasonic levels (D’Angelo et al. 2006). However, only three of six different brands tested by Scheifele et al. (2003) actually produced ultrasound, with a dominant frequency of only 12 kHz; the other three had a much lower dominant frequency of 3.3 kHz. More importantly, none of the six could be detected above vehicle noise (Scheifele et al. 2003). Unsurprisingly, free-ranging mule deer (Odocoilieus hemionus) did not alter their behaviour in response to whistles fitted to a moving vehicle (Romin and Dalton 1992). However, even a range of frequencies produced by a pure-tone generator, each calibrated to 25 dB above the sound of a moving vehicle, did not elicit appropriate road-crossing behaviour in white-tailed deer (Valitzski et al. 2009): deer were more likely to enter the road in response to the lowest frequency (0.28 kHz), and showed no behavioural change for other frequencies.
Taken together, these findings indicate that artificial, tonal sounds, even if detectable by deer, are not effective for reducing roadkill. As described for artificial light stimuli in the previous section, tonal sounds may elicit no response at all, or elicit an inappropriate response, such as entering the roadway (e.g. Valitzski et al. 2009). Habituation to tonal sound is also evident: fallow deer exhibited a moderate level of alarm and flight from the roadside in response to low-frequency tones (5–14 kHz), followed by habituation after five nights (Ujvári et al. 2004). In contrast, broadcasting a sequence of natural sounds (e.g. alarm calls and wolf howls) reduced reaction time and increased flight frequency of roe deer (Capreolus capreolus) when a train approached; moreover, the magnitude of these responses did not decline over the five years of the study, suggesting that the natural sounds were resistant to habituation (Babińska-Werka et al. 2015).
Fox et al. (2019) described the ‘virtual fence’ unit as having two frequency settings: 3.5–6.5 kHz for rural areas, and a higher frequency range (7–13 kHz) for residential areas. Fox et al. did not specify which setting was used in their trial of the ‘virtual fence’. However, behavioural and electrophysiological studies of the auditory system of Australian marsupial taxa (Aitkin 1995; Aitkin and Shepherd 2010) suggest that the Tasmanian species would be sensitive to sound produced at both settings, but more so for the higher setting. On the basis of brainstem responses, the tammar wallaby showed highest neural sensitivity in the 8–16 kHz range (Cone-Wesson et al. 1997), while the pinnae provided greatest amplification at lower frequencies, with maximum gain at 5 kHz (Coles and Guppy 1986). Brainstem responses of the northern quoll (Dasyurus hallucatus), a dasyurid, were triggered over a wide frequency range of 0.5–40 kHz, but were most sensitive at 8 kHz (Aitkin et al. 1994). The common brushtail possum (Trichosurus vulpecula), a phalangerid that occurs in Tasmania, had more sensitive brainstem responses than the quoll’s overall, and was most sensitive at a higher frequency, 17–19 kHz (Gates and Aitkin 1982). Behavioural assays of possums showed that hearing sensitivity increased from 2 to 15 kHz, and was sustained from 20 to 35 kHz (Osugi et al. 2011).
Although sensitivity tests indicate the potential to detect a stimulus, sound intensity determines whether the target species can actually perceive a stimulus over a distance and above background noise. For example, the signal generated by the Shu-Roo, an electronic vehicle-mounted device, was not detectable above the noise produced by any of four different types of vehicles when they were moving (Bender 2001). Unfortunately, Fox et al. (2019) did not specify the intensity of the signal produced by the ‘virtual fence’ device, or provide any other specifications for its signal characteristics. They also did not provide any data on traffic volume or speed over time (see Ramp et al. 2016). Traffic noise comes mainly from the tyres and engine, and is dependent on the number of vehicles passing, the mix of vehicle types, their speed, and the road surface gradient (Department of State Growth 2015).
In the absence of any other information, we assume that the signal produced by the ‘virtual fence’ unit is artificial rather than a replica of some natural sound. Artificial sounds designed as deterrents have been tested in several Australian marsupials, and have consistently been shown to be ineffective. One such device, the Roo-Guard, did not change the behaviour of tammar wallabies (Muirhead et al. 2006), eastern grey kangaroos (Bender 2003) or red kangaroos (Bender 2003). Similarly, the behaviour of eastern grey and red kangaroos did not change in response to the Shu-Roo (Bender 2001). In the only Australian test of a wildlife warning whistle, the Hobi ‘Ultrasonic Animal Alert’ had no detectable effect on behaviour and did not change the total rate of wildlife roadkill in northern Tasmania (Magnus et al. 2004). In contrast, several studies have shown appropriate responses to natural sounds. Playback of foot-thumps, which are given by macropods in predation contexts (Bender 2006; Rose et al. 2006), increased the vigilance of tammar wallabies (Blumstein et al. 2000), red-necked wallabies (Ramp et al. 2011), red-necked pademelons (Thylogale thetis) (Blumstein et al. 2002; Ramp et al. 2011), eastern grey kangaroos (Bender 2005) and western grey kangaroos (Macropus fuliginosus) (Biedenweg et al. 2011). Other natural acoustic stimuli, such as dingo howls, bird alarm calls and conspecific distress calls, affected behaviour in some studies (Blumstein et al. 2002; Ramp et al. 2011) but not in others (Blumstein et al. 2000; Biedenweg et al. 2011). Like deer, marsupials appear to be more likely to respond innately to natural rather than artificial sounds, particularly those signifying predation risk (Blumstein et al. 2000, 2002). If the sound emitted by the ‘virtual fence’ is artificial, then it is less likely to produce an appropriate response.
Combining acoustic and visual stimuli
The ‘virtual fence’ produces both acoustic and visual stimuli (Fox et al. 2019). In their comprehensive review of roadkill mitigation in Europe, Langbein et al. (2011) identified several products that produce combinations of sound and light stimuli (e.g. WEGU-GFT acoustic wildlife warning reflector, Eurocontor Ecopollars and WIWASOL-II) intended to deter deer and other ungulates from roads. The underlying assumption is that mixing signal modalities will increase the response rate or reduce the likelihood of habituation by the target species. The efficacy of such devices was incisively appraised by Langbein et al. (2011): ‘As has often been the case regarding optical reflectors and other wildlife deterrents in the past, various preliminary findings reported in the hunting press or other general media claim good results with such devices in reduction of ungulate collisions, mostly during the first one or two years after installation. However, firm evidence for lasting effects remains lacking in the published scientific literature and results of trials undertaken in differing countries or situations … remain contradictory’. We contend that the same caveats apply to the ‘virtual fence’ tested by Fox et al. (2019).
Study design and analysis
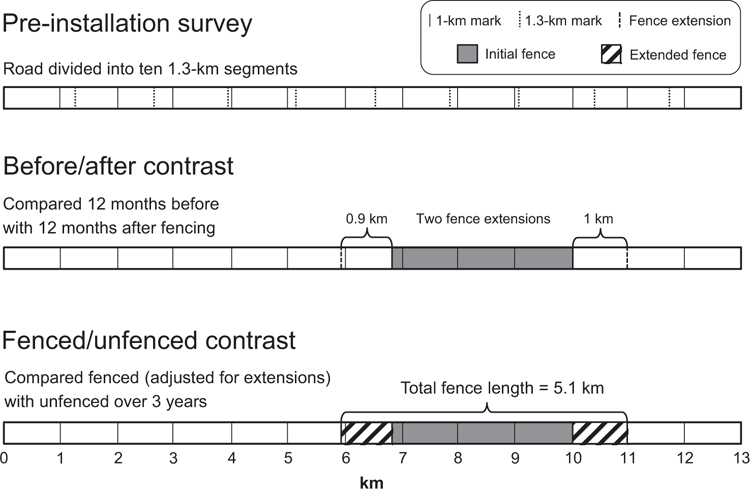
Fox et al. (2019) conducted their study on a 13-km stretch of rural road from Arthur River to Marrawah (Route C214) in north-west Tasmania. The study took place over three phases: a preinstallation survey, a before/after contrast of ‘virtual fence’ extensions, and a longer contrast of ‘virtual fenced’ and unfenced sections of road (Fig. 1).
|
Preinstallation survey
Prior to installation of the ‘virtual fence’, roadkill data were collected each day for four months (October 2013 to January 2014). The data recorded were the distance (in kilometres) of each roadkill from the starting point of the stretch of road, and the species of each roadkill where possible. Carcasses were then removed from the road to prevent double counting. The authors used this initial dataset to examine the distribution of roadkills along the stretch of road, in order to determine whether the planned location of the ‘virtual fence’ had a noticeably different roadkill rate than the rest of the road. The authors divided the road into 10 1.3-km segments and used a goodness-of-fit Chi-square test to compare the observed frequency of roadkills (of all species) per segment with a uniform expected distribution; the null hypothesis of uniformity was accepted (P = 0.45). Three flaws are evident in this first step of the design and analysis: (1) the data were collected at a linear resolution of 1 km, using a vehicle odometer; (2) the preinstallation survey was conducted over only a four-month period, in spring and summer; and (3) the statistical power of the goodness-of-fit test to a uniform distribution of roadkill was low, given the small sample size. We deal with each of these in turn.
The low resolution (1 km) for the locations of roadkill records did not match the 10 1.3-km road segments before installation of the ‘virtual fence’. This would give a 300-m difference between the end of the first kilometre, as measured with the vehicle odometer, and the end of the first 1.3-km segment. The ends of the second kilometre on the odometer and the second 1.3-km segment would then be 600 m apart, and so on. We cannot envisage how each roadkill record could have been allocated to the 10 segments without substantial error.
The preinstallation survey sampled only one portion of one year, so the extent to which these data represented the true ‘background’ level of roadkills, as claimed by the authors, was unknown. Annual, seasonal and lunar variation in roadkill rate is observed around the world (Farmer and Brooks 2012; Steiner et al. 2014; Seo et al. 2015; Canova and Balestrieri 2019), including Australia (e.g. Coulson 1982, 1989; Taylor and Goldingay 2004). In Tasmania, Hobday and Minstrell (2008) reported strong temporal variation in roadkills, although the most common species (rufous-bellied pademelon, Thylogale billardierii, and Bennett’s wallaby, Notamacropus rufogriseus) recorded by Fox et al. did not show a statewide seasonal pattern, despite strong seasonality of breeding (Rose and McCatney 1982; Curlewis 1989).
The small sample size of roadkills (n = 54) and the large number of categories (ten 1.3-km road segments) used in this analysis would have resulted in low statistical power. The power of standard goodness-of-fit tests is typically low: even samples of 200 would achieve a power value of only 0.8 for an effect size of 60% over 2–4 categories (Watkins and Di Stefano 2013). Fox et al. thus risked committing a Type II error, failing to reject a false null hypothesis because their test lacked the power to detect real differences in roadkill rates along the stretch of road. A partial solution might have been to reduce the road categories to two (unfenced and later ‘virtual fenced’) and adjust the expected frequencies by the total length of each category. Unfortunately, the data were not presented in a form that allowed us to conduct that analysis.
Before/after contrast
The ‘virtual fence’ was installed along a 3.2-km stretch of the road in February 2014 and daily surveys continued as earlier. Nine months later (November 2015), the ‘virtual fence’ was extended by a total of 1.9 km, 0.9 km at one end and 1.0 km at the other, increasing the virtual fence length to 5.1 km. Fox et al. calculated the monthly roadkill rate within this 1.9-km extension over the 12 months before the ‘virtual fence’ was extended, then compared it with the corresponding rate for 12 months after the extension, using a paired t-test. Fox et al. did not present mean monthly data, but reported raw totals of 28 roadkills before the extension and 8 roadkills after, and rejected the null hypothesis of no difference (P = 0.04). This second step of the design and analysis has one flaw identified earlier and three additional flaws: (1) the data were collected at a linear resolution of 1 km as before; (4) the monthly replicates were not independent; (5) the ends of the existing ‘virtual fence’ were not considered; and (6) the before and after data were collated over only 12-month periods. We expand on these in turn.
The 1-km resolution of roadkill records did not match the northern end of either the initial installation of the ‘virtual fence’, beginning at the 6.8-km mark, or extension to the ‘virtual fence’, beginning at the 5.9-km mark. As previously, we cannot envisage how each roadkill record could have been allocated to portions of the road that were subsequently ‘virtual fenced’, or continued to be unfenced, without error at the northern end.
A key assumption of a t-test is that observations are independent of each other (Zar 1974). However, the monthly roadkill data in this analysis were not independent: an animal killed in one month was simply unavailable to be killed in another month. It could be assumed that the individuals of each species were drawn from very large populations, so that deaths of individuals were effectively independent events. However, Fox et al. did not stipulate this assumption, and without data on population density and turnover for each species, the validity of the assumption cannot be assessed.
Roadkill data used in the before/after comparison were collected near each end of the initial ‘virtual fence’. This design raises the ‘fence-end’ issue, which confounds interpretation of the effectiveness of wildlife fencing (Rytwinski et al. 2016). If a fence of any type forms an effective barrier to movement, animals may attempt to cross the road at the end of the fence, resulting in a concentration of roadkill. This exaggerates the disparity between fenced and unfenced roadkill rates, so the benefit of the fence becomes overestimated. The ‘fence-end’ issue is best known from studies of physical fences to exclude deer from highways (e.g. Feldhamer et al. 1986; Parker et al. 2008). However, an experimental study of rufous-bellied pademelons and Bennett’s wallabies showed that both species shifted their home range centres and changed their habitat in response to the erection of a physical macropod-proof fence (Wiggins et al. 2010), so the ‘fence-end’ problem could apply to the study by Fox et al. (2019). Assuming that the ‘virtual fence’ had some effect in their study, as expected for a novel stimulus, the roadkill rate in the unfenced section before the ‘virtual fence’ extension may have been inflated, so the reduction in roadkill rate in the fenced section after the ‘virtual fence’ extension appeared more marked than in reality.
Fox et al. compared the 12-month period before the ‘virtual fence’ extension with the corresponding 12-months after as a way of accounting for monthly variation. While this approach made some allowance for potential seasonal effects, it could not allow for any variation between years. Given that a larger monthly dataset was available, it is unclear why the authors did not use more of their data from the beginning of the study before the ‘virtual fence’ extension (October 2013 to October 2015) and contrast it with matching monthly data collected up to the end of the study in March 2017.
Fenced/unfenced contrast
Fox et al. (2019) continued to record roadkill data as earlier until March 2017, three years after the initial installation of the ‘virtual fence’. The authors then compared the monthly roadkill rates in the ‘virtual fenced’ section of road with the two unfenced sections of road (pooled), using a paired t-test. Roadkill rate was expressed per kilometre of road, adjusted for the different lengths of road before and after the ‘virtual fence’ extensions were installed. The null hypothesis of no difference was rejected for all species combined (P = 0.0001), and for rufous-bellied pademelons (P = 0.0001) and Bennett’s wallabies (P = 0.013) separately; mean monthly roadkill rates were lower in the ‘virtual fenced’ section than in the unfenced sections in each comparison. This third step of the design and analysis had three flaws identified earlier: (1) the data were collected at a linear resolution of 1 km; (4) the monthly replicates were not independent; and (5) the ends of the existing virtual fence were not considered. The third step also had two additional flaws: (7) measures of sampling variation were not presented; and (8) possible habituation to the stimuli was not addressed. We consider these flaws in turn.
As previously, there was a mismatch between the 1-km resolution of roadkill records and the northern ends of the ‘virtual fence’ as initially installed (6.8-km mark) and later extended (5.9-km mark). Presumably, the boundaries between ‘virtual fenced’ and unfenced would have been apparent once the ‘virtual fence’ units were installed, so roadkills could be confidently allocated to their appropriate categories. However, Fox et al. never specified precisely what they considered to be the fenced segment of road: was it from the most northerly unit to the most southerly, or did it perhaps include a zone of influence extending 12.5 m beyond the last unit (see Fox et al. 2019, fig. 2)?
Use of monthly roadkill data in this analysis violated the t-test’s assumption of independent observations (Zar 2010). As pointed out above, an animal killed in one month logically cannot be killed in another. The data presented for rufous-bellied pademelons by Fox et al. (2019, fig. 3) illustrate the likely strength of this effect: marked seasonal peaks in roadkills occurred in summer and early autumn each year, potentially depleting the local population. An experimental cull of rufous-bellied pademelons and Bennett’s wallabies showed that surviving individuals of both species shifted their home range centres and changed their habitat within a month (Wiggins et al. 2010), but it is unlikely that these fine-scale movements would rapidly restore the population of susceptible individuals near the road.
Roadkill data were collected for 9 months at each end of the initial ‘virtual fence’, then collected for another 27 months at the ends of the extension to the ‘virtual fence’. This sampling regime was thus at risk of a double ‘fence-end’ problem (Rytwinski et al. 2016). If the ‘virtual fence’ formed even a partial barrier to movement, roadkills may have been concentrated beyond the ends of the initial ‘virtual fence’, followed by a second set of roadkills concentrated beyond the extended ‘virtual fence’. As previously, this effect would inflate the roadkill rate along unfenced stretches of road, falsely reducing the apparent roadkill rate within the ‘virtual fence’.
Fox et al. (2019) did not present any measures of sampling variation in their estimates of roadkill rates. Without data on standard parameters such as minima and maxima, range, standard deviation, standard error or coefficient of variation, it is impossible to appreciate the variability in the data or the implications of the patterns being described. For the Tasmanian devil (Sarcophilus harissii), for example, Fox et al. reported monthly roadkill rates of 0.035 km–1 within the ‘virtual fence’ and 0.041 km–1 in the unfenced area, and argued that these data ‘suggest that the ‘virtual fence’ reduced the number of devils hit on the road’. This is a specious argument because of the inherent variability in these data from only 19 incidents spread over 36 months and split between two treatment groups.
Fox et al. (2019) did not give any consideration to habituation. This is a pervasive problem in the entire field of wildlife deterrence (Shivik et al. 2003; Blumstein 2016), including roadkill mitigation. Indeed, the occurrence of any wildlife on roadsides, despite the loud noises and bright lights produced by passing vehicles, illustrates the strength of this phenomenon (e.g. Ben-Ami and Ramp 2013, Goldingay et al. 2018). Inspection of the monthly roadkill rates recorded by Fox et al. (2019, fig. 2) suggests that the rates in the ‘virtual fenced’ and ‘unfenced’ sectors have essentially converged by the third year of the trial, but formal analysis would be required to determine the role of time as a factor.
Conclusion
Discussing their findings, Fox et al. (2019) stated that ‘this study clearly demonstrates that virtual fence technology shows great potential in reducing roadkill rates’. We strongly disagree. Rather, we argue that ‘virtual fence’ technology follows a familiar path in roadkill mitigation, which too often leads to failure. The technology is based on misconceptions about wildlife behaviour, which apply equally to marsupials in Tasmania. A clear evaluation of the effectiveness of the ‘virtual fence’ is simply not possible, due to the many flaws in the design and analysis of this study.
Fox et al. (2019) concluded their paper with ‘The obvious reduction in roadkill in this trial of virtual fence technology is encouraging and should support roll-out of these devices at other identified roadkill hotspots in Tasmania, which, with concurrent monitoring, may be used to demonstrate the widespread applicability of the device in reducing roadkill state-wide’. Again, we disagree. At best, this trial should be considered a pilot study, which has generated data on roadkill rates, albeit with poor spatial resolution and no measure of sampling variation. These data could make a useful contribution to the design of future trials, with acceptable statistical power, for a robust assessment of the ‘virtual fence’. We concur with Rytwinski et al. (2015), who outlined strong design principles for evaluating road mitigation measures, while also acknowledging that ideal designs may be difficult to achieve in practice. Nonetheless, a higher degree of experimental rigor is required to properly evaluate the ‘virtual fence’, and far more caution is required before this technology is ever applied on a broader scale.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
Aitkin, L. (1995). The auditory neurobiology of marsupials: a review. Hearing Research 82, 257–266.| The auditory neurobiology of marsupials: a review.Crossref | GoogleScholarGoogle Scholar | 7775290PubMed |
Aitkin, L. M., and Shepherd, R. K. (2010). Auditory system. In ‘The Neurobiology of Australian Marsupials: Brain Evolution in the Other Mammalian Radiation’. (Ed. K. Ashwell.) pp. 184–191. (Cambridge University Press: Cambridge.)
Aitkin, L. M., Nelson, J. E., and Shepherd, R. K. (1994). Hearing, vocalization and the external ear of a marsupial, the northern quoll, Dasyurus hallucatus. The Journal of Comparative Neurology 349, 377–388.
| Hearing, vocalization and the external ear of a marsupial, the northern quoll, Dasyurus hallucatus.Crossref | GoogleScholarGoogle Scholar | 7852631PubMed |
Arrese, C. A., Oddy, A. Y., Runham, P. B., Hart, N. S., Shand, J., Hunt, D. M., and Beazley, L. D. (2005). Cone topography and spectral sensitivity in two potentially trichromatic marsupials, the quokka (Setonix brachyurus) and quenda (Isoodon obesulus). Proceedings of the Royal Society of London. Series B, Biological Sciences 272, 791–796.
| Cone topography and spectral sensitivity in two potentially trichromatic marsupials, the quokka (Setonix brachyurus) and quenda (Isoodon obesulus).Crossref | GoogleScholarGoogle Scholar |
Aspinall, I. (1994). Evaluation of wildlife warning reflectors. Vic Roads Report No. GR 94-12, Kew, Victoria.
Babińska-Werka, J., Krauze-Gryz, D., Wasilewski, M., and Jasińska, K. (2015). Effectiveness of an acoustic wildlife warning device using natural calls to reduce the risk of train collisions with animals. Transportation Research Part D, Transport and Environment 38, 6–14.
| Effectiveness of an acoustic wildlife warning device using natural calls to reduce the risk of train collisions with animals.Crossref | GoogleScholarGoogle Scholar |
Beazeley, L. D., Arrese, C., and Hunt, D. M. (2010). Visual system. In ‘The Neurobiology of Australian Marsupials: Brain Evolution in the Other Mammalian Radiation’. (Ed. K. Ashwell.) pp. 155–183. (Cambridge University Press: Cambridge.)
Ben-Ami, D., and Ramp, D. (2013). Impact of roadside habitat on swamp wallaby movement and fitness. Wildlife Research 40, 512–522.
| Impact of roadside habitat on swamp wallaby movement and fitness.Crossref | GoogleScholarGoogle Scholar |
Bender, H. (2001). Deterrence of kangaroos from roadways using ultrasonic frequencies – efficacy of the Shu Roo. Department of Zoology, University of Melbourne.
Bender, H. (2003). Deterrence of kangaroos from agricultural areas using ultrasonic frequencies: efficacy of a commercial device. Wildlife Society Bulletin 31, 1037–1046.
Bender, H. (2005). Effectiveness of the eastern grey kangaroo foot thump for deterring conspecifics. Wildlife Research 32, 649–655.
| Effectiveness of the eastern grey kangaroo foot thump for deterring conspecifics.Crossref | GoogleScholarGoogle Scholar |
Bender, H. (2006). Structure and function of the eastern grey kangaroo foot thump. Journal of Zoology 268, 415–422.
| Structure and function of the eastern grey kangaroo foot thump.Crossref | GoogleScholarGoogle Scholar |
Benten, A., Annighöfer, P., and Vor, T. (2018a). Wildlife warning reflectors’ potential to mitigate wildlife–vehicle collisions – a review on the evaluation methods. Frontiers in Ecology and Evolution 6, 37.
| Wildlife warning reflectors’ potential to mitigate wildlife–vehicle collisions – a review on the evaluation methods.Crossref | GoogleScholarGoogle Scholar |
Benten, A., Hothorn, T., Vor, T., and Ammer, C. (2018b). Wildlife warning reflectors do not mitigate wildlife–vehicle collisions on roads. Accident; Analysis and Prevention 120, 64–73.
| Wildlife warning reflectors do not mitigate wildlife–vehicle collisions on roads.Crossref | GoogleScholarGoogle Scholar | 30096449PubMed |
Biedenweg, T. A., Parsons, M. H., Fleming, P. A., and Blumstein, D. T. (2011). Sounds scary? Lack of habituation following the presentation of novel sounds. PLoS One 6, e14549.
| Sounds scary? Lack of habituation following the presentation of novel sounds.Crossref | GoogleScholarGoogle Scholar | 21267451PubMed |
Blumstein, D. T. (2016). Habituation and sensitization: new thoughts about old ideas. Animal Behaviour 120, 255–262.
| Habituation and sensitization: new thoughts about old ideas.Crossref | GoogleScholarGoogle Scholar |
Blumstein, D. T., Daniel, J. C., Griffin, A. S., and Evans, C. S. (2000). Insular tammar wallabies (Macropus eugenii) respond to visual but not acoustic cues from predators. Behavioral Ecology 11, 528–535.
| Insular tammar wallabies (Macropus eugenii) respond to visual but not acoustic cues from predators.Crossref | GoogleScholarGoogle Scholar |
Blumstein, D. T., Daniel, J. C., Schnell, M. R., Ardron, J. G., and Evans, C. S. (2002). Antipredator behaviour of red-necked pademelons: a factor contributing to species survival? Animal Conservation 5, 325–331.
| Antipredator behaviour of red-necked pademelons: a factor contributing to species survival?Crossref | GoogleScholarGoogle Scholar |
Bomford, M., and O’Brien, P. H. (1990). Sonic deterrents in animal damage control: a review of device tests and effectiveness. Wildlife Society Bulletin 18, 411–422.
Brieger, F., Hagen, R., Vetter, D., Dormann, C. F., and Storch, I. (2016). Effectiveness of light-reflecting devices: a systematic reanalysis of animal–vehicle collision data. Accident; Analysis and Prevention 97, 242–260.
| Effectiveness of light-reflecting devices: a systematic reanalysis of animal–vehicle collision data.Crossref | GoogleScholarGoogle Scholar | 27716546PubMed |
Canova, L., and Balestrieri, A. (2019). Long-term monitoring by roadkill counts of mammal populations living in intensively cultivated landscapes. Biodiversity and Conservation 28, 97–113.
| Long-term monitoring by roadkill counts of mammal populations living in intensively cultivated landscapes.Crossref | GoogleScholarGoogle Scholar |
Cohen, B. S., Osborn, D. A., Gallagher, G. R., Warren, R. J., and Miller, K. V. (2014). Behavioral measure of the light‐adapted visual sensitivity of white‐tailed deer. Wildlife Society Bulletin 38, 480–485.
| Behavioral measure of the light‐adapted visual sensitivity of white‐tailed deer.Crossref | GoogleScholarGoogle Scholar |
Coles, R. B., and Guppy, A. (1986). Biophysical aspects of directional hearing in the tammar wallaby, Macropus eugenii. The Journal of Experimental Biology 121, 371–394.
Cone-Wesson, B. K., Hill, K. G., and Liu, G. B. (1997). Auditory brainstem response in tammar wallaby (Macropus eugenii). Hearing Research 105, 119–129.
| Auditory brainstem response in tammar wallaby (Macropus eugenii).Crossref | GoogleScholarGoogle Scholar | 9083809PubMed |
Coulson, G. M. (1982). Road-kills of macropds on a section of highway in central Victoria. Wildlife Research 9, 21–26.
| Road-kills of macropds on a section of highway in central Victoria.Crossref | GoogleScholarGoogle Scholar |
Coulson, G. (1989). The effect of drought on road mortality of macropods. Wildlife Research 16, 79–83.
| The effect of drought on road mortality of macropods.Crossref | GoogleScholarGoogle Scholar |
Curlewis, J. D. (1989). The breeding season of Bennett’s wallaby (Macropus rufogriseus rufogriseus) in Tasmania. Journal of Zoology 218, 337–339.
| The breeding season of Bennett’s wallaby (Macropus rufogriseus rufogriseus) in Tasmania.Crossref | GoogleScholarGoogle Scholar |
D’Angelo, G., and van der Ree, R. (2015). Use of reflectors and auditory deterrents to prevent wildlife–vehicle collisions. In ‘Handbook of Road Ecology’. (Eds R. van der Ree, D. J. Smith and C. Grilo.) pp. 213–218. (Wiley: Hoboken, NJ.)
D’Angelo, G. J., D’Angelo, J. G., Gallagher, G. R., Osborn, D. A., Miller, K. V., and Warren, R. J. (2006). Evaluation of wildlife warning reflectors for altering white‐tailed deer behavior along roadways. Wildlife Society Bulletin 34, 1175–1183.
| Evaluation of wildlife warning reflectors for altering white‐tailed deer behavior along roadways.Crossref | GoogleScholarGoogle Scholar |
de Vries, M. (2015). Road kills of roe deer (Capreolus capreolus) in the Netherlands: assessment of impacts and mitigation measures. M.Sc. internship report, Copernicus Institute of Sustainable Development, Utrecht University, Utrecht, The Netherlands.
Deeb, S. S., Wakefield, M. J., Tada, T., Marotte, L., Yokoyama, S., and Marshall Graves, J. A. (2003). The cone visual pigments of an Australian marsupial, the tammar wallaby (Macropus eugenii): sequence, spectral tuning, and evolution. Molecular Biology and Evolution 20, 1642–1649.
| The cone visual pigments of an Australian marsupial, the tammar wallaby (Macropus eugenii): sequence, spectral tuning, and evolution.Crossref | GoogleScholarGoogle Scholar | 12885969PubMed |
Department of State Growth (2015). Tasmanian state road traffic noise management guidelines.
Dimovski, A. M., and Robert, K. A. (2018). Artificial light pollution: shifting spectral wavelengths to mitigate physiological and health consequences in a nocturnal marsupial mammal. The Journal of Experimental Zoology 329, 497–505.
| Artificial light pollution: shifting spectral wavelengths to mitigate physiological and health consequences in a nocturnal marsupial mammal.Crossref | GoogleScholarGoogle Scholar |
Ebeling, W., Natoli, R. C., and Hemmi, J. M. (2010). Diversity of color vision: not all Australian marsupials are trichromatic. PLoS One 5, e14231.
| Diversity of color vision: not all Australian marsupials are trichromatic.Crossref | GoogleScholarGoogle Scholar | 21151905PubMed |
Farmer, R. G., and Brooks, R. J. (2012). Integrated risk factors for vertebrate roadkill in southern Ontario. Journal of Wildlife Management 76, 1215–1224.
| Integrated risk factors for vertebrate roadkill in southern Ontario.Crossref | GoogleScholarGoogle Scholar |
Feldhamer, G. A., Gates, J. E., Harman, D. M., Loranger, A. J., and Dixon, K. R. (1986). Effects of interstate highway fencing on white-tailed deer activity. Journal of Wildlife Management 50, 497–503.
| Effects of interstate highway fencing on white-tailed deer activity.Crossref | GoogleScholarGoogle Scholar |
Fox, S., Potts, J.M., Pemberton, D., and Crosswell, D. (2019). Roadkill mitigation: trialing virtual fence devices on the west coast of Tasmania. Australian Mammalogy 41, 205–211.
| Roadkill mitigation: trialing virtual fence devices on the west coast of Tasmania.Crossref | GoogleScholarGoogle Scholar |
Gates, G. R., and Aitkin, L. M. (1982). Auditory cortex in the marsupial possum Trichosurus vulpecula. Hearing Research 7, 1–11.
| Auditory cortex in the marsupial possum Trichosurus vulpecula.Crossref | GoogleScholarGoogle Scholar | 7096213PubMed |
Goldingay, R. L., Taylor, B. D., Parkyn, J. L., and Lindsay, J. M. (2018). Are wildlife escape ramps needed along Australian highways? Ecological Management & Restoration 19, 198–203.
| Are wildlife escape ramps needed along Australian highways?Crossref | GoogleScholarGoogle Scholar |
Hemmi, J. M. (1999). Dichromatic colour vision in an Australian marsupial, the tammar wallaby. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 185, 509–515.
| Dichromatic colour vision in an Australian marsupial, the tammar wallaby.Crossref | GoogleScholarGoogle Scholar |
Hemmi, J. M., Maddess, T., and Mark, R. F. (2000). Spectral sensitivity of photoreceptors in an Australian marsupial, the tammar wallaby (Macropus eugenii). Vision Research 40, 591–599.
| Spectral sensitivity of photoreceptors in an Australian marsupial, the tammar wallaby (Macropus eugenii).Crossref | GoogleScholarGoogle Scholar | 10824263PubMed |
Hobday, A. J., and Minstrell, M. L. (2008). Distribution and abundance of roadkill on Tasmanian highways: human management options. Wildlife Research 35, 712–726.
| Distribution and abundance of roadkill on Tasmanian highways: human management options.Crossref | GoogleScholarGoogle Scholar |
Hunt, D. M., Carvalho, L. S., Cowing, J. A., and Davies, W. L. (2009). Evolution and spectral tuning of visual pigments in birds and mammals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364, 2941–2955.
| Evolution and spectral tuning of visual pigments in birds and mammals.Crossref | GoogleScholarGoogle Scholar | 19720655PubMed |
Jacobs, G. H., Deegan, J., Neitz, J., Murphy, B. P., Miller, K. V., and Marchinton, R. L. (1994). Electrophysiological measurements of spectral mechanisms in the retinas of two cervids: white-tailed deer (Odocoileus virginianus) and fallow deer (Dama dama). Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 174, 551–557.
| Electrophysiological measurements of spectral mechanisms in the retinas of two cervids: white-tailed deer (Odocoileus virginianus) and fallow deer (Dama dama).Crossref | GoogleScholarGoogle Scholar |
Langbein, J., Putman, R. J., and Pokorny, B. (2011). Traffic collisions involving deer and other ungulates in Europe. In ‘Ungulate Management in Europe: Problems and Practices’. (Eds R. J. Putman, M. Apollonio, and R. Andersen.) pp. 215–259. (Cambridge University Press: Cambridge.)
Magnus, Z., Kriwoken, L. K., Mooney, N. J., and Jones, M. E. (2004). Reducing the incidence of wildlife roadkill: improving the visitor experience in Tasmania. Technical report, CRC for Sustainable Tourism.
Moser, E. (2007). Acoustic wildlife warning modules under test. OÖ Jäger 4, 1–2.
Muirhead, S., Blache, D., Wykes, B., and Bencini, R. (2006). Roo-Guard® sound emitters are not effective at deterring tammar wallabies (Macropus eugenii) from a source of food. Wildlife Research 33, 131–136.
| Roo-Guard® sound emitters are not effective at deterring tammar wallabies (Macropus eugenii) from a source of food.Crossref | GoogleScholarGoogle Scholar |
Osugi, M., Foster, T. M., Temple, W., and Poling, A. (2011). Behavior‐based assessment of the auditory abilities of brushtail possums. Journal of the Experimental Analysis of Behavior 96, 123–138.
| Behavior‐based assessment of the auditory abilities of brushtail possums.Crossref | GoogleScholarGoogle Scholar | 21765549PubMed |
Parker, I. D., Braden, A. W., Lopez, R. R., Silvy, N. J., Davis, D. S., and Owen, C. B. (2008). Effects of US 1 Project on Florida Key deer mortality. Journal of Wildlife Management 72, 354–359.
| Effects of US 1 Project on Florida Key deer mortality.Crossref | GoogleScholarGoogle Scholar |
Ramp, D., and Croft, D. B. (2006). Do wildlife warning reflectors elicit aversion in captive macropods? Wildlife Research 33, 583–590.
| Do wildlife warning reflectors elicit aversion in captive macropods?Crossref | GoogleScholarGoogle Scholar |
Ramp, D., Foale, C. G., Roger, E., and Croft, D. B. (2011). Suitability of acoustics as non-lethal deterrents for macropodids: the influence of origin, delivery and anti-predator behaviour. Wildlife Research 38, 408–418.
| Suitability of acoustics as non-lethal deterrents for macropodids: the influence of origin, delivery and anti-predator behaviour.Crossref | GoogleScholarGoogle Scholar |
Ramp, D., Wilson, V. K., and Croft, D. B. (2016). Contradiction and complacency shape attitudes towards the toll of roads on wildlife. Animals (Basel) 6, 40.
| Contradiction and complacency shape attitudes towards the toll of roads on wildlife.Crossref | GoogleScholarGoogle Scholar |
Reeve, A. F., and Anderson, S. H. (1993). Ineffectiveness of Swareflex reflectors at reducing deer–vehicle collisions. Wildlife Society Bulletin 21, 127–132.
Riginos, C., Graham, M. W., Davis, M. J., Johnson, A. B., May, A. B., Ryer, K. W., and Hall, L. E. (2018). Wildlife warning reflectors and white canvas reduce deer–vehicle collisions and risky road‐crossing behavior. Wildlife Society Bulletin 42, 119–130.
| Wildlife warning reflectors and white canvas reduce deer–vehicle collisions and risky road‐crossing behavior.Crossref | GoogleScholarGoogle Scholar |
Romin, L. A., and Dalton, L. B. (1992). Lack of response by mule deer to wildlife warning whistles. Wildlife Society Bulletin 20, 382–384.
Rose, R. W., and McCatney, D. J. (1982). Reproduction of the red-bellied pademelon Thylogale billardierii (Marsupialia). Wildlife Research 9, 27–32.
| Reproduction of the red-bellied pademelon Thylogale billardierii (Marsupialia).Crossref | GoogleScholarGoogle Scholar |
Rose, T. A., Munn, A. J., Ramp, D., and Banks, P. B. (2006). Foot-thumping as an alarm signal in macropodoid marsupials: prevalence and hypotheses of function. Mammal Review 36, 281–298.
| Foot-thumping as an alarm signal in macropodoid marsupials: prevalence and hypotheses of function.Crossref | GoogleScholarGoogle Scholar |
Rytwinski, T., van der Ree, R., Cunnington, G. M., Fahrig, L., Findlay, C. S., Houlahan, J., Jaeger, J. A. G., Soanes, K., and van der Grift, E. A. (2015). Experimental study designs to improve the evaluation of road mitigation measures for wildlife. Journal of Environmental Management 154, 48–64.
| Experimental study designs to improve the evaluation of road mitigation measures for wildlife.Crossref | GoogleScholarGoogle Scholar | 25704749PubMed |
Rytwinski, T., Soanes, K., Jaeger, J. A. G., Fahrig, L., Findlay, C. S., Houlahan, J., van der Ree, R., and van der Grift, E. A. (2016). How effective is road mitigation at reducing road-kill? A meta-analysis. PLoS One 11, e0166941.
| How effective is road mitigation at reducing road-kill? A meta-analysis.Crossref | GoogleScholarGoogle Scholar | 27870889PubMed |
Schakner, Z. A., and Blumstein, D. T. (2013). Behavioral biology of marine mammal deterrents: a review and prospectus. Biological Conservation 167, 380–389.
| Behavioral biology of marine mammal deterrents: a review and prospectus.Crossref | GoogleScholarGoogle Scholar |
Scheifele, P. M., Browning, D. G., and Collins-Scheifele, L. M. (2003). Analysis and effectiveness of deer whistles for motor vehicles: frequencies, levels, and animal threshold responses. Acoustics Research Letters Online 4, 71–76.
| Analysis and effectiveness of deer whistles for motor vehicles: frequencies, levels, and animal threshold responses.Crossref | GoogleScholarGoogle Scholar |
Seo, C., Thorne, J. H., Choi, T., Kwon, H., and Park, C. H. (2015). Disentangling roadkill: the influence of landscape and season on cumulative vertebrate mortality in South Korea. Landscape and Ecological Engineering 11, 87–99.
| Disentangling roadkill: the influence of landscape and season on cumulative vertebrate mortality in South Korea.Crossref | GoogleScholarGoogle Scholar |
Shivik, J. A., Treves, A., and Callahan, P. (2003). Nonlethal techniques for managing predation: primary and secondary repellents. Conservation Biology 17, 1531–1537.
| Nonlethal techniques for managing predation: primary and secondary repellents.Crossref | GoogleScholarGoogle Scholar |
Sielecki, L. E. (2001). Evaluating the effectiveness of wildlife accident mitigation installations with the wildlife accident reporting system (WARS) in British Columbia. In ‘Proceedings of the 2001 International Conference on Ecology and Transportation’. (Eds C. L. Irwin, P. Garrett and K. P. McDermott.) pp. 473–489. (North Carolina University: Raleigh, NC.)
Steiner, W., Leisch, F., and Hackländer, K. (2014). A review on the temporal pattern of deer–vehicle accidents: impact of seasonal, diurnal and lunar effects in cervids. Accident; Analysis and Prevention 66, 168–181.
| A review on the temporal pattern of deer–vehicle accidents: impact of seasonal, diurnal and lunar effects in cervids.Crossref | GoogleScholarGoogle Scholar | 24549035PubMed |
Taylor, B. D., and Goldingay, R. L. (2004). Wildlife road-kills on three major roads in north-eastern New South Wales. Wildlife Research 31, 83–91.
| Wildlife road-kills on three major roads in north-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Ujvári, M., Baagøe, H. J., and Madsen, A. B. (1998). Effectiveness of wildlife warning reflectors in reducing deer–vehicle collisions: a behavioral study. Journal of Wildlife Management 62, 1094–1099.
| Effectiveness of wildlife warning reflectors in reducing deer–vehicle collisions: a behavioral study.Crossref | GoogleScholarGoogle Scholar |
Ujvári, M., Baagøe, H. J., and Madsen, A. B. (2004). Effectiveness of acoustic road markings in reducing deer–vehicle collisions: a behavioural study. Wildlife Biology 10, 155–159.
| Effectiveness of acoustic road markings in reducing deer–vehicle collisions: a behavioural study.Crossref | GoogleScholarGoogle Scholar |
Valitzski, S. A., D’Angelo, G. J., Gallagher, G. R., Osborn, D. A., Miller, K. V., and Warren, R. J. (2009). Deer responses to sounds from a vehicle-mounted sound-production system. Journal of Wildlife Management 73, 1072–1076.
| Deer responses to sounds from a vehicle-mounted sound-production system.Crossref | GoogleScholarGoogle Scholar |
VerCauteren, K. C., and Pipas, M. J. (2003). A review of color vision in white-tailed deer. Wildlife Society Bulletin 31, 684–691.
| A review of color vision in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
VerCauteren, K. C., Gilsdorf, J. M., Hygnstrom, S. E., Fioranelli, P. B., Wilson, J. A., and Barras, S. (2006). Green and blue lasers are ineffective for dispersing deer at night. Wildlife Society Bulletin 34, 371–374.
| Green and blue lasers are ineffective for dispersing deer at night.Crossref | GoogleScholarGoogle Scholar |
Waring, G. H., Griffis, J. L., and Vaughn, M. E. (1991). White-tailed deer roadside behavior, wildlife warning reflectors, and highway mortality. Applied Animal Behaviour Science 29, 215–223.
| White-tailed deer roadside behavior, wildlife warning reflectors, and highway mortality.Crossref | GoogleScholarGoogle Scholar |
Watkins, E., and Di Stefano, J. (2013). Quantifying annual patterns in the frequency of mammalian births: do goodness-of-fit tests provide adequate inferences? Australian Journal of Zoology 60, 381–387.
| Quantifying annual patterns in the frequency of mammalian births: do goodness-of-fit tests provide adequate inferences?Crossref | GoogleScholarGoogle Scholar |
Wiggins, N. L., Williamson, G. J., McCallum, H. I., McMahon, C. R., and Bowman, D. M. J. S. (2010). Shifts in macropod home ranges in response to wildlife management interventions. Wildlife Research 37, 379–391.
| Shifts in macropod home ranges in response to wildlife management interventions.Crossref | GoogleScholarGoogle Scholar |
Zar, J. H. (2010). ‘Biostatistical Analysis.’ (Pearson: Upper Saddle River, NJ.)


