Typing of Plum pox virus (PPV) strains in Cyprus
L. C. Papayiannis A B , A. Kyriakou A and T. Kapari-Isaia AA Agricultural Research Institute, PO Box 22016, Nicosia 1516, Cyprus.
B Corresponding author. Email: l.papayiannis@arinet.ari.gov.cy
Australasian Plant Disease Notes 2(1) 29-30 https://doi.org/10.1071/DN07013
Submitted: 6 December 2007 Accepted: 5 March 2007 Published: 29 March 2007
Abstract
A survey was conducted in the main stone fruit producing areas of Cyprus during 2004–05 in order to determine the identity and distribution of Plum pox virus strains using serological and molecular methods. A total of 72 peach, apricot and plum tree samples were analysed by ELISA and RT-PCR and results showed that PPV-M was the predominant strain, while PPV-D was identified for the first time in the island.
Plum pox virus (PPV) is a member of the genus Potyvirus (family Potyviridae) and is considered to be one of the most devastating diseases of stone fruit crops. The disease was first described to infect plums in Bulgaria around 1917, where it was named ‘sharka’ (Atanasoff 1935), and has since spread to a large part of Europe, many Mediterranean countries (Myrta et al. 1998), America (Stobbs et al. 2005) and Asia (Spiegel et al. 2004; Navratil et al. 2005). Contaminated plant-propagating material is considered the most important means of long distance spread of the disease. The virus is transmitted from plant to plant by grafting or by several aphid species in a non-persistent manner (Labonne et al. 1995). PPV infects various members of the genus Prunus, such as plums (Prunus domestica), apricots (P. armeniaca), peaches and nectarines (P. persica), causing important economic losses. Foliar symptoms include chlorotic rings and yellow patterns which are more prominent during spring (Fig. 1) while fruits can be severely deformed and bumpy. Severity of the symptoms in diseased plants depends on the susceptibility of Prunus cultivars, the virus strains and the simultaneous infection with other viruses and/or viroids. To the present, four PPV strains have been described worldwide. PPV-M (Marcus), originating from peach trees in Greece and PPV-D (Dideron) from apricots in France, are the two most common strains (Kernal and Dunez 1979). PPV-EA (El Amar) and PPV-C (Cherry) are two strains described on apricots in Egypt and sour cherry in Mouldova, respectively (Wetzel et al. 1991). The above PPV isolates exhibit large differences in symptomatology of infected trees, aphid transmissibility and disease epidemiology. The M isolates spread more aggressively than D isolates and cause more severe symptoms and heavier crop losses (Boscia et al. 1997).

|
In Cyprus, PPV-M was first detected in 1982 in the western part of the island on apricots, peaches and plums. A stone fruit certification program was initiated in 1997 in order to produce and distribute certified virus-free propagating material. A regional study using strain-specific monoclonal antibodies (MAbs), showed that PPV-M was the only virus strain present in Cyprus (Myrta et al. 1998).
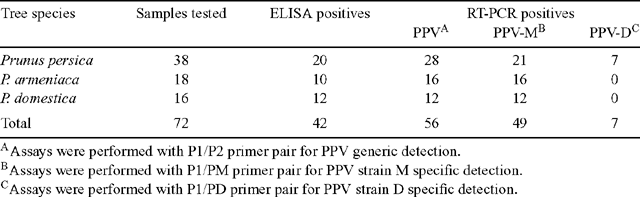
During 2004–05, a survey was conducted in the main stone fruit producing areas of Cyprus in order to investigate the identity and prevalence of PPV strains by the use of molecular methods. A total of 72 leaf samples from peaches, apricots and plums with or without symptoms were collected from the areas of Agros, Kyperounda, Galata, Pera, Psimolophou, Akchelia and Avdimou (Table 1). All samples were tested using the standard double-antibody sandwich ELISA, as described by Clark and Adams (1977), using polyclonal antibodies against PPV. In addition, total RNA from all samples was extracted using a commercial kit and a reverse transcription–polymerase chain reaction (RT-PCR) test was performed in order to confirm serology results, using the broad reactivity PPV-specific primers P1/P2 (Wetzel et al. 1991). Moreover, samples were also analysed using the PPV-M and PPV-D strain-specific primers P1/PM and P1/PD (Olmos et al. 1997). All reactions were carried out in a PTC-200 thermocycler (MJ Research, Inc., USA). Amplified products were analysed by gel electrophoresis on 1.5% agarose gels in TAE buffer, stained with ethidium bromide and photographed under UV light.
|
Out of the 72 samples tested by the above methods, 42 tested positive using ELISA, while a 0.24-kb fragment was amplified from 56 samples, including the 42 ELISA-positive samples, tested by RT-PCR using the PPV generic P1/P2 primer pair (73% peach, 88% apricot and 75% plum trees). Strain-specific primer results showed that 49 PPV isolates gave an amplification product with primers against the M type, whereas 7 peach cultivars located at Agros area gave a positive reaction with the D-specific primer pair (Table 1).
Molecular tests for the detection of PPV were used for the first time in Cyprus. RT-PCR was proven to be a more accurate and sensitive tool for the detection of PPV in comparison with the serological ELISA techniques. In addition, RT-PCR was shown to be a quick and reliable tool for the discrimination of sharka disease strains. To our knowledge this is the first report of the PPV-D strain infecting P. persica trees in Cyprus. More research is required to determine the current situation of the disease on the island. A new survey using modern detection techniques and an eradication project needs to be established in addition to a valid certification program for preventing the introduction and spread of possible more destructive exotic strains.
Atanasoff D
(1935) Mosaic of stone fruits. Phytopathologie Zeitschrift 8, 259–284.

Boscia D,
Zeramdini H,
Cambra M,
Potore O,
Gorris MT,
Myrta A,
Di Terlizzi B, Savino V
(1997) Production and characterization of a monoclonal antibody specific to the M serotype of plum pox potyvirus. European Journal of Plant Pathology 103, 477–480.
| Crossref | GoogleScholarGoogle Scholar |

Clark MF, Adams AN
(1977) Characteristics of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. The Journal of General Virology 34, 475–483.
| PubMed |

Kernal C, Dunez J
(1979) Differentiation biologique et serologique des souches du virus de la sharka. Annals de Phytopathologie 11, 541–555.

Labonne G,
Yvon M,
Quiot JB,
Avinent L, Llácer G
(1995) Aphids as potential vectors of plum pox virus: Comparison of methods of testing and epidemiological consequences. Acta Horticulturae 386, 207–218.

Myrta A,
Di Terlizzi B,
Boscia D,
Caglayan K,
Gavriel I,
Ghanem G,
Varveri C, Savino V
(1998) Detection and serotyping of Mediterranean plum pox virus isolates by means of strain-specific monoclonal antibodies. Acta Virologica. English Ed. 42, 251–254.

Navratil M,
Safarova D,
Karesova R, Petrzik K
(2005) First incidence of Plum pox virus on apricot trees in China. Plant Disease 89, 338.

Olmos A,
Cambra M,
Dasi MA,
Candresse T,
Esteban O,
Gorris MT, Asensio M
(1997) Simultaneous detection and typing of plum pox potyvirus (PPV) isolates by heminested-PCR and PCR-ELISA. Journal of Virological Methods 68, 127–137.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Spiegel S,
Kovalenko EM,
Varga A, James D
(2004) Detection and partial molecular characterization of two Plum pox virus isolates from plum and wild apricot in southeast Kazakhstan. Plant Disease 88, 973–979.

Stobbs LW,
Van Driel L,
Whybourne K,
Carlson C,
Tulloch M, Van Lier J
(2005) Distribution of Plum pox virus in residential sites, commercial nurseries and native plant species in the Niagara Region, Ontario, Canada. Plant Disease 89, 822–827.

Wetzel T,
Candresse T,
Ravelonandro M, Dunez J
(1991) A polymerase chain reaction adapted to plum pox potyvirus detection. Journal of Virological Methods 33, 355–365.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



