First report of Tubercularia lateritia as the causal agent of canker on macadamia
O. A. Akinsanmi A B and A. Drenth AA Tree Pathology Centre, The University of Queensland and Department of Primary Industries and Fisheries, 80 Meiers Road, Indooroopilly, Qld 4068, Australia.
B Corresponding author. Email: uqoakins@uq.edu.au
Australasian Plant Disease Notes 1(1) 49-51 https://doi.org/10.1071/DN06019
Submitted: 26 October 2006 Accepted: 22 November 2006 Published: 30 November 2006
Abstract
Tubercularia lateritia was recorded for the first time as causing canker, characterised by a sunken centre surrounded by galls or callus, on macadamia. The fungus was isolated and inoculated on young macadamia trees in the glasshouse and produced characteristic disease symptoms from which the fungus was successfully reisolated.
Macadamia (Macadamia integrifolia Maiden & Betche and M. tetraphylla L.A.S. Johnson) are native to Australia. Both macadamia species and their hybrids are commercially grown in many countries including the United States of America (USA), Kenya, Costa Rica, South Africa, Brazil and Australia. At present, Australia is the world’s largest producer of nut-in-shell followed by the USA, and production in other countries such as South Africa is increasing rapidly. Macadamia stems and branches are affected by several diseases including trunk canker and tree decline caused by Phytophthora spp., Dothiorella canker caused by Botryosphaeria ribis Grossenb. & Duggar and pink limb blight caused by Phanerochaete salmonicolor (Berk. & Broome) Jülich (Fitzell 1994).
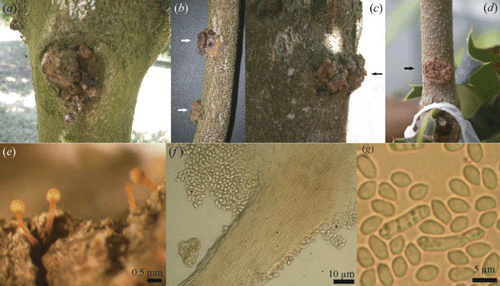
In late 2004, we observed canker with callus formation on the main trunk and on old and young branches of several macadamia varieties in three orchards in the Northern Rivers region of New South Wales (NSW). In some cases, gum exudates occurred on infected parts of the trees and the tissue under the canker and callus was discoloured. The galls sometimes coalesced and the bark was completely sloughed off or irregular in shape (Fig. 1a–c). Fungal structures were evident singly or in clusters on the canker margin as creamy-white or orange pinhead-shaped elongated synnemata (Fig. 1e), bearing conidia in clusters of acropetal chains (Fig. 1f). When culture plates were incubated at room temperature (25°C ± 2°C) under diurnal 12 h fluorescent light : dark conditions, mycelium was whitish on oat meal agar (20 g rolled oats, 12.5 g Davis J3 agar in 1 L distilled water), whereas on potato dextrose agar (PDA, 200 g potatoes, 20 g dextrose, 12.5 g Davis J3 agar in 1 L distilled water) only scant aerial mycelium was produced. The mycelium was superficial, composed of branched, septate, hyaline, smooth thin-walled hyphae. Synnemata and scattered masses of orange-coloured sporodochia were produced on both of the culture media. Some orange-brown coloured perithecia-like structures were also produced on PDA, but no ascospores were visible. Measurements of the synnemata produced in culture and field samples were similar, up to 800–1076 μm long, with a stipe (907 long × 70–94 μm wide) and were capitate (340–354.5 μm wide), parallel determinate, slender, creamy-white to orange, unbranched and erect. The conidiophores were undifferentiated with globose capitulum bearing monophialidic conidiogenous cells. The stromatic pigment did not change in KOH (Seifert 1985). Conidia were oblong-elipsoidial, aseptate, hyaline, smooth and thin-walled, measuring 6.3–8.0 × 2.7–3.6 μm (Fig. 1g). Based on the morphological characteristics, the fungus was identified as the anamorphic stage, Tubercularia lateritia (Berk.) Seifert of Nectria pseudotrichia Berk. & M.A. Curtis (syn. Thyronectria pseudotrichia (Berk. & M.A. Curtis) Seeler (Seifert 1985; Rossman et al. 1999) by Dr Amy Rossman (Herbarium BPI, USA). A specimen has been deposited in Queensland Department of Primary Industries and Fisheries Plant Pathology Herbarium as BRIP 46556. Sequences of the ITS region of the rDNA obtained after PCR amplification performed with ITS4 and ITS5 primers (White et al. 1990) were congruent with BRIP herbarium cultures (BRIP 46786 and BRIP 46787) of the teleomorph state, N. pseudotrichia from different hosts.
|
To conduct Koch’s postulates and confirm the pathogenicity of T. lateritia on macadamia, five replicate pots (10-cm diameter) containing steam-pasteurised potting mix (1 peat : 1 soil : 1 sand, v/v) with glasshouse-grown macadamia cultivar H2 seedlings of ~60 cm height were wound-inoculated on the main stem. A small incision in the bark of each seedling was made with a sterile scalpel and a 40 µL T. lateritia spore suspension (106 spores/mL) was added beneath the bark before wrapping the cut section with parafilm. Seedlings inoculated with sterile distilled water served as controls. Parafilm was removed after 14 days and the inoculated plants were kept in the glasshouse at 20–28°C for six months and were regularly inspected for canker with gall formation. Appearance of canker symptoms with galls similar to those observed in field trees indicated successful infection (Fig. 1d). Symptoms were visible from three months after inoculation. Pieces of the symptomatic tissue were taken with a sterile scalpel, surface-sterilised in 2.5% sodium hypochlorite solution for three minutes and then rinsed in three changes of sterile distilled water before plating on PDA. Cultural characteristics were similar to those produced from field isolates. When the galls were removed, the underlying parts were darkened and this extended up and below the point of inoculation and deeper in the stem tissue. The fungal fruiting structures were not produced on the inoculated seedlings within the six months observation period.
Both the asexual and sexual stages of N. pseudotrichia are widely distributed in tropical and subtropical regions and it is one of the most conspicuous and common nectrioid fungi occurring on recently killed dicotyledonous wood (Rossman et al. 1999). It has also been associated with stem-end rot in avocado (Persea Americana Mill.) in Australia (Pegg et al. 2002) and stem canker in Japanese pear (Pyrus pyrifolia (Burm.) Nakai) in Brazil (Becker 2003). Although the economic losses on macadamia have not been determined, similar situations on apple and pear caused by Neonectria galligena (Bres.) Rossman & Samuels have resulted in the removal of infected trees (McCracken et al. 2003). The incidence of this canker disease in macadamia does not appear to be restricted to particular cultivars or specific geographical locations. The economic importance of the disease to macadamia and the potential of cross-pathogenicity with other crops including avocado will be investigated in further studies.
Becker WF
(2003) Nectria pseudotrichia, como agente causal de cancro de ramos, ocorrendo em pereira japonesa no Brasil. Fitopatologia Brasileira 28, 107.
| Crossref | GoogleScholarGoogle Scholar |

McCracken AR,
Berrie AM,
Barbara DJ,
Locke T,
Cooke LW,
Phelps K,
Swinburne TR,
Brown AE,
Ellerker B, Langrell SRH
(2003) Relative significance of nursery infections and orchard inoculum in the development and spread of apple canker (Nectria galligena) in young orchards. Plant Pathology 52, 553–566.
| Crossref | GoogleScholarGoogle Scholar |

Rossman AY,
Samuels GJ,
Rogerson CT, Lowen R
(1999) Genera of bionectriaceae, hypocreaceae and nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42, 1–248.

Seifert K
(1985) A monograph of Stilbella and some allied Hyphomycetes. Studies in Mycology 27, 1–235.



