A catch-all leguminous tree: Erythrina velutina visited and pollinated by vertebrates at an oceanic island
Ivan Sazima A , Cristina Sazima B and Marlies Sazima C DA Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil (retired and associated as voluntary researcher).
B Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil.
C Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil.
D Corresponding author. Email: msazima@unicamp.br
Australian Journal of Botany 57(1) 26-30 https://doi.org/10.1071/BT08179
Submitted: 30 September 2008 Accepted: 24 November 2008 Published: 23 March 2009
Abstract
Species of the pantropical genus Erythrina (Fabaceae) are visited by perching and/or hovering birds in the mainland. At the oceanic island of Fernando de Noronha, north-eastern Brazil, we found that Erythrina velutina Willd. blooms during the dry season and the flowers are visited by a small vertebrate assemblage. Flowers last 2 days and their stigmas remain receptive, although only first-day flowers produce nectar. Nectar is dilute and produced copiously. All terrestrial native vertebrates (three of them endemics), the dove Zenaida auriculata noronha, the perching birds Vireo gracilirostris and Elaenia ridleyana, and the lizard Euprepis atlanticus are regular visitors and pollinators. The features of E. velutina conform to those of passerine-pollinated species within the genus. Its nectar is a resource sought by the vertebrates, which visit the inflorescences from dawn to sunset. Since none of the visitors depends on nectar as a major food source, the flowers are likely to serve a dual purpose, i.e. water balance and energy intake, similarly to the findings for some Erythrina species in Neotropic and Palaeotropic mainlands. However, E. velutina is the only species within the genus that is visited and pollinated by doves and lizards.
Introduction
Oceanic islands provide some remarkable case stories of relationships between vertebrates and plants, including pollination by lizards (e.g. Eifler 1995; Olesen and Valido 2003, 2004; Sazima et al. 2005). At the oceanic island of Fernando de Noronha, north-eastern Brazil, we found that flowers of E. velutina are visited by a small assemblage of native vertebrates (three of them endemics, see Sick 1997; Carleton and Olson 1999). Mainland species of the pantropical genus Erythrina (Fabaceae) are visited by perching and/or hovering birds (e.g. Morton 1979; Bruneau 1997; Buzato et al. 2000; Etcheverry and Alemán 2005), although an arboreal mammal is reported in one instance as well (Rangaiah et al. 2004). Some studies on the pollination of Erythrina species in Central and South America mainland (Toledo and Hernández 1979; Etcheverry and Alemán 2005) and India (Rangaiah et al. 2004) indicate that birds as well as mammals (India) visit the flowers for energetic requirements and water intake, which also seems to be the case of a lizard at an oceanic island off Brazil (Sazima et al. 2005). We present here a short study on the relationship between the island-dwelling E. velutina and its visitors to examine whether there is any similarity with findings reported for other plants in oceanic islands, or whether this Erythrina species conforms to the findings reported for mainland species within the genus (e.g. Toledo and Hernández 1979; Rangaiah et al. 2004; Etcheverry and Alemán 2005).
Materials and methods
Field observations were made at several sites from sea level to ~50 m asl on Fernando de Noronha Island (3°50′S, 32°15′W), off north-eastern Brazil (see Carleton and Olson 1999 for map and description). Most data were obtained from seven 3–18-m-tall trees studied at the peak of the dry season in October (2002, 2004) and November (2003). Number of flowers and maturing fruits per inflorescence was obtained in situ from 17 inflorescences on lower branches of four 5–6-m-tall trees. Flower biology and morphological features were recorded in situ and in the laboratory according to Faegri and van der Pijl (1980). Nectar volume and concentration were measured in situ with micro syringe and pocket refractometer (Kearns and Inouye 1993) in early morning (0630–0715 hours) from 22 flowers previously bagged in early afternoon, from 10 flowers bagged for 55–60 min after nectar depletion by visitors, and from nine flowers after visits ceased in late afternoon (1745 hours). Stigma receptivity was tested in situ by the H2O2-catalase activity method (Zeisler 1938). Pollen viability was estimated in laboratory (n = 40 anthers from 14 flowers from three trees) by cytoplasmic staining (Radford et al. 1974). Sugar composition in nectar was analysed from samples taken from four flowers of two trees grown in the campus of the Universidade Estadual de Campinas. The sugar composition was analysed with gas chromatography, following the procedure described in Galetto and Bernardello (2005). Plant vouchers are in the herbarium of the Universidade Estadual de Campinas (UEC), São Paulo, Brazil. Visitors to flowers were observed with naked eye or through binoculars at a distance of 1.5–12 m. Observational sessions lasted 15–60 min, totalling 1865 min during 14 non-consecutive days. ‘All occurrences’ sampling (Lehner 1979), in which all behaviours are recorded, was used throughout the sessions. To count visit frequency of each bird species throughout the day, one focal 9-m-tall tree with ~150 inflorescences was selected and watched throughout 1 day, with observational sessions beginning at dawn (0530 hours) and ending at sunset (1730 hours). Sessions were 60 min long and were interspersed with 90 min with no observations. Because of their small size and camouflaging colour on the branches, lizards were recorded throughout 1 day on a branch section with four inflorescences in five ‘scan’ or ‘instantaneous’ samplings (Lehner 1979), in which the behaviour of all visible subjects was recorded. Newly opened flowers (n = 13) were examined for pollen load left after the visits of each visitor type. Photographs and tape sequences of visits to flowers were used to analyse and describe the visitors’ behaviour.
Results
Floral biology
Most E. velutina trees bloom synchronously (Fig. 1A). Each inflorescence develops 10–26 flowers (18.0 ± 4.87 (mean ± s.d.), n = 17), with daily 1–8 (3.81 ± 1.91, n = 32) newly opened ones. The flowers are large and odourless, and the typical papilionaceous corolla is pale yellow to rich orange and composed of a poorly developed keel and wing petals on the upper side and the expanded vexillum below (Fig. 1B, E). Nine stamens are united until one-third of their length, forming the staminal tube from where on the tube ends and the apical portions of the stamens are displayed in a fan-shaped array (Fig. 1B, E). The gynoecium is curved, enclosed by and longer than the staminal tube, and thus the terminal stigma is slightly ahead (~3–5 mm) of the anthers. The fan-shaped array of the reproductive organs promotes a broad contact surface with a flower visitor (Fig. 1E). The dark purple nectary is at the base of the ovary, attached to the receptacle and forms a ring whose upper part is divided into 10 short lobes.

|
Flower opening occurred at 0600–0630 hours. The anthers dehisce at that time and face downward, which results in a nototribic pollen deposition. The powdery pollen, dispersed on the first day a flower opens, adheres easily to any surface. The stigma is wet and reacts intensely with hydrogen peroxide on flowers of the first day, which coincides with the anther opening and indicates that the flowers are homogamous. Nectar accumulates within a chamber formed by the staminal furrow and from there it overflows into the keel and wings where it is retained as a large drop (Fig. 1C). Nectar was produced continuously and during the first day of anthesis only. Freshly open flowers from the first day had a nectar volume of 182–281 μL (233.86 ± 28.41, n = 22) and sugar concentration of 11–14% (11.75 ± 0.79, n = 22). In early afternoon flowers whose nectar was depleted by visitors replaced 67–106 μL (89.50 ± 10.88, n = 10) in 1 h, with sugar concentration of 10–12% (10.95 ± 0.76, n = 10). In late afternoon, after visits to flowers ceased, nectar volume was 61–112 μL (74.11 ± 16.04, n = 9) and concentration was 13–19% (15.33 ± 2.59, n = 9). Sugar composition had high and similar percentages of glucose and fructose (50.59–56.07 and 42.2–49.2%, respectively) and a very low percentage of saccharose (0.0–1.73%), which characterises the nectar as hexose-dominant [S/(G+F) < 0.1]. Second-day flowers produced no more nectar, although they still were visited for nectar that remained from the previous day; there was almost no more pollen available and the stigma remained receptive. Viability of pollen grains was high (91–100%). Under natural conditions the fruit : flower ratio (61 : 306) was on average 20% and varied from 10% (1 : 10) to 33% (8 : 24).
Visitors and their behaviour on flowers
The flowers of E. velutina were visited by all four vertebrate species native to Fernando de Noronha Island, the Noronha eared dove (Zenaida auriculata noronha (Columbidae)), the Noronha elaenia (Elaenia ridleyana (Tyrannidae)), the Noronha vireo (Vireo gracilirostris (Vireonidae)) and the Noronha skink (Euprepis atlanticus (Scincidae)), this latter being a lizard. With the exception of the dove, which also occurs on the mainland, the three other vertebrates are endemics (Sick 1997; Carleton and Olson 1999). The dove and the lizard were the most ubiquitous flower visitors, found on almost every blooming tree on the island (including those in urbanised areas), whereas the two passerine birds were more common in less disturbed habitats.
On landing on a tree, the dove walked on the branches or over inflorescences, and mostly visited the flowers legitimately, i.e. taking nectar with its head between the vexillum and the stamens and stigma (Fig. 1D). In doing so, the bird’s forehead and crown came into contact with the flower’s reproductive organs. Additionally, when the dove crawled over the inflorescences to take nectar from contiguous flowers, it became dusted with pollen on the belly and feet, contacting the stigmas this way as well. The smaller elaenia also contacted the flower’s reproductive organs while taking nectar during legitimate visits; however, sometimes it took nectar without touching the stamens and stigma. In contrast, the small vireo rarely contacted the reproductive organs, mostly owing to its small size relative to the flower, even while making legitimate visits (Fig. 1F). The lizard crawled over the inflorescences and, irrespective of its position on the flower while taking nectar, it contacted the stamens and stigma owing to the fan-shaped array of this set (Fig. 1E). During its visits, the lizard received pollen on the head and neck, back and underside. After the visits of doves and lizards (less so for the two passerine birds) the stigmas showed variable, although clearly visible pollen loads. Both the birds and the lizards moved between adjacent trees, although the lizards moved for shorter distances.
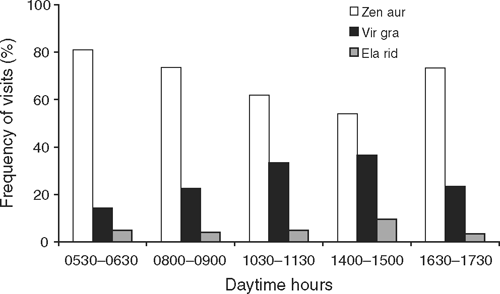
The dove Z. auriculata was the most frequent visitor and its visit frequency remained almost steady throughout the day (Fig. 2). Visits of the two passerines, E. ridleyana and V. gracilirostris, were less frequent than those of the doves and peaked between 1400 and 1500 hours, the warmest period of the day. At any given day period, the focal tree had from one to ~10 visitors taking nectar. Visits of the lizard E. atlanticus were recorded from early morning (0635 hours) to late afternoon (1735 hours). Frequency of lizard visits on the branch section yielded the following results: 0635 hours (n = 2 lizards), 0905 hours (n = 3), 1135 hours (n = 3), 1505 hours (n = 2), 1735 hours (n = 1), from which three lizards were individually recognisable and only one was observed twice on the branch section.
|
Discussion
Floral biology
The flower features of E. velutina conform to the pattern reported for species within the genus pollinated by passerine birds (Bruneau 1997). The sugar composition of nectar – hexose dominant – is another trait that supports a passerine-pollination system (Baker and Baker 1983; Bruneau 1997). Copious amounts of diluted nectar are reported for several passerine-pollinated Erythrina species (e.g. Toledo and Hernández 1979; Rangaiah et al. 2004; Etcheverry and Alemán 2005), a trait that contrasts with much smaller amounts of more concentrated nectar found in hummingbird-pollinated species within the genus, such as E. speciosa Andr., with sugar composition ~35% and 15 μL of nectar per flower (Buzato et al. 2000; Sazima et al. 2005). The slightly higher nectar concentration we recorded for E. velutina at the end of the day is likely to be due to water evaporation, as temperature increases and relative humidity decreases throughout the day (see Rangaiah et al. 2004 for similar results in E. variegata L.).
The position of anthers and stigma indicates very low possibility of contact with each other, which would make self-pollination rare or unlikely. Further, a low fruit set may indicate self-incompatibility and, thus, dependence on pollen vectors. Cross-pollination is a trait reported for other species within the genus (e.g. Rangaiah et al. 2004; Etcheverry and Alemán 2005). Overall, E. velutina follows the pattern found for other species within the genus studied to some detail (see Rangaiah et al. 2004; Etcheverry and Alemán 2005, and references therein).
Visitors and their behaviour on flowers
Doves and lizards are the main pollinators of E. velutina, although all vertebrate species native to Fernando de Noronha and able to reach the nectar act as pollinators. The doves are likely to be the most efficient pollinators, owing to their behaviour and larger size, thus carrying larger pollen loads. Owing to their basking on E. velutina branches and their crawling mostly between closely adjacent trees (Sazima et al. 2005), the lizards would be less efficient pollinators than the doves. The two passerines are rarer and smaller than the doves and may be regarded as occasional pollinators. Pollination by small but varied vertebrate assemblages, including lizards, is reported for several island-dwelling flowering plant species (e.g. Olesen and Valido 2003, 2004, and references therein).
The day-round flower visitation by birds and lizards, the large amount of diluted nectar produced continuously and the scarcity of natural freshwater sources on the island during the dry season, all are indicative of the terrestrial vertebrates at Fernando de Noronha Island seeking nectar primarily as a water supply. The increase of the small vireo’s visits during the warmest period of the day lends support to our suggestion. Additionally, none of the visitors depends on nectar as a major food source; the lizard eats anything edible, the dove is a seed-eater and the perching birds feed mostly on insects (Sick 1997; Gasparini et al. 2007). Thus, we suggest that visits of Noronha Island native vertebrates to E. velutina flowers are likely to serve a dual purpose, i.e. water balance and energy acquisition (see Sazima et al. 2005 for this view about the lizard visitor). This dual purpose is already reported for Erythrina species in mainland Central and South America (Toledo and Hernández 1979; Etcheverry and Alemán 2005) and India (Rangaiah et al. 2004).
In conclusion, the island-dwelling E. velutina conforms to the general pattern found for passerine-pollinated species within the genus, both in flower features and nectar composition (Bruneau 1997), although its main visitor and pollinator is a dove, a trait unrecorded for other Erythrina species so far. Additionally, this is the only Erythrina pollinated by lizards (Sazima et al. 2005), a phenomenon reported for plant species dwelling on the generally arid environment of oceanic islands (Olesen and Valido 2003, 2004 and references therein).
Acknowledgements
We thank José Martins Silva Jr (Centro Golfinho Rotador) for unfailing logistic support, Marco Aurélio Silva (IBAMA) for logistic support in the Parque Nacional Marinho do Arquipélago de Fernando de Noronha, João Paulo Krajewski for allowing use of his superb photographs (Fig. 1A, E, F), Leonardo Galetto and Kayna Agostini for allowing the use of chemical analyses of the nectar and the CNPq and FAPESP for financial support.
Bruneau A
(1997) Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae). American Journal of Botany 84, 54–71.
| Crossref | GoogleScholarGoogle Scholar |

Buzato S,
Sazima M, Sazima I
(2000) Hummingbird pollinated floras at three Atlantic Forest sites. Biotropica 32, 824–841.

Carleton MD, Olson SL
(1999) Amerigo Vespucci and the rat of Fernando de Noronha: a new genus and species of Rodentia (Muridae: Sigmodontinae) from a volcanic island off Brazil’s continental shelf. American Museum Novitates 3256, 1–59.

Eifler DA
(1995) Patterns of plant visitation by nectar-feeding lizards. Oecologia 101, 228–233.
| Crossref | GoogleScholarGoogle Scholar |

Etcheverry AV, Alemán CET
(2005) Reproductive biology of Erythrina falcata (Fabaceae: Papilionoideae). Biotropica 37, 54–63.

Gasparini JL,
Peloso PL, Sazima I
(2007) New opportunities and hazards brought by humans to the island habitat of the skink Euprepis atlanticus. Herpetological Bulletin 100, 30–32.

Morton ES
(1979) Effective pollination of Erythrina fusca by the orchard oriole (Icterus spurius): coevolved behavioral manipulation? Annals of the Missouri Botanical Garden 66, 482–489.
| Crossref | GoogleScholarGoogle Scholar |

Olesen JM, Valido A
(2003) Lizards as pollinators and seed dispersers: an island phenomenon. Trends in Ecology & Evolution 18, 177–181.
| Crossref | GoogleScholarGoogle Scholar |

Rangaiah K,
Solomon Raju AJ, Rao SP
(2004) Passerine bird-pollination in the Indian coral tree, Erythrina variegata var. orientalis (Fabaceae). Current Science 87, 736–739.

Sazima I,
Sazima C, Sazima M
(2005) Little dragons prefer flowers to maidens: a lizard that laps nectar and pollinates trees. Biota Neotropica 5, 1–8.
| Crossref | GoogleScholarGoogle Scholar |

Toledo VM, Hernández HM
(1979) Erythrina oliviae: a new case of oriole pollination in Mexico. Annals of the Missouri Botanical Garden 66, 503–511.
| Crossref | GoogleScholarGoogle Scholar |

Zeisler M
(1938) Über die Abgrenzung der eigentlichen Narbenfläche mit Hilfe von Reaktionen. Beihefte zum Botanischen Zentralblatt 58, 308–318.



