Leaf blight of Syzygium cumini and its management in vitro
A. Bhanumathi A and V. Ravishankar Rai A BA Department of Studies in Applied Botany and Biotechnology, University of Mysore, Manasagangotri, Mysore 570 006, Karnataka, India.
B Corresponding author. Email: rrai33@hotmail.com
Australasian Plant Disease Notes 2(1) 117-121 https://doi.org/10.1071/DN07049
Submitted: 18 July 2007 Accepted: 2 August 2007 Published: 13 August 2007
Abstract
A Pestalotiopsis sp. identified as the major pathogen causing leaf blight disease of Syzygium cumini was isolated from naturally infected leaf samples of S. cumini collected from forest nurseries of the Mysore district, India. The fungus was pathogenic on 4-month-old seedlings, which exhibited leaf blight symptoms within 15 days of inoculation. The effects of five systemic and two contact fungicides were evaluated against the pathogen in vitro using the poison food technique. Among the seven fungicides studied in vitro only two systemic fungicides namely, Bavistin and Roko were proven to be effective against Pestalotiopsis sp. at concentrations of 50, 100 and 150 mg/L; these fungicides showed 100% growth inhibition and nil fungal growth. The effectiveness of systemic fungicides was higher than that of contact fungicides. Thus, the present study recommends the use of Bavistin and Roko at a minimal concentration of 50 mg/L for maximum inhibition of Pestalotiopsis sp.
Introduction
Jambolan (Syzygium cumini L. Skeel) is a fast-growing, medicinally important plant of the family Myrtaceae and is one of the most common plants grown in forest nurseries of the Mysore district. The leaves are antibacterial and are used locally for strengthening teeth and gums. The fruits and seeds are sweet, acrid, sour, tonic and cooling and are used medicinally in diabetes, diarrhoea and ringworm (Prajapati et al. 2003). The bark is astringent, sweet, sour, diuretic, digestive and antihelminthic. The juice of the ripe fruit or a decoction of the fruit or jambolan vinegar may be administered in India in cases of enlargement of the spleen, chronic diarrhoea and urine retention. Water-diluted juice is used as a gargle for sore throats and as a lotion for ringworm of the scalp (www.alternative-healthguide.com, verified 2 August 2007).
Pestalotiopsis has always been considered to be a weak parasite and of minor importance in different crops and is being managed by manipulating existing cultural practices (Bilgrami et al. 1979). This pathogen has been recorded on a wide variety of hosts, mostly on their leaves, fruits and in the rhizosphere (Bilgrami et al. 1979). Early studies indicated that Pestalotiopsis sp. is ubiquitous in distribution, occurring on wide range of substrata. Many of them are saprobes (Wu et al. 1982), while others are pathogenic on a wide range of hosts causing various diseases including stem canker, necrotic lesions on leaf, seed and root rots in different hosts (Madar et al. 1991; Yuan 1996; Yuan and Mohammed 1999; Vujanovic et al. 2000; Dhingra et al. 2002, 2003). In the present study, a Pestalotiopsis sp. was identified as the major pathogen causing leaf blight on seedlings of S. cumini. Therefore, studies were conducted to evaluate the response of seven fungicides under in vitro conditions.
Methods
Isolation of the pathogen
Leaves of S. cumini with leaf blight symptoms (Fig. 1) were cut into 1 cm2 pieces and surface sterilised for 5 min using a 2% sodium hypochlorite solution followed by washing three times with distilled water. The leaf pieces were blotter-dried and placed on 2–3 layers of moistened blotters in Petri plates. The plates were incubated under 12 h/12 h cycles of lightness and darkness for 7 days. On 8th day the plates were screened for the pathogen.

|
Test for pathogenicity
A spore suspension of the leaf blight pathogen of S. cumini was prepared using a 7-day-old actively growing culture that was crushed in sterile distilled water using a pestle and mortar. The spore suspension was adjusted to 108 spores/mL using a haemocytometer and was then sprayed onto 4-month-old seedlings of S. cumini and covered with polythene covers to avoid secondary contamination. The polycovers containing S. cumini seedlings were watered daily to maintain humidity and observed regularly for symptoms.
In vitro management
Five systemic (Bavistin, Calixin, Contaf Plus, Roko and Tilt) and two contact (Blitox and Indofil M45) fungicides were tested at three different concentrations of 50, 100 and 150 mg/L for their efficacy against this leaf blight pathogen of S. cumini using the poison food technique (Dhingra and Sinclair 1985). Different concentrations of fungicides were prepared by dissolving the requisite quantity of each fungicide in warm potato dextrose agar before autoclaving. The autoclaved media were poured into Petri plates and allowed to cool. Actively growing 7-day-old culture of Pestalotiopsis sp. was cut into 0.4-cm diameter discs using a cork borer and was placed at the centre of each treatment. Each treatment was maintained in triplicate. Media without fungicide served as a control. The plates were incubated under 12 h/12 h cycles of lightness and darkness for 7 days. On the 8th day the radial growth of the mycelial colony was recorded and the percentage growth inhibition was calculated using the formula,
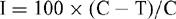
where I is percentage inhibition, C is growth of fungus in the control and T is growth of fungus in the treatment.
The experiment was repeated three times and the data was analysed statistically by analysis of variance and the means were compared by Duncan’s Multiple Range Test (P < 0.05).
Results
Isolation of pathogen
The pathogen was identified as a Pestalotiopsis sp. based on its morphological and conidial characters. Sooty black curls of conidia are the characteristic feature of the pathogen (Fig. 2a). Conidia have 3–5 septae, the middle cell is broad, the apical cell hyaline with three branched filiform appendages known as setulae. The basal cell is hyaline with a stipe (Fig. 2b).
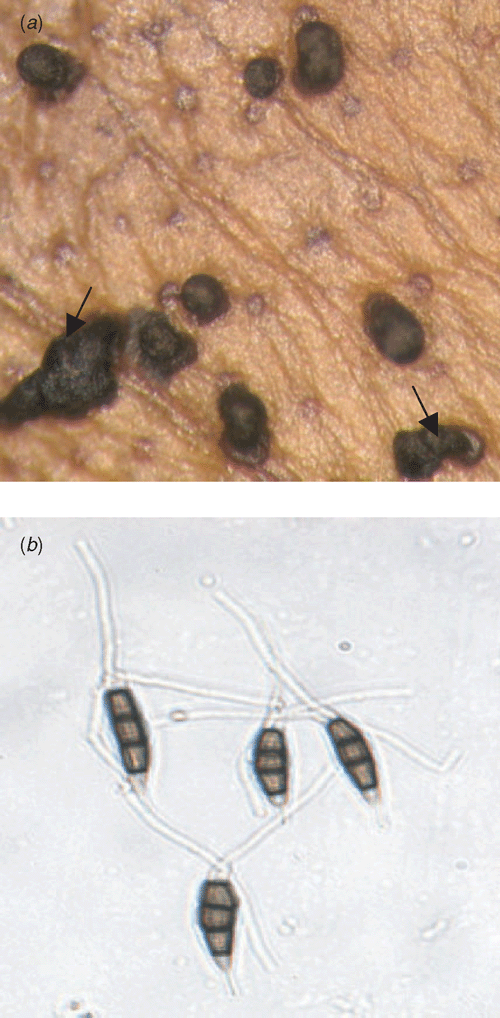
|
Test for pathogenicity
Initial symptoms occurred after 30 days and became prominent after 2 months (Fig. 3). The leaves with blight symptoms were surface sterilised using a 2% sodium hypochlorite solution and subjected to the standard blotter method and the pathogen was reisolated.

|
In vitro management
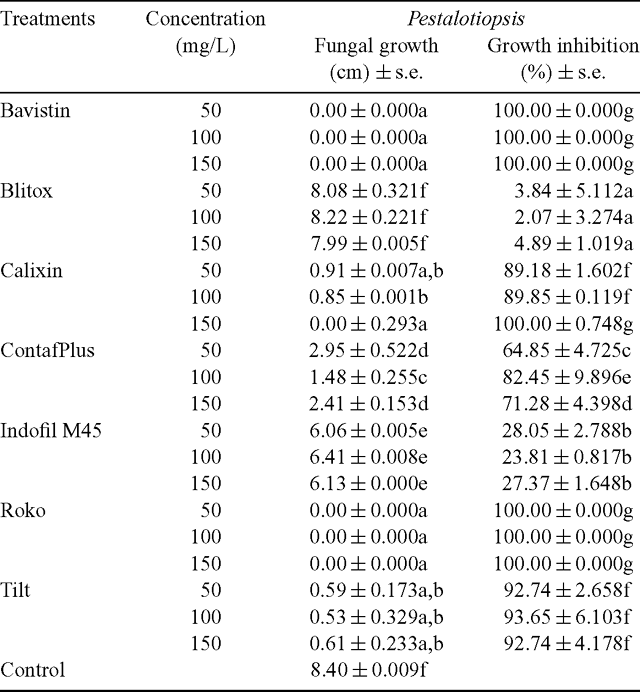
Effect of fungicides against Pestalotiopsis sp. significantly differed from the control in response of radial growth irrespective of concentrations at 1% level of significance and percentage growth inhibition (Table 1).
|
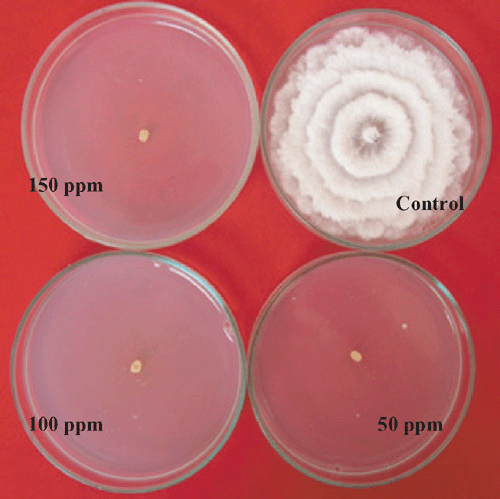
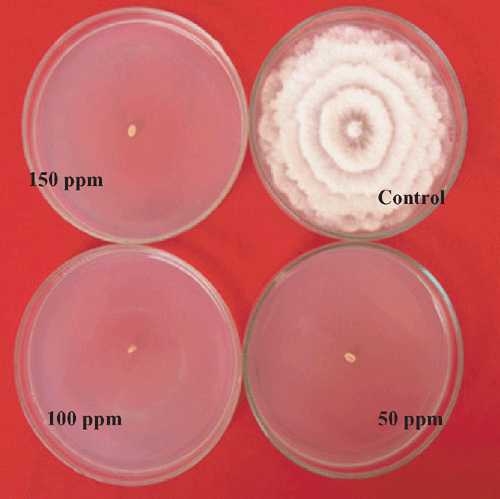
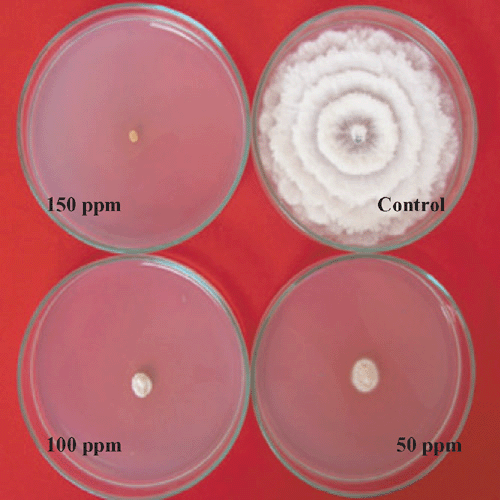
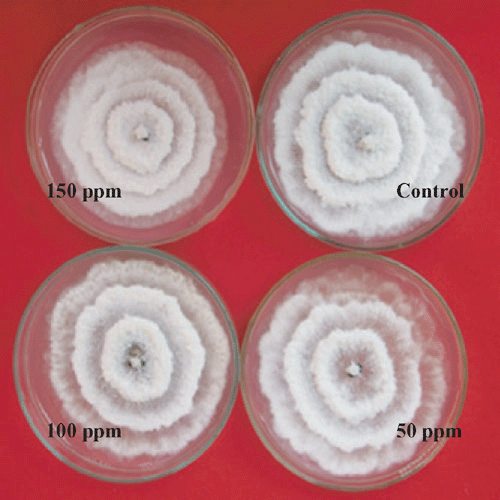
Nil growth was recorded in all the three concentration of Bavistin (Carbendazim 50% WP) (Fig. 4) and Roko (Thiophanate methyl 70% WP) (Fig. 5) and 150 mg/L Calixin (Tridemorph 80% EC) (Fig. 6). Maximum growth of mycelial colony was recorded in control followed by all the three concentrations of Blitox (Copperoxychlorite 50% WP) (Fig. 7) followed by 100 mg/L Indofil M45 (Mancozeb 75% WP).
|
|
|
|
Maximum growth inhibition of 100% was found in case of all the three concentrations of Bavistin (Carbendazim 50% WP) and Roko (Thiophanate methyl 70% WP) and 150 mg/L Calixin (Tridemorph 80% EC). Minimum growth inhibition was found in all the three concentrations of Biltox (Copper oxychlorite 50% WP).
Discussion
In this trial, the performance of systemic fungicides was better compared with contact fungicides against Pestalotiopsis sp. Harsh et al. (1987) reported the effectiveness of Bavistin at 0.1% concentration and Dithane M45 at 0.3% concentration against Pestalotiopsis versicolor, which causes foliar disease in Diospyros melanoxylon Roxb. Various workers have reported that Bavistin (Carbendazim), Tilt 250 EC, Cupravit and Dithane M45 (Mancozeb) performed best against Pestalotia palmarum in vitro (Kundalkar et al. 1991; Joshi and Raut 1992; Selvan et al. 1993; Saw and Raut 1995; Khalequzzaman et al. 1998; Islam 2001). Complete inhibition of Pestalotiopsis mangiferae colony growth at the lowest concentration (0.11%) of Carbendazim was reported by Pandey et al. (2006). Even in the present study, Bavistin at all three concentrations (50, 100 and 150 mg/L) has been proved to be one of the most effective fungicides for causing complete inhibition of Pestalotiopsis sp. In contrast to the above reports, all the three concentrations of Indofil M45 (Mancozeb 75% WP) were less effective against Pestalotiopsis in the present study. Similarly, the performance of Dithane M45 against Pestalotia palmarum, the causal agent of leaf spot of Betelnut, was poor under in vitro tests (Islam et al. 2004). Our study suggests the use of minimal concentrations of fungicides for maximum inhibition of the fungus Pestalotiopsis sp. under in vitro condition.
The Pestalotiopsis sp. identified and isolated from the naturally infected leaf samples of S. cumini was pathogenic. Bavistin and Roko were the effective fungicides against Pestalotiopsis of S. cumini compared with all the systemic and contact fungicides studied. Therefore, Bavistin and Roko may be recommended in field trail for management of the disease caused by Pestalotiopsis sp.
Acknowledgements
Financial assistance from the University Grants Commission, New Delhi, through a major R & D project is gratefully acknowledged.
Dhingra OD,
Maia CB,
Lustosa DC, Mesquita JB
(2002) Seedborne pathogenic fungi that affect seedling quality of red angico (Anadenanthera macrocarpa) trees in Brazil. Journal of Phytopathology 150, 451–455.
| Crossref | GoogleScholarGoogle Scholar |

Dhingra OD,
Lustosa DC,
Maia CB, Mesquita JB
(2003) Seedborne fungal pathogens of Jacaranda (Dalbergia nigra) tree. Seed Science and Technology 31, 341–349.

Harsh NSK,
Nath V,
Tiwari CK, Rehill PS
(1987) Studies on a new foliar disease of Diospyros melanoxylon Roxb. Van Vigyan 25, 16–20.

Islam MR,
Hossazin MK,
Bahar MH, Ali MR
(2004) Identification of the causal agent of leaf spot of Betelnut and in vitro evaluation of fungicides and plant extracts against it. Pakistan Journal of Biological Sciences 7, 1758–1761.

Joshi MS, Raut SP
(1992) Grey leaf blight disease of clove in Konkan region of Maharashtra. Indian Cocoa. Areacanut and Spices Journal 15, 73–74.

Khalequzzaman K,
Hossain MI, Hosssain MM
(1998) Effect of fungicides and potash in controlling grey leaf spot of coconut. Bangladesh Journal of Training and Development 11, 151–156.

Kundalkar SK,
Joshi MS, Pawar DR
(1991) Control of leaf blight of coconut caused by Pestalotia palmarum CKE. Indian Coconut Journal Cochin 22, 18–19.

Madar Z,
Solel Z, Kimchi M
(1991) Pestalotiopsis canker of cypress in Israel. Phytoparasitica 19, 79–81.

Pandey A,
Shukla AN, Chandra S
(2006) Pestalotiopsis stem canker of Jatropha curcas. Indian Forester 132, 763–766.

Saw NV, Raut SP
(1995) Studies on Pestalotiopsis mangiferae Butl. Journal of Maharashtra Agricultural University 20, 126–128.

Selvan MS,
Bhaskaran R, Ramadoss N
(1993) Laboratory screening of fungicides against grey blight of coconut. Madras Agricultural Journal 80, 240–241.

Vujanovic V,
St-Arnaud M, Neumann PJ
(2000) Susceptibility of cones and seeds to fungal infection in a pine (Pinus spp.) collection. Forest Pathology 30, 305–320.
| Crossref | GoogleScholarGoogle Scholar |

Wu CG,
Tseng HY, Chen ZC
(1982) Fungi inhabiting on Schoenoplectus triqueter (L.) Palla (I). Taiwania 27, 35–38.

Yuan ZQ
(1996) Fungi and associated tree diseases in Melville Island, Northern Territory, Australia. Australian Systematic Botany 9, 337–360.
| Crossref | GoogleScholarGoogle Scholar |

Yuan ZQ, Mohammed C
(1999) Pathogenicity of fungi associated with stem cankers of eucalypts in Tasmania, Australia. Plant Disease 83, 1063–1069.
| Crossref | GoogleScholarGoogle Scholar |



