Mating types of Phytophthora colocasiae from the Pacific region, India and South-east Asia
J. L. Tyson A C and R. A. Fullerton BA Biosecurity New Zealand, Ministry of Agriculture and Forestry, PO Box 2526, Wellington, New Zealand.
B HortResearch, Private Bag 92-169, Auckland, New Zealand.
C Corresponding author. Email: tysonj@maf.govt.nz
Australasian Plant Disease Notes 2(1) 111-112 https://doi.org/10.1071/DN07046
Submitted: 28 March 2007 Accepted: 13 July 2007 Published: 26 July 2007
Abstract
Mating type interactions were examined between 54 Phytophthora colocasiae isolates from the Pacific region, India and South-east Asia. Forty isolates were found to be A2 mating type and 14 did not form oospores with either mating type. No A1 or self-fertile isolates were found.
Phytophthora colocasiae is the causal agent of leaf blight and corm rot of taro (Colocasia esculenta), a destructive disease that destroyed Samoa’s taro industry following its introduction in 1993 (Chan et al. 1998). It is present throughout most of Asia and is scattered through the Pacific region (CMI 1997). Research on mating types suggests that Hainan Island, China, is within the centre of origin (Zhang et al. 1994).
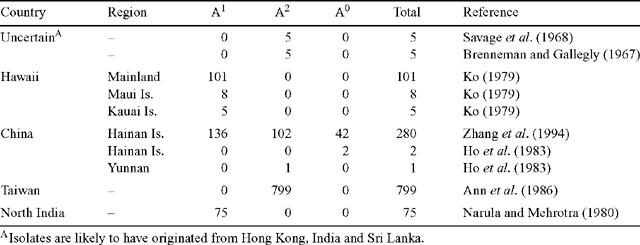
Phytophthora colocasiae is heterothallic, requiring the presence of opposite mating types (A1 and A2) for the formation of oospores. Heterothallic species of Phytophthora readily produce oospores in intra- or interspecific pairings of two compatible types (Ko 1979). The aim of this study was to examine the occurrence of the A1 and A2 mating types of P. colocasiae in the Pacific region, India and South-east Asia. The results of earlier determinations of mating type in P. colocasiae are summarised in Table 1.
|
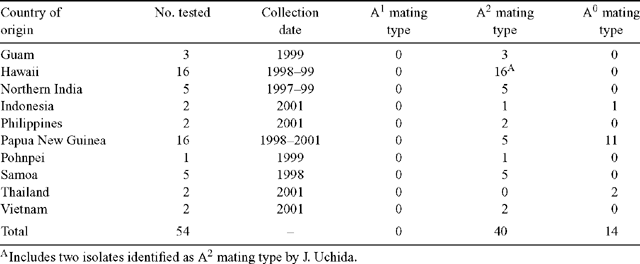
Fifty-four isolates of P. colocasiae were obtained from Guam, Hawaii, Northern India, Indonesia, Philippines, Papua New Guinea, Pohnpei, Samoa, Thailand, and Vietnam. Representative isolates have been deposited in the International Collection of Microorganisms from Plants (ICMP), Manaaki Whenua Landcare Research, Auckland, New Zealand. Two tester isolates of P. nicotianae were obtained from L.W. Timmer, University of Florida (P91-28 = mating type A1, Riv-1 = mating type A2). Among the isolates of P. colocasiae obtained from Hawaii were two previously determined to be mating type A2 by J. Uchida, University of Hawaii.
Isolates were placed 10–15 mm apart on unclarified V8 agar (100 mL V8 juice, 2 g CaCO3, 15 g agar, 900 mL distilled water) and on rape-malt agar (500 mL rapeseed extract, 1 g malt extract, 17 g agar and 500 mL distilled water), and incubated at 24 ± 1°C in the dark.
Each isolate was paired with itself and with P. nicotianae A1 and A2 mating types and a Hawaiian A2 isolate (Pc37) of P. colocasiae on separate plates. In addition, the two P. nicotianae mating type isolates were paired with each other. Pairs of cultures were examined for the formation of oospores after 7, 10 and 14 days. The process was repeated at least once for those isolates that did not form oospores with either of the tester isolates.
Oospores formed between fertile cultures in 1–2 weeks and between the tester isolates in 5–6 days. The results are summarised in Table 2.
|
This study has found only one mating type (A2) throughout the Pacific region, including Guam, Hawaii, Indonesia, Philippines, Papua New Guinea and Samoa. An earlier study (Ko 1979) records only the A1 mating type in Hawaii. More recently all isolates tested in Hawaii have been found to be mating type A2 (J. Uchida, pers. comm.). Only the A2 mating type was found among the Hawaiian isolates in the present study, and the mating type of the two A2 isolates provided by J. Uchida is confirmed.
Apart from the Hawaiian report by Ko (1979), P. colocasiae mating type A1 has been recorded only from Hainan Island and Northern India. Mating type A2 was previously known from Hainan Island and Yunnan in China, Taiwan, and Hawaii. That range can now be extended to include Guam, Indonesia, Northern India, Papua New Guinea, Philippines, Pohnpei, Samoa, Thailand and Vietnam.
Fourteen of the 54 isolates tested did not form oospores when paired with either of the tester isolates. Non-mating strains (A0 mating type) were found in Indonesia, Thailand and Papua New Guinea. Strains of A0 mating type have been previously reported from mainland China and Hainan Island (Ann et al. 1986; Zhang et al. 1994). In this study, the majority of isolates from Papua New Guinea were A0. The failure of the strains to mate is unlikely to be due to insufficient incubation time. Zhang et al. (1994) and Narula and Mehrotra (1980), using similar incubation conditions, found that P. colocasiae formed oospores with the opposite mating type in 3 days and 7–10 days, respectively. It is also unlikely to be due to the interspecific pairing as both P. parasitica (P. nicotianae) and P. palmivora have been used successfully in the past for mating type determination of various species of Phytophthora (Ko 1979; Narula and Mehrotra 1980; Ann et al. 1986; Zhang et al. 1994). Savage et al. (1968) found that an A2 isolate of P. colocasiae formed oospores when paired with A1 isolates of 11 different Phytophthora species. In this study, all of the A2 isolates from Papua New Guinea formed oospores sparingly when paired with the opposite mating type. It is possible that the A0 isolates from Papua New Guinea are of the A2 mating type but have lost the ability to reproduce sexually. The affinities of A0 strains may be able to be resolved by the use of strain-specific molecular markers.
Because of the apparently restricted distribution of the A1 mating type and the geographical separation from the areas in which it is found (Hainan Is., Northern India), the likelihood of its introduction to the Pacific region is considered to be relatively low. Nevertheless, its introduction would constitute a risk to the region. Not only would the resultant oospores create a new, longer-lived form of inoculum, but the potential for new, more virulent genotypes is increased both by the new incursions and by their genetic recombination with existing locally adapted strains.
Acknowledgements
The authors thank L.W. Timmer for the P. nicotianae A1 and A2 tester isolates; J. Uchida for Hawaiian isolates and advice on methods; K. Engelberger, T. Gunua, D. Hunter, G. Wall, Y. R. Sharma, V. Lebot and M. Duchamp for P. colocasiae isolates from various countries. Additional isolates were collected by R.A. Fullerton. This research was funded by the New Zealand Ministry of Foreign Affairs and Trade.
Ann PJ,
Kao CW, Ko WH
(1986) Mating-type distribution of Phytophthora colocasiae in Taiwan. Mycopathologica 93, 193–194.
| Crossref | GoogleScholarGoogle Scholar |

Brenneman JA, Gallegly ME
(1967) Sexual patterns of seven species of Phytophthora. Phytopathology [Abstr.] 57, 805.

Chan E,
Milne M, Fleming E
(1998) The causes and consequences of taro leaf blight in Samoa and the implications for trade patterns in the South Pacific region. Tropical Agriculture (Trinidad) 75, 93–98.

Ho HH,
Yu Y,
Zhuang W, Liang Z
(1983) Mating types of heterothallic species of Phytophthora in China. I. Acta Mycologica Sinica 2, 187–191.

Ko WH
(1979) Mating-type distribution of Phytophthora colocasiae on the island of Hawaii. Mycologia 71, 434–437.
| Crossref | GoogleScholarGoogle Scholar |

Narula KL, Mehrotra RS
(1980) Occurrence of A1 mating type of Phytophthora colocasiae. Indian Phytopathology 33, 603–604.

Savage EJ,
Clayton CW,
Hunter JH,
Brenneman JA,
Laviola C, Gallegly ME
(1968) Homothallism, heterothallism, and interspecific hybridization in the genus Phytophthora. Phytopathology 58, 1004–1021.

Zhang KM,
Zheng FC,
Li YD,
Ann PJ, Ko WH
(1994) Isolates of Phytophthora colocasiae from Hainan Island in China: evidence suggesting an Asian origin of this species. Mycologia 86, 108–112.
| Crossref | GoogleScholarGoogle Scholar |



