An introduction to Caladenia R.Br. – Australasia’s jewel among terrestrial orchids
Kingsley W. Dixon A B D and Stephen D. Hopper A CA School of Plant Biology, The University of Western Australia, Nedlands, WA 6009, Australia.
B Botanic Gardens and Parks Authority, Kings Park and Botanic Garden, West Perth, WA 6005, Australia.
C Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK.
D Corresponding author. Email: kdixon@bgpa.wa.gov.au
Australian Journal of Botany 57(4) i-vii https://doi.org/10.1071/BT09999
Published: 29 July 2009
Abstract
Caladenia is a genus of more than 250 species of geophytic orchids in the Tribe Diurideae endemic to the Australasian Region. The genus in this broad sense has an exceptional diversity of insect pollination adaptations among its colourfully adorned species, from food-rewarding generalists to specialists achieving pollination by sexual deception of male thynnid wasps. The exploration of diversity in Caladenia involves many of the great names in the foundation of Australasian plant systematics, as well as reflecting a remarkable second phase of discovery and description over the past three decades. Molecular phylogenetics has greatly clarified relationships of Caladenia and established six major clades within the genus. Some researchers regard these clades as genera themselves, whereas they are treated as subgenera herein to maximise nomenclatural stability and information retrieval. More work is needed to adequately document relationships within each of these clades, and disputed matters of typification greatly influence nomenclature applied to many species if the six clades are recognised as genera. Given the relatively recent and ongoing discovery of so many new species in Caladenia, the biology of these orchids is only now being documented comprehensively. Significant advances in pollination ecology, mycorrhizal studies, horticulture and conservation biology are emerging that highlight the extraordinary ecological sensitivity and conservation vulnerability of the genus. Indeed, the high species number and complex biotic connections have resulted in no other genus of terrestrial orchids possessing such a large number of rare and threatened taxa. Some of this rich body of new data is presented by a diverse range of laboratories and researchers in this special issue.
Introduction
Few who encounter species of Caladenia R.Br. in the field fail to be captivated by the beauty of these colourful geophytic orchids. Indeed, the prodigious but dour Scottish naturalist on Flinders’ Investigator expedition, Robert Brown, not taken to hyperbole, named the genus from the Greek calos – beautiful + aden – gland (Brown 1810). In so doing, he was celebrating the ornately structured calli on the labellum of Caladenia compared with the absence of such in two other related genera he named (Glossodia and Eriochilus).
This paper briefly reviews aspects of the systematics, biology and conservation of Caladenia, as an introduction to the rich array of papers on the genus that follow.
Historical perspectives and systematics
Caladenia is a genus of more than 250 species in the Tribe Diurideae endemic to the Australasian Region (Jones et al. 2001; Kores et al. 2001; Hopper and Brown 2004). The precise number of species is not yet clear, given the ongoing pace of systematic discovery, although a maximum of 300 would seem likely on present evidence.
The majority of species are split across southern Australia between the South-west Australian Floristic region (SWAFR, sensu Hopper and Gioia 2004) and south-eastern Australia. A few species extend north into Queensland and across the Tasman Sea to New Zealand, with the high mountains of the Indonesian Archipelago having the most northerly representatives of the genus (Jones et al. 2001).
Aboriginal Australians undoubtedly know species of Caladenia intimately, especially those deemed useful, but we have been unable to trace specific records of such knowledge.
The earliest European collections of Caladenia were either by Archibald Menzies, surgeon and naturalist aboard Captain George Vancouver’s H.M.S. Discovery, or by Surgeon General of New South Wales, John White, on the First Fleet. Menzies was the first European to make collections of Western Australian orchids. The Discovery was anchored in King George’s Sound from 28 September to 11 October 1791, when Menzies collected the type of Caladenia flava, named by Brown (1810).
John White was based at Port Jackson from 26 January 1788 to 17 December 1794, and at some point collected or received the type of C. catenata (Sm.) Druce from an unknown collector. This species was first named as Arethusa catenata by Smith (1805), who remarked in the protologue: ‘Specimens of this elegant little plant, both dried and in spirits, accompanied by a coloured drawing, were long ago communicated to us by Dr White from New South Wales’. The type specimen is undated. The species was not illustrated nor mentioned by White (1790) in his journal, dispatched to London for publication in 1788.
As with so many Australian plants, Robert Brown made a seminal contribution on Caladenia, establishing the genus, based primarily on collections made by himself and others, including the Investigator’s artist Ferdinand Bauer, who had a special interest in orchids (Mabberley 1999). Brown’s (1810) protologue of Caladenia included a treatment of 15 species divided among two groups – Caladenia verae (13 species) and Leptoceras (2 species). The inclusion of Leptoceras rendered Brown’s concept of Caladenia polyphyletic in the light of contemporary molecular phylogenetic data.
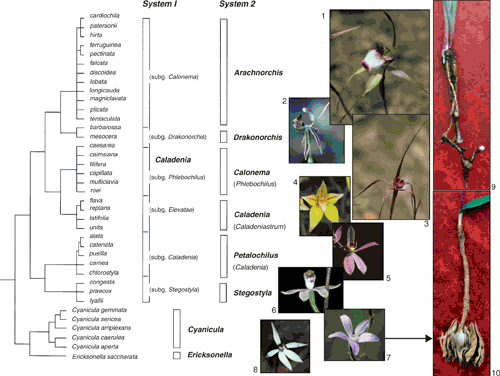
His section Eucaladenia included two species each considered now to belong to different genera (i.e. Cyanicula caerulea and Pheladenia deformis), but the other 11 species represented five of the six clades now recognised in Caladenia from DNA sequence data (Hopper and Brown 2001; Jones et al. 2001; Kores et al. 2001). Thus, Brown named C. alba, C. carnea and C. alata in Caladenia subg. Caladenia; C. flava and C. latifolia in Caladenia subg. Elevatae; C. gracilis, C. testacea and C. congesta in Caladenia subg. Stegostyla; C. filamentosa in Caladenia subg. Phlebochilus; and C. patersonii and C. dilatata in Caladenia subg. Calonema. The only subgenus, none of whose species were known at the time, is Caladenia subg. Drakonorchis, which has four species endemic to inland parts of south-western Australia (Hopper and Brown 2001), the first of which was collected by Swan River colonial botanist James Drummond in 1839 from near Toodyay and named as Caladenia barbarossa by H.G. Reichenbach (1871).
Many of Drummond’s other collections of Caladenia were examined and named by Lindley (1840a), who added significantly to knowledge of Caladenia, and was the first author to circumscribe the genus as a monophyletic entity as presently understood. In the same year, Lindley (1840b) enumerated all known Caladenia species, in both eastern and western Australian, listing 30 in total.
Reichenbach (1871) considerably expanded the circumscription of Caladenia, rendering the genus polyphyletic by including species of Glossodia, Cyrtostylis, Adenochilus, Chiloglottis, Rimacola and Lyperanthus. He also sank Leptoceras back into Caladenia as a section, and described four new Western Australian species, including the distinctive C. saccharata (now Ericksonella saccharata – Hopper and Brown 2004).
Bentham (1873) did not accept this broadest of concepts for Caladenia, but favoured Brown’s (1810) treatment of Leptoceras as a section of Caladenia rather than as a distinct genus as proposed by Lindley (1840a). Bentham (1873) also retained Lyperanthus suaveolens and L. serratus in Caladenia, following Reichenbach (1871). Among the new species that Bentham described was C. aphylla, a distinctive Western Australian orchid subsequently to be placed in the monotypic genus Praecoxanthus (Hopper and Brown 2000). Bentham (1873) acknowledged considerable difficulty with generic delimitation. He recognised 27 species of Caladenia.
Ferdinand von Mueller had less of an interest in orchids than the above botanists, his solitary taxonomic contribution in Caladenia being the description of C. cairnsiana from a collection he made in 1869 north of the Stirling Range in the SWAFR.
Thereafter, authors largely followed Bentham (1873) for more than 100 years, as the description of new species of Caladenia slowly progressed. Over the past three decades, there has been a remarkable second phase of discovery of species in the genus, accompanied by the resolution of taxonomic relationships through DNA sequence studies. Key contributions have come from research groups on both sides of southern Australia (reviewed by Hopper and Brown 2004; Hopper 2009a). This reflects a wider surge in Australian systematic botany studies, highlighted, for example, by Hopper and Gioia (2004).
Molecular phylogenetics has greatly clarified relationships of Caladenia within the Diurideae (Kores et al. 2001) and established six major clades within the genus. Some researchers regard these clades as genera themselves, whereas they are treated as subgenera herein and elsewhere (Hopper and Brown 2001, 2004) to maximise nomenclatural stability and information retrieval. More work is needed to adequately document relationships within each of these clades, and disputed matters of typification greatly influence nomenclature applied to many species if the six clades are recognised as genera (Fig. 1). Hopper and Brown (2004) and Hopper (2009a) elaborate on this complex situation.
|
Biology
Caladenia are some of the most conspicuous of Australia’s 900 taxa of geophytic orchids. Most species are restricted to the temperate southern regions of Australia and only a few taxa are found beyond (Phillips et al. this issue). Caladenia species have a distinctive growth form comprising a single, often erect, hairy leaf arising from a (deeply) buried, underground tuber and often large and colourful (or complex) floral forms.
Most species occur in dryland habitats although several taxa favour swamp margins, moist moss aprons of granite rocks or the edges of salt lakes (e.g. Caladenia cristata). Caladenia paludosa from Western Australia has ‘taken the plunge’ and can live for part of the growing season with its leaf fully submerged with flowers perched just above the waterline (see Dixon and Tremblay this issue).
The phenology of growth and development in Caladenia is based on a cycle of winter (wet season) active growth and summer (dry season) dormancy. Initiation of growth is usually in concert with a drop in temperature and an increase in soil moisture (Pate and Dixon 1982). A shoot arising from the tuber grows toward the soil surface usually within the persistent remains of previous year’s underground stems (Brown et al. 2008). Arriving at the soil surface, the shoot converts into a green vegetative apex producing a single green leaf subtended by a swollen region referred to as the collar (Ramsay et al. 1986; Dixon 1991; Brown et al. 2008). The inflorescence arises from the base of the leaf. Dormancy in Caladenia is usually associated with a rise in temperature and drying of soil usually with the replacement tuber fully formed and packed with starch and other storage compounds by the time leaf and stem tissues have senesced (Pate and Dixon 1982). Failure to develop a replacement tuber will result in death of the plant for those taxa unable to produce daughter tubers.
The collar region is the major site of mycorrhizal infection in all Caladenia species. This is a defining feature of the Caladeniineae, setting the clade apart from all other terrestrial orchids. The position of the collar region at the soil surface or just in the leaf litter layer may afford the mycorrhizal fungus the best opportunity to exploit newly arrived leaf litter. Such an attribute may provide Caladenia with a competitive nutritional advantage over other orchids that engage in root-infected mycorrhiza and indicate a need to compete with root systems of other plants for the access to organic materials and nutrients. However, this competitive advantage comes at a price, with the collar region being especially vulnerable to the deleterious impacts of drought, soil disturbance including changed fire regimes (combustion loss of organic material from the soil surface) and physical disturbance that may interrupt the mycorrhizal network.
Pollination mechanisms vary from selfing, food deception and sexual deception with reports of potential food rewarding taxa (see Phillips et al., Faast et al. and Dixon and Tremblay, this issue). Seed set is rapid and seed pods mature in 4–6 weeks depending on species and location. Seed is dust-like, comprising a thin papery testa surrounding a spherical pro-embryo.
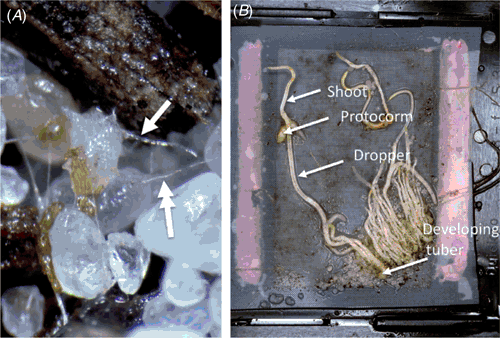
Seed germination in Caladenia requires intercession by a mycorrhizal fungus and under laboratory conditions, germination takes 4–6 weeks for swelling of the pro-embryo and for production of the trichomes leading to development of the distinctive protocorm phase common to all orchids (Fig. 2A). Trichomes are principally associated with controlling movement of the mycorrhizal fungus into and out of the developing protocorm and seedling. A single leaf emerges 6–12 weeks after germination followed by production of a depth-seeking structure known as the dropper that carries the developing tuber to depth (Pate and Dixon 1982; Dixon 1991). Effective propagation for conservation of terrestrial orchids, including Caladenia, is therefore linked to an efficacious mycorrhizal fungus as shown by Anderson (1991) for terrestrial orchid taxa from North America. Symbiotic propagation compared with asymbiotic approaches, resulted in a hundred-fold improvement in seedling survival. Similar benefits are likely to be applicable to Caladenia (Ramsay and Dixon 2003).
|
Mycorrhiza form a critical part of the annual life cycle of Caladenia with the fungus capable of providing much of the necessary nourishment for seedling growth and tuber development. This was aptly demonstrated by Batty et al. (2006b) where buried seed encased in protective nylon gauze sachets were able to produce healthy, fully developed dormant tubers without sprouting leaves or photosynthetic tissues (Fig. 2B). Compared with other Australian terrestrial orchids, the specificity of mycorrhiza in Caladenia is well established with some taxa possessing highly specific, one-on-one fungal associations (Ramsay et al. 1986; Bonnardeaux et al. 2007) while other taxa appear able to engage with a wider diversity of fungi (Huynh et al. 2004). One Caladenia species appears to use a ‘super-fungus’ capable of germinating a variety of other Caladenia species (Hollick et al. 2005). In comparison, research with the rare and threatened grand spider orchid (Caladenia huegelii) from Western Australia highlighted how fungi isolated from common sympatric congeners could germinate the rare species, but not vice versa, and have resulted in niche occupancy by the common species to the exclusion of the rare species (Swarts 2007). The importance of ‘winner takes all’ niche competition by common orchid species to the exclusion of rare species when a shared symbiont is involved needs further investigation.
Conservation
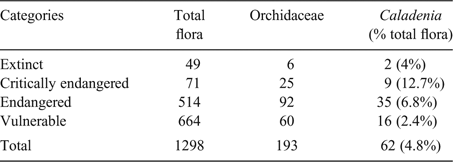
Caladenia has more species under threat than any other orchid genus in Australia (see http://www.environment.gov.au for details of listings of conservation taxa), with Caladenia alone contributing almost 5% of all threatened flora for Australia (Table 1). Caladenia, more than any other orchid genus, has a disproportionately high number of taxa in the most critical state of conservation and representing over a third of critically endangered orchids and almost 40% of endangered orchids.
|
The reasons for this remarkably high, genus-specific level of threat are probably site- and species-specific, that is, types and degree of threats for orchids in urban versus rural remnants may be very different and require different conservation approaches. For example, there is limited understanding of the ecological importance for Caladenia species of edaphic requirements such as composition and quantity of organic materials, microsite specialisation (light, moisture, co-habiting vegetation) with management practices such as prescribed burning (Dixon and Barrett 2003) likely to play a significant and possibly negative role in the edaphic equilibrium required to sustain the plant and mycorrhiza.
What is clear is that the obligate biotic-interactions associated with many species of Caladenia (specific mycorrhiza; often highly specific pollinator interactions; ecological niche specialisation) predispose the genus to a higher level of vulnerability to ecological change than taxa with broader ecological tolerance (ability for ‘ecological substitution’ Dixon and Tremblay, this issue) (Fig. 3).

|
For a genus experiencing such high levels of threat, there is a surprising dearth of information on plant population demography and dynamics, including individual plant longevity, both areas that are in need of research if conservation and management of Caladenia is to be effective across the diversity of biomes in which they occur. The few studies that are available (Batty et al. 2001; Dixon and Tremblay, this issue) show that Caladenia are restricted to sites with sufficient fungal abundance to support seed germination and plant establishment and that the soil seed-bank is transitory and limited to just one growing season. Of note is that in old, stable landscapes that support high diversity of Caladenia, such as the SWAFR (Hopper and Gioia 2004), dispersal adjacent to the parent plant represents the niche most favourable for maximising recruitment success (see Cramer and Hobbs 2007; and Hopper 2009b; for discussion of this concept), a phenomenon successfully applied in direct seeding practices for Caladenia, particularly rare species such as Caladenia hastata from Victoria (Hill et al. 1999).
With less than 40% of the Australian continent considered wilderness (Booth and Traill 2008), the prospects are remote for the conservation estate to adequately protect all Caladenia species as self-sustaining populations. Extinction-proofing Caladenia will therefore require an integrated approach (Swarts and Dixon 2009) including population and genetic diversity studies, biology of obligate biotic associates (mycorrhiza and pollinators), off-site genebanks of seed and mycorrhiza fungi and translocation research linked to a robust horticultural science program. Significant progress has occurred in improving the success of generating orchid plants from seed under laboratory conditions (Batty et al. 2006a). However, these techniques remain labour-intensive and further research is required to determine whether large-scale conservation translocations will be possible for Caladenia.
Ultimately, the dire predictions of impacts of climate change (Fig. 4) for species existence will require consideration of ‘assisted migration’ where species, including common taxa, will need to be translocated to new, climatically buffered safe-sites as home ranges contract (McLachlan et al. 2007).
|
This special issue
This volume of papers arose from a workshop held in Adelaide during December 2007 on the biology and conservation of Caladenia. The workshop was held in response to a request from the Australian Orchid Foundation to determine whether a more co-ordinated research effort across a significant number of research scientists could be harnessed to improve conservation outcomes for the genus.
The workshop was attended by national and international participants engaged in Caladenia research. Invited papers covered the key research areas of taxonomy and phylogeny, population ecology, mycorrhizal associations, impact of climate change and conservation management, concluding with a special session on research and management knowledge gaps. Several research papers from these themes form the basis for this volume.
The papers in this special issue of the Australian Journal of Botany are arranged into sections, with the present contribution providing an overview of the systematics, diversity and intriguing biological attributes of the genus that have attracted naturalists and scientists alike to admire and study Caladenia in a broad range of scientific disciplines.
Within the subsection Biology and Biogeography is a review by Dixon and Tremblay that provides the current state of knowledge on the biology and natural history of the genus. The authors highlight particular attributes of the genus that may predispose species to higher levels of threat in the environment. Then Phillips et al. investigate the biogeography of Caladenia, highlighting how the conservation estate may be under-representing some threatened habitats favoured by Caladenia. The final paper in this section by Farrington et al. deals with the emerging molecular opportunities for resolving the often vexed taxonomic complexities associated with Caladenia. They discuss how current molecular tools with further development will play an important role in resolving the conflict.
The diverse and often complex pollination syndromes in Caladenia represent fundamental knowledge necessary for effective conservation planning for the genus. The section, Pollination Biology, highlights principles across the genus and within species. The lead paper by Phillips et al. shows how the food to sexual deception pollination continuum found in Caladenia species plays a critical role in determining pollination success in several species and where this information underpins conservation planning for species. Using an array of ‘baiting’ approaches, Faast et al. in their paper on pollination in Caladenia rigida demonstrate the potential for both food and sexual deceptive pollination syndromes, with the first quantitative evidence of nectar production for the genus. The study highlights the risks of assuming a pollination syndrome for Caladenia and the need for carefully designed empirical research to elucidate possible pollinators. Developing more effective hand pollination for conservation of threatened species is part of the study by Petit et al. where they investigate the rare Caladenia behrii. The study demonstrates that seed production is correlated with leaf size, with improvement in seed quality linked to the amount of pollen on the stigma with outcrossed seed exhibiting higher germinability.
The challenge for conservation agencies is integrating the complete life cycle of an endangered species within its community and predicting the future of the species of interest. Moreover, species that are included in conservation programs are often included on such lists as a consequence of few extant individuals or populations. Ecological studies of small populations are problematic because statistical tests are limited by sample size. In the Ecology and Population Biology section, Coates and Duncan overcome the small sample size issue by using longitudinal data from at least eight years of survey. Coates and Duncan show that short-term dynamics may be influenced by reproductive success while long-term persistence of the populations will require maintenance of the dominant shrub community. In a another paper, Tremblay et al. likewise used longitudinal surveys of up to 12 years to estimate the rates of dormancy as well as transition rates. They show that dormancy in Caladenia, although variable among species, is mainly limited to very short periods of one or two years. In their study of population dynamics in Caladenia, Tremblay et al. used a Bayesian approach to facilitate the incorporation of transition information from multiple species to estimate the life cycle of nine species, thus acquiring information for each species even though sample size for some species was limited in number or years. Population persistence of many of the Caladenia species will require large investment in management of the species to assure recruitment, otherwise there is a high probability of population extinction. Finally, the intriguing conservation-based study by Faast and Facelli demonstrate how the preferential florivorous selection for Caladenia flowers by native chuffs can result in substantial depression in seed set for the study species. This study provides a timely reminder of the subtleties in orchid conservation science and how terrestrial taxa with their multitude of complex associations typified by Caladenia can result in rapid loss of plants.
A recent and promising development in Caladenia conservation has been that of effective propagation techniques for translocation of artificially propagated plants to habitat. These advances are reviewed in the final paper of this special issue, where Wright et al. outline current advances in symbiotic techniques that have resulted in significant improvement in success rates in the transfer of plants to soil, and ultimately to habitat. The research presented offers great promise for more effective use of in situ conservation technologies for rebuilding depleted species and populations, particularly critically endangered species or species where introduction (to new habitats) will be necessary to conserve a species.
Anderson AB
(1991) Symbiotic and asymbiotic germination and growth of Spiranthes magnicamporum (Orchidaceae). Lindleyana 6, 183–186.

Batty AL,
Brundrett MC,
Dixon KW, Sivasithamparam K
(2006a) New methods to improve symbiotic propagation of temperate terrestrial orchid seedlings from axenic culture to soil. Australian Journal of Botany 54, 367–374.
| Crossref | GoogleScholarGoogle Scholar |

Batty AL,
Brundrett MC,
Dixon KW, Sivasithamparam K
(2006b) In situ symbiotic seed germination and propagation of terrestrial orchid seedlings for establishment in field sites. Australian Journal of Botany 54, 375–381.
| Crossref | GoogleScholarGoogle Scholar |

Batty AL,
Dixon KW,
Brundrett MC, Sivasithamparam K
(2001) Constraints to symbiotic germination of terrestrial orchid seed in a mediterranean bushland. New Phytologist 152, 511–520.
| Crossref | GoogleScholarGoogle Scholar |

Bonnardeaux Y,
Brundrett M,
Batty AL,
Dixon KW,
Koch J, Sivasithamparam K
(2007) Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research 111, 51–61.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hollick PS,
Taylor RJ,
McComb JA, Dixon KW
(2005) If orchid mycorrhizal fungi are so specific, how do natural hybrids cope? Selbeyana 26, 159–170.

Hopper SD
(2009a) Taxonomic turmoil down-under: recent developments in Australian orchid systematics. Annals of Botany (in press). ,
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hopper SD
(2009b) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil (in press). ,

Hopper SD, Brown AP
(2000) New genera, subgenera, combinations, and species in the Caladenia alliance (Orchidaceae: Diurideae). Lindleyana 15, 120–126.

Hopper SD, Brown AP
(2001) Contributions to Western Australian Orchidology: 2. New taxa and circumscriptions in Caladenia (Spider, Fairy and Dragon Orchids of Western Australia). Nuytsia 14, 27–314.

Hopper SD, Brown AP
(2004) Robert Brown’s Caladenia revisited, including a revision of its sister genera Cyanicula, Ericksonella and Pheladenia (Caladeniinae: Orchidaceae). Australian Systematic Botany 17, 171–240.
| Crossref | GoogleScholarGoogle Scholar |

Hopper SD, Gioia P
(2004) The Southwest Australian Floristic Region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution and Systematics 35, 623–650.
| Crossref | GoogleScholarGoogle Scholar |

Huynh TT,
McLean CB,
Coates F, Lawrie AC
(2004) Effect of developmental stage and peloton morphology on success in isolation of mycorrhizal fungi in Caladenia formosa (Orchidaceae). Australian Journal of Botany 52, 231–241.
| Crossref | GoogleScholarGoogle Scholar |

Jones DL,
Clements MA,
Sharma IK, Mackenzie AM
(2001) A new classification of Caladenia R.Br. (Orchidaceae). Orchadian 13, 389–419.

Kores PJ,
Molvray M,
Weston PH,
Hopper SD,
Brown AP,
Cameron KM, Chase MW
(2001) A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. American Journal of Botany 88, 1903–1914.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

McLachlan JS,
Hellman JJ, Schwartz MW
(2007) A framework for debate of assisted migration in an era of climate change. Conservation Biology 21, 297–302.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ramsay RR,
Dixon KW, Sivasithamparam K
(1986) Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana 1, 203–214.

Swarts N, Dixon KW
(2009) Orchid conservation in the age of extinction. Annals of Botany (in press)15 February 2009). ,
| Crossref | GoogleScholarGoogle Scholar | PubMed |



