Pittosporum undulatum is a potential Australian host of Phytophthora ramorum
D. Hüberli A B C , C. Wilkinson A , M. A. Smith A , M. Meshriy A , T. Y. Harnik A and M. Garbelotto AA Department of ESPM-ES, University of California, 137 Mulford Hall, Berkeley, CA 94720, USA.
B Current address: Centre for Phytophthora Science and Management, School of Biological Sciences and Biotechnology, Murdoch University, Murdoch, WA 6150, Australia.
C Corresponding author. Email: d.huberli@murdoch.edu.au
Australasian Plant Disease Notes 1(1) 19-21 https://doi.org/10.1071/DN06009
Accepted: 16 September 2006 Published: 27 September 2006
Abstract
Pittosporum undulatum is a potential Australian host of Phytophthora ramorum, the causal agent of sudden oak death in California. It was susceptible and supported sporulation in zoospore inoculations of detached leaves. Susceptibility and sporulation potential were low when compared to Umbellularia californica. Two independent positives were obtained from symptomatic trees in a PCR-based assay using species-specific primers. Foliar symptoms observed on the trees were replicated in the detached leaf inoculations.
Additional keywords: horticulture, oomycete, nursery, sporulation, sudden oak death.
Australian, New Zealand and other southern hemisphere native plants are grown and traded throughout the ornamental industry both in the USA and in Europe. Recently in Europe, the Australian species Eucalyptus haemastoma (scribbly gum), the Chilean Nothofagus obliqua (southern beech) and the New Zealand Griselinia littoralis (New Zealand broadleaf) have been listed as natural hosts of Phytophthora ramorum, the causal agent of sudden oak death (DEFRA 2006). The pathogen has become a major disease concern in coastal forest ecosystems of western USA and in nurseries and private estates of Europe (Werres et al. 2001; Rizzo et al. 2002). Thus far, P. ramorum has not been detected in Australia and New Zealand, although a preliminary study has identified possible ecosystems with similar climatic conditions to those in the USA and Europe that could be conducive to disease development (W. Smith, unpublished data). Furthermore, the European example of the recent isolations of P. ramorum from several tree species in the field that had previously been identified as potential hosts in artificial inoculations (Brasier et al. 2004), highlights the importance in identification of potential hosts from disease-free countries. Knowledge of potential hosts and, therefore, carriers of the pathogen, will provide data on which robust quarantine guidelines can be built and thus help prevent the inadvertent introduction of P. ramorum into Australia and other regions of the world. It will also assist to reduce the further spread of the pathogen via the nursery industry in USA and Europe. However, the potential hosts’ role in transmission of the pathogen, should an incursion occur in disease-free countries, has not been elucidated in any of the previous studies.
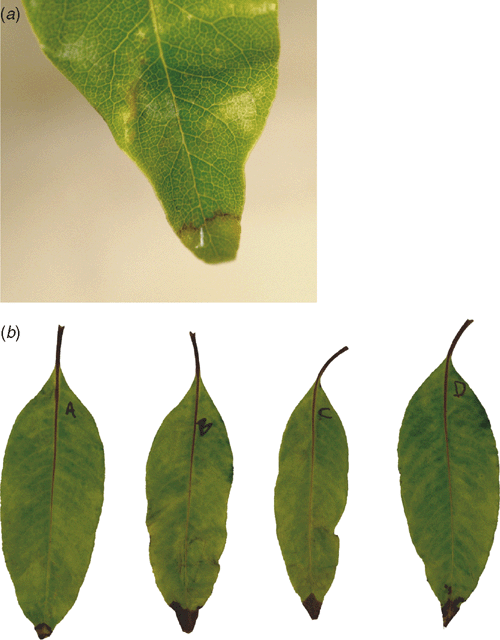
In October 2001 to April 2003, numerous foliar lesions were observed on six Pittosporum undulatum (Victorian Box) growing on the campus of the University of California, Berkeley. These trees were growing ~500 m from Umbellularia californica (bay laurel) trees infected with P. ramorum; these trees are now known to be one of the most epidemiologically important hosts of P. ramorum (Davidson et al. 2005). Foliar symptoms on P. undulatum included numerous dark brown leaf spots (>0.5 cm in diameter) and/or dark brown lesions on leaf tips (Fig. 1).
|
Internal transcribed spacer (ITS) rDNA sequences (GenBank accession number DQ665269) of 288 bp obtained from the symptomatic leaves of P. undulatum were identical to those of P. ramorum S. Werres & A.W.A.M. de Cock (Werres et al. 2001; Rizzo et al. 2002). Symptomatic leaves were also plated onto pimaricin–ampicillin–rifampicin–PCNB agar (P10ARP) containing 25 mg PCNB, a selective medium for Phytophthora spp. (Erwin and Ribeiro 1996). Although the PCR-primer test was positive on two independent runs, several attempts to isolate the pathogen from the leaves during 2001 to 2003 were unsuccessful. Since 2003, these trees have not displayed the described symptoms, which has hindered further attempts to culture the pathogen from this host.
Isolation of Phytophthora spp. is often difficult and may lead to false-negative isolations and misdiagnosis of infected plants (Hüberli et al. 2000). Direct PCR amplification from symptomatic plant tissue has been shown to be more sensitive and reliable than culturing onto a selective agar medium for initial detection of P. ramorum (Garbelotto et al. 2003). In fact, of the 13 non-oak host species first identified in California, 10 were detected using the PCR method before the isolation of a pure culture. For one particular host, Acer macrophyllum (big leaf maple), it took more than one year after the PCR positive for P. ramorum to isolate the culture.
To test for pathogenicity to P. undulatum, asymptomatic leaves were inoculated using two different methods. In the first inoculation trial, small twigs containing 5–6 asymptomatic and mature leaves were cut from one tree with symptoms and each twig placed into a 150-mL conical flask containing sterile deionised water. Two leaves for each of the 5 twigs were inoculated with zoospores of P. ramorum culture Pr-52 (American Type Culture Collection, Manassas, VA, ATCC MYA-2436; Centraal Bureau voor Schimmelcultures, Baarn, the Netherlands, CBS110537) originally isolated from a Rhododendron sp. in California. This isolate had been shown to be highly pathogenic in previous inoculation studies (Hüberli et al. in press). The zoospores were produced by placing agar discs (1 cm in diameter) taken from the margin of 8 to 14 day-old colonies growing on V8 juice agar into 20–30 mL sterile deionized water in Petri dishes. After 2 days incubation at 20°C in the dark, zoospore release was induced by placing dishes at 4°C for 20 min, and then returned to room-temperature for 45–60 min. Three hundred µL of the zoospore suspension (~2 × 104 zoospores/mL) were pipetted into 500-µL modified microcentrifuge tubes in which tips of leaves were submerged. One control leaf per twig was dipped in sterile deionised water. Plants were placed in a humid chamber consisting of moist paper towels placed on the tray and covered with a clear-plastic lid that was sprayed with sterile water. The chambers were maintained at 20–24°C in the laboratory. All inoculum tubes were removed after 12 h. Twigs were incubated for 20 days and sprayed occasionally with sterile water when leaves were almost dry. At harvest, leaves were detached from twigs and the lesion length from leaf tip to the lesion margin measured. To confirm the presence of P. ramorum, two small leaf pieces from the lesion margin or region of inoculation were plated onto P10ARP. The described inoculation method has been used for previous confirmations of Koch’s postulates (Hüberli et al. 2003).
In the second inoculation trial, 21 asymptomatic and mature leaves were collected from four of the symptomatic P. undulatum trees and from one U. californica tree; the latter was included as a known susceptible host with a high sporulation potential (Davidson et al. 2005). Leaves were submerged, leaf tip first, into individual 50-mL conical tubes to which 300 µL of zoospores were added. Leaves in tubes were incubated for 12 h at 19°C and then removed and placed into humid chambers at 19°C for 6 days in darkness. One leaf per tree was submerged in sterile water as the negative control. Lesion area was determined using image analysis software, Assess (Version 1.01, APS Press, St Paul, MN). The lesion area or the region of inoculation was gently scraped with a flat spatula on both surfaces into individual wells of a microtiter plate containing 300 µL sterile deionised water. One drop of phenol-cotton blue was added to each well and the number of sporangia per well was counted under the microscope. Two leaf pieces of the lesion or region of inoculation were plated onto P10ARP.
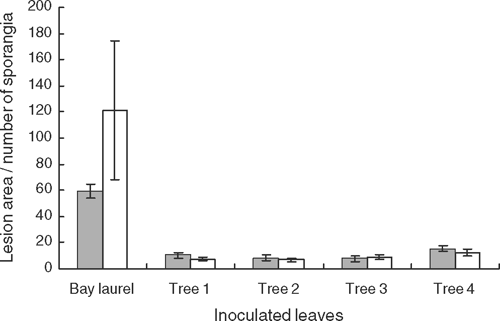
In both trials, water-soaked lesions on the leaves of P. undulatum were first observed 32 h after inoculation with P. ramorum (Fig. 1). At 3–4 days after inoculation, dark brown lesions identical to those observed on the symptomatic trees were present on all inoculated leaves. In trial 1, foliar lesion lengths averaged 18.4 ± 1.4 mm (± s.e.) 20 days after inoculation. Foliar lesion areas for P. undulatum in trial 2 averaged 7.6 ± 2.5 mm2 (± s.e.) to 15.4 ± 2.3 mm2 (± s.e.), and were more than 3-fold smaller than the lesions on U. californica leaves (Fig. 2). While P. undulatum supported sporulation on the leaves, the numbers of sporangia produced were more than 9-fold lower than those produced on U. californica leaves (Fig. 2). Phytophthora ramorum was reisolated on P10ARP from all lesions in both trials. Control leaves had no lesions, nor was P. ramorum reisolated from control leaves.
|
Koch’s postulates have not been strictly fulfilled as they state that the organism must be cultured from the original diseased plant to prove that it is the causal agent. However, with the use of known isolates of the pathogen it is reasonable to show the susceptibility of a host where difficulties occur in the initial isolation of the pathogen (either environmental or host), particularly where molecular methods have shown the presence of the pathogen in host tissue. In this case, two independent PCR positives were obtained from field samples and the symptoms observed in the field were replicated in two artificial zoospore inoculations. This approach, in the absence of direct isolation, has been an integral component in determining natural hosts of P. ramorum in California (Garbelotto et al. 2003). Further isolations from field plants will be attempted when fresh symptoms reoccur.
This is the first report of a plant from the Pittosporaceae as a potential host for P. ramorum and adds to the list of potential Australian hosts. Pittosporum undulatum is a widespread landscape and forest understorey tree in parts of south-eastern Australia and is also popular in the horticultural industry in California. While less susceptible than U. californica, foliar sporulation was supported, and thus the pathogen could be disseminated in the horticultural industry or plants could possibly act as a natural transmissive host as does U. californica in California (Davidson et al. 2005). Further screening for susceptibility and transmission potential of Australian plants, particularly from the eastern states where climatic conditions would be conducive for P. ramorum disease, will commence shortly.
Acknowledgements
The project was funded by the Pacific South-West Research Station USDA Forest Service and the Betty and Gordon Moore Foundation.
Brasier C,
Denman S,
Brown A, Webber J
(2004) Sudden oak death (Phytophthora ramorum) discovered on trees in Europe. Mycological Research 108, 1108–1110.
| Crossref | GoogleScholarGoogle Scholar |
(verified 18 September 2006)
Garbelotto M,
Davidson JM,
Ivors K,
Maloney PE,
Hüberli D,
Koike ST, Rizzo DM
(2003) Non-oak native plants are main hosts for sudden oak death pathogen in California. California Agriculture 57, 18–23.

Hüberli D,
Tommerup IC, Hardy GEStJ
(2000) False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australasian Plant Pathology 29, 164–169.
| Crossref | GoogleScholarGoogle Scholar |

Hüberli D,
Van Sant-Glass W,
Tse JG, Garbelotto M
(2003) First report of foliar infection of starflower by Phytophthora ramorum. Plant Disease 87, 599.

Rizzo DM,
Garbelotto M,
Davidson JM,
Slaughter GW, Koike ST
(2002) Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Disease 86, 205–214.

Werres S,
Marwitz R,
Man In’T Veld WA,
De Cock AWAM,
Bonants PJM,
De Weerdt M,
Themann K,
Ilieva E, Baayen RP
(2001) Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycological Research 105, 1155–1165.



