First report of Tobacco streak virus in sunflower (Helianthus annuus), cotton (Gossypium hirsutum), chickpea (Cicer arietinum) and mung bean (Vigna radiata) in Australia
M. Sharman A B , J. E. Thomas A and D. M. Persley AA Department of Primary Industries and Fisheries, 80 Meiers Road, Indooroopilly, Qld 4068, Australia.
B Corresponding author. Email: Murray.Sharman@dpi.qld.gov.au
Australasian Plant Disease Notes 3(1) 27-29 https://doi.org/10.1071/DN08012
Submitted: 14 January 2008 Accepted: 19 March 2008 Published: 4 April 2008
Abstract
Tobacco streak virus (genus Ilarvirus) is recorded on sunflower (Helianthus annuus), cotton (Gossypium hirsutum), chickpea (Cicer arietinum) and mung bean (Vigna radiata) in Australia for the first time.
A significant proportion of the Australian total production of sunflower (Helianthus annuus), chickpea (Cicer arietinum), mung bean (Vigna radiata) and cotton (Gossypium hirsutum) occurs in the Queensland grain belt. Sunflower is used primarily for domestic consumption, whilst over 90% of the chickpea, mung bean and cotton production is exported (Anon. 2004, 2008; Douglas 2007).
Since the summer of 2004–05, an unidentified necrotic disorder of sunflower has caused significant production losses across a large area of the Central Highlands of Queensland, Australia, between the towns of Springsure in the south and Clermont in the north. Subsequently, necrotic symptoms have also been observed in crops of chickpea, mung bean and cotton grown in the same region. While limited field observations in 2007 indicated that disease incidence on chickpea and cotton was low, significant losses were reported from numerous mung bean crops across the Central Highlands in early 2007.
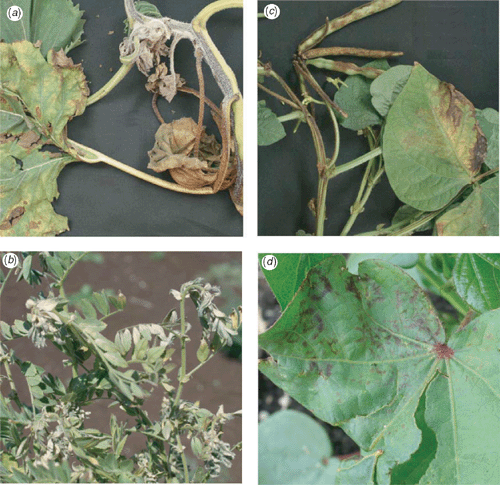
Symptoms on sunflower included necrosis of leaf lamina, petiole, stem and floral calyx, often with lodging of taller plants or stunted growth and plant death (Fig. 1a). On chickpea, symptoms included stem necrosis, tip wilting and necrotic etching on leaves (Fig. 1b). On mung beans there were necrotic line patterns on leaves, severe petiole, stem and tip necrosis, stunted growth and plant death (Fig. 1c). On cotton, symptoms ranged from young plants with single diffuse necrotic lesions to older plants with many necrotic lesions, ring spots, chlorotic mottle and leaf deformation (Fig. 1d).
|
Representative samples from each of the four crops were selected for detailed analysis. Sunflower and chickpea samples were collected in 2006 while mung bean and cotton samples were collected in 2007. Quasi-isometric virions typical of members of the genus Ilarvirus were observed in sap extracts of isolate 1974 from sunflower, when negatively contrasted with 1% ammonium molybdate pH 7.0 and examined by electron microscopy. Leaf samples of isolates 1974 (sunflower, from Clermont), 1979 (chickpea, from Clermont), 2027 (mung bean, from Springsure) and 2120 (cotton, from Emerald) tested positive for Tobacco streak virus (TSV) by ELISA (AGDIA ELISA reagent set, Cat. No. SRA25500/0500) with A405 nm values of 15–90 times greater than the means of their respective healthy controls.
For confirmation of ELISA results by RT-PCR, RNA was extracted from leaf tissue of the four isolates mentioned above using the Concert RNA Reagent (Invitrogen) before preparation of cDNA using SuperScript III reverse transciptase (Invitrogen) as per the manufacturer’s instructions. Previously published sequences from GenBank (accessions NC_003845, AY354406 and DQ323518) were used to design TSV-specific PCR primers flanking the coat protein gene. Primers TSVcpR2 (5′ CCA CAT CGC ACA CAA GTA TTA C 3′) and TSVcpF2 (5′ GCT TCT CGG ACT TAC CTG AGA T 3′) were used at an annealing temperature of 58°C and primed amplification of an 802 bp fragment from each of the four isolates, containing the entire coat protein gene of 717 nt. The nucleotide sequence obtained for sunflower isolate-1974 (TSV-1974; GenBank accession EU375481) had >98% identity with a Brazilian TSV isolate reported by Almeida et al. (2005) from soybean (GenBank accession AY354406). While sequence data have not yet been obtained for isolates 1979, 2027 and 2120, the strong positive results by two independent diagnostic methods indicate that TSV is present. TSV isolates 1974, 1979, 2027 and 2120 have been lodged in the DPI&F Indooroopilly Plant Virus Collection.
TSV-1974 was isolated from the field sample by manual inoculation to Nicotiana tabacum cv. Xanthi nc, which developed systemic necrotic etching and notched leaf margins typical of TSV infection (Greber 1971). When inoculated from tobacco back to sunflower cv. Suncross 53, the range of symptoms observed was similar to that seen in natural field infections, including chlorotic local lesions, and midrib, petiole and stem necrosis (Fig. 2).

|
TSV was first reported from Australia in 1971 and has subsequently been reported from tobacco, strawberry, dahlia and various weed species, mostly from south-eastern Queensland (Greber 1971, 1979; Greber et al. 1991). This is the first report of TSV naturally infecting sunflower, cotton, mung bean and chickpea in Australia. Natural field infections with TSV have previously been reported on sunflower, mung bean and cotton from India (Prasada Rao et al. 2000; Bhat et al. 2002a, 2002c) and also on cotton from Pakistan and Brazil (Costa and Carvalho 1961; Ahmed et al. 2003). In India, TSV-induced sunflower necrosis disease has been responsible for serious economic losses (Bhat et al. 2002b). Kaiser et al. (1991) reported TSV naturally infecting chickpea growing adjacent to plots of inoculated plants in the United States of America.
While TSV has been present in south-eastern Queensland since at least the early 1970s (Greber 1971), it remains to be determined why it has only recently become prominent in the Central Highlands region. Research is continuing to determine the relationship between TSV isolates from different regions of Australia. Further studies on the epidemiology of TSV from the Central Highlands of Queensland, including identification of alternative hosts and thrips vectors will be important for development of effective management strategies.
Acknowledgements
This work was funded by the Grains Research and Development Corporation, the Cotton Research and Development Corporation of Australia, and the Queensland Department of Primary Industries and Fisheries. We are grateful for the supply of sunflower isolate-1974 by John Ladewig and mung bean isolate-2027 by Graham Spackman and Associates.
Ahmed W,
Butt TB,
Ihsan J, Rehman A
(2003) Natural occurence of Tobacco streak virus in cotton in Pakistan and screening for its resistant sources. Pakistan Journal of Botany 35, 401–408.
[Verified 27 March 2008]
Bhat AI,
Jain RK,
Chaudhary V,
Krishna Reddy M,
Ramiah M,
Chattannavar SN, Varma A
(2002a) Sequence conservation in the coat protein gene of Tobacco streak virus isolates causing necrosis in cotton, mungbean, sunflower and sunn-hemp in India. Indian Journal of Biotechnology 1, 350–356.
[Verified 27 March 2008]
Greber RS
(1971) Some characteristics of tobacco streak virus isolates from Queensland. Queensland Journal of Agricultural and Animal Sciences 28, 105–114.

Greber RS
(1979) Virus diseases of Queensland strawberries and epidemiological effects of the strawberry runner approval scheme. Queensland Journal of Agricultural and Animal Sciences 36, 93–103.

Greber RS,
Klose MJ,
Teakle DS, Milne JR
(1991) High incidence of Tobacco streak virus in tobacco and its transmission by Microcephalothrips abdominalis and pollen from Ageratum houstonianum. Plant Disease 75, 450–452.

Kaiser WJ,
Wyatt SD, Klein RE
(1991) Epidemiology and seed transmission of two Tobacco streak virus pathotypes associated with seed increases of legume germ plasm in easern Washington. Plant Disease 75, 258–264.

Prasada Rao RDVJ,
Reddy AS,
Chander Rao AS,
Varaprasad KS,
Thirumala-Devi K,
Nagaraju ,
Muniyappa V, Reddy DVR
(2000) Tobacco streak ilarvirus as causal agent of sunflower necrosis disease in India. Journal of Oilseeds Research 17, 400–401.



