Natural infection of periwinkle (Catharanthus roseus) with Cucumber mosaic virus, subgroup IB
A. Samad A B , P. V. Ajayakumar A , M. K. Gupta A , A. K. Shukla A , M. P. Darokar A , B. Somkuwar A and M. Alam AA Central Institute of Medicinal and Aromatic Plants (CIMAP), P.O., CIMAP, Lucknow-226 015, India.
B Corresponding author: Email: samad_cimap@yahoo.co.in
Australasian Plant Disease Notes 3(1) 30-34 https://doi.org/10.1071/DN08013
Submitted: 30 January 2008 Accepted: 19 March 2008 Published: 9 April 2008
Abstract
This paper reports the characterisation of a naturally occurring Cucumber mosaic virus (CMV) infection, causing mosaic, leaf distortion and stunting of periwinkle (Catharanthus roseus). The virus isolate showed wide host range, biophysical properties similar to CMV, isometric particles of 28 nm, capsid protein of 26 kDa, serological reaction with CMV-S and non-persistent transmission by aphids. The CP gene of the virus was amplified using reverse transcriptase–polymerase chain reaction (RT-PCR) with CP-specific primers, cloned and sequenced (657 bp). Sequence analysis of the PCR product with other CMV isolates revealed the closest identity with Rauvolfia serpentina isolate of CMV (98%) and the phylogram revealed that CMV naturally infecting periwinkle belongs to subgroup IB.
Catharanthus roseus, commonly known as periwinkle (Fig. 1), is a tropical perennial herb of the family Apocyanaceae. It is cosmopolitan in distribution and cultivated in Madagascar, India, Israel and USA (Stearn 1975). The species contains a large variety of alkaloids (Svoboda and Blake 1975) and some of them are of importance mainly for their anti-cancer (vincristine and vinblastine) medicinal values.

|
Cucumber mosaic virus (CMV, genus: Cucumovirus, family: Bromoviridae) is one of the most widespread plant viruses in the world with an extensive host range infecting ~1000 species including cereals, fruits, vegetables, ornamentals, medicinal and aromatic plants (Roossinck 1999). More than 75 species of aphids can transmit CMV in a non-persistent manner (Palukaitis et al. 1992). CMV was first reported in Cucumis sativus from the USA (Prince 1934). In India, the occurrence of CMV has been reported from many hosts such as Egyptian henbane (Samad et al. 2000), gladiolus (Raj et al. 2002), Lycopersicum esculentum (Sudhakar et al. 2006), geranium (Verma et al. 2006), banana (Aglave et al. 2007), Rauvolfia serpentina and Jatropha curcas (Raj et al. 2007, 2008). However, only limited reports are available on biological and molecular characterisation of these isolates and their exact identification remained unaddressed. In this article, we report a natural occurrence of CMV on C. roseus and its biological and molecular properties.
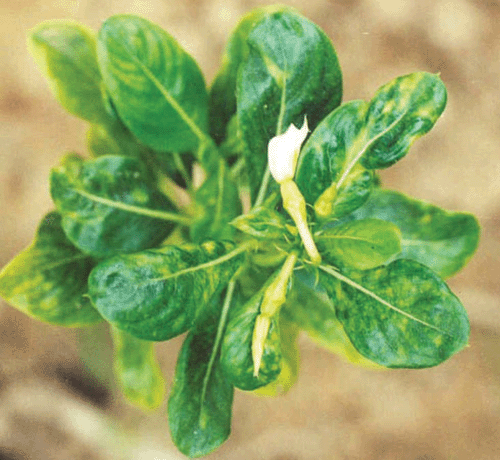
In early summer 2003 (March–May), several plants of C. roseus exhibited bright chlorotic spots on the upper leaves, gradually developing to a green mosaic with leathery, irregular and deformed leaves and an overall stunted growth in comparison to healthy plants (Fig. 2). Flowers exhibited symptoms of faded petals and were malformed. Incidence in commercial fields was from 70 to 80%, with highest incidence in poorly drained areas of the field. Flower production in commercial fields was reduced by ~30%. In advanced stages of the disease, premature death and drying of infected plants was observed.
|
Several diseases caused by bacteria, fungi, nematodes, phytoplasmas and viruses are reported on periwinkle from different parts of the world. A review of literature revealed that periwinkle has been reported as a susceptible host of many distinct viruses such as Pepper ringspot virus, Narcissus mosaic virus, Poplar mosaic virus, Cacao yellow mosaic virus, Tobacco streak virus, Alfalfa mosaic virus, Tobacco ringspot virus (Plant viruses online, http://image.fs.uidaho.edu/vide/famly011.htm#Catharanthus%20roseus, verified 27 March 2008) Zantedeschia mild mosaic virus (ZaMMV, Huang and Chang 2005), Carnation mottle virus (Singh et al. 2005), Potato yellow vein virus (PYVV, Salazar et al. 2000), Tomato spotted wilt virus (TSWV, Chatzivassiliou et al. 2000). Recently R. serpentina, another member of the Apocyanaceae is recorded as a natural host of CMV (Raj et al. 2007). However, natural occurrence of CMV on C. roseus has not been previously recorded.
Infected plant tissues were homogenised in 0.1 M phosphate buffer (pH 7.2) containing 0.02% thioglycollic acid and sodium sulfite, squeezed through muslin cloth and mechanically applied onto leaves using carborundum (600 mesh) as an abrasive. Seedlings treated with buffer only served as negative controls for inoculation. Both inoculated and control plants were kept under observation in an insect-proof glasshouse (Noordam 1973). The virus isolate was efficiently sap transmitted from naturally infected periwinkle to healthy periwinkle and a variety of plant species mainly from Chenopodiaceae, Cucurbitaceae and Solanaceae. Our studies used a single lesion from periwinkle obtained by passage through C. amaranticolor and further propagated in Nicotiana benthamiana. Periwinkle plants inoculated mechanically reacted with symptoms similar to those observed on naturally infected plants. The mechanically inoculated plant species that showed local and/or systemic symptoms included; Gomphrena globosa, N. benthamiana, N. glutinosa, N. occidentalis, N. rustica, N. tabacum cv.DR-1 (Harrison Special), N. tabacum Samsun NN, N. tabacum White Burley, N. xanthi, Cucumis melo, C. sativus, Pisum sativum, Capsicum annuum, Solanum lycopersicum, Lycopersicum esculentum, Petunia hybrida, Chenopodium album, C. amaranticolor, C. quinoa, Cucurbita pepo, Datura stramonium, D. inoxia, Glycine max, Solanum melongena, Vigna mungo and Solanum nigrum. However, the following plants failed to develop either local or systemic symptoms; Arachis hypogaea, Brassica campestris, Dahlia hybrida, Chrysanthemum cinerariifolium, Dianthus barbatus, Helianthus annuus, Luffa cylindrical, Mirabilis jalapa, Tagetes erecta, Zinnia elegans, Mentha arvensis, M. spicata, M. citrata, Withania somnifera and Physalis floridana. Selected hosts of the periwinkle CMV isolate are compared with the host range studies of others CMV isolates (Table 1) from vanilla (Madhubala et al. 2005), Egyptian and black henbane (Zaim and Khan1988; Samad et al. 2000), R. serpentina (Zaim and Khan 1988), banana (Aglave et al. 2007) and lily (Terami et al. 2004).
Groups of five aphids (Myzus persicae) were transferred to glass tubes and starved for 2 h. They were allowed to feed for 15 min on infected periwinkle or N. benthamiana leaves and transferred to a healthy periwinkle or N. benthamiana plant for a 24 h inoculation/feeding period. Myzus persicae transmitted the virus in a non-persistent manner to healthy periwinkle (5/10 plants) and N. benthamiana (7/10 plants). Symptoms developed after 15–20 days of feeding. For soil transmission, periwinkle plants bearing viral symptoms along with soil collected from infested fields were planted individually in pots. Two weeks later, three healthy periwinkle seedlings at the five to six leaf stage were planted next to the symptom bearing diseased plants. Control arrangements were set up using apparently healthy periwinkle in autoclaved soil. Plants were grown in a glasshouse and observed daily for symptom development over 6 weeks. Neither disease symptoms nor virus particles were detected in the seedlings transplanted adjacent to infected plants.
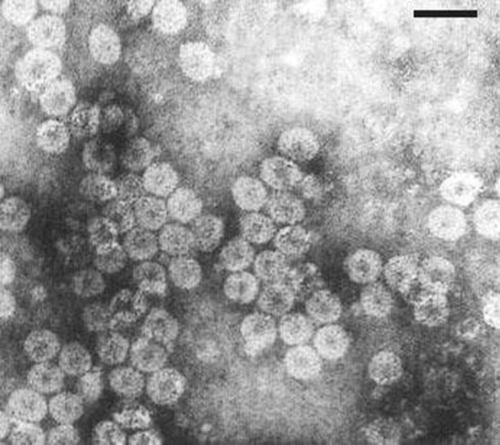
Serial 10-fold dilutions of crude sap in 0.1 M phosphate buffer (pH 7.0) were prepared from infected N. benthamiana for determination of bio-physical studies, dilution end point (DEP), thermal inactivation point (TIP) and longevity in vitro (LIV) of the virus (Noordam 1973). Infectivity was assayed on C. amaranticolor. Virus retained infectivity in crude sap up to a dilution of 10−3 TIP at 65°C and LIV at 4°C for 7 days. Fresh N. benthamiana leaves showing distinct symptoms were harvested after 2 weeks of inoculation and used as a source for virus purification as described previously (Samad et al. 2000). The purified virus suspension revealed a typical UV absorbance spectrum (Gibbs and Harrison 1976) of nucleoprotein showing a A260/A280 ratio of 1.7. Purified virus preparations stained with 2% uranyl acetate revealed the presence of typical isometric particles of ~28 nm diameter under Philips EM420 transmission electron microscope (Fig. 3). The periwinkle virus isolate reacted positively with antiserum of CMV (CMV; PVAS 242a, American Type Culture Collection, USA) in a gel double diffusion test (Raj et al. 2007). Electrophoretic analysis of SDS-dissociated virus revealed the presence of a single polypeptide with a relative molecular weight of ~26 kDa (Sambrook and Russell 2001). Leaf dip preparations from infected periwinkle and N. benthamiana plants contained spherical particles similar to those in purified preparation. Virion particles with similar profiles were frequently observed in ultrathin sections of infected tissues from leaves and petioles.
|
Total RNA was extracted using an RNeasy Mini kit (QIAGEN) following the manufacturer’s instructions. The isolated RNA was used for cDNA synthesis using reverse transcriptase for polymerase chain reaction (PCR) amplification. The cDNA synthesis was carried out using Avian Myeloblastosis Virus Reverse Transcriptase AMVRTase (10U) at 42°C for 90 min, in a total volume of 20 μL containing RNase inhibitor (25U), 1 mM each dNTP and a downstream primer (5′GCATGGTACCTCAAACTGGGAGCAC-3′). The PCR (25 μL) was performed using 2 μL of cDNA and primers specific to CMV coat protein (CP, Srivastava et al. 2004) (F: 5′-GCATTCTAGATGGACAAATCTGAATC-3′ and R: 5′GCATGGTACCTCAAACTGGGAGCAC-3′) in an automated thermal cycler (Perkin Elmer-Gene Amp PCR system 2400). The PCR cycles were, one initial cycle of denaturation at 94°C for 5 min followed by denaturation at 94°C (1 min), annealing at 52°C (1 min) and extension at 72°C for 1.30 min. The final extension at 72°C was maintained for 5 min. The PCR amplification product was separated by electrophoresis in 1.2% agarose gel in 1X TAE and stained with ethidium bromide revealing an amplified DNA fragment of the expected size (~650 bp) from virus infected samples only but not from healthy samples (Fig. 4). DNA marker λ/EcoRI+HindIII double digests were used as size markers.
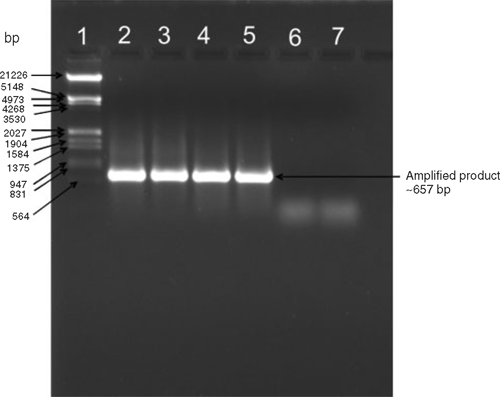
|
The amplified PCR product was cloned into pGEM-T Easy vector kit (Promega, USA), recombinant clones were identified by restriction digestion using the restriction endonuclease enzyme EcoR1 and selected clones were sequenced using XL 3130 Genetic Analyser (ABS, USA). The sequenced region was submitted to GenBank (Accession number EU310928) and contained a single open reading frame, which comprised 657 bases of nucleotides potentially coding for 218 amino acids. The new sequence was compared using bioinformatics tools DiAlign (Genomatrix) MEGA 4.0 with CP gene sequences of all available CMV isolates from India as well as representative isolates from other parts of the world belonging to both the subgroups (I and II) (Table 2). Nucleotide and deduced amino acid sequence of the CP gene of CMV infecting periwinkle showed closest identity (98%) with the subgroup IB CMV isolates from Rauvolfia serpentina (EF 593025) and with GenBank accession numbers. DQ640743 and EF593026 (Fig. 5). On the basis of these biological and molecular studies it is concluded that the virus naturally infecting periwinkle is a subgroup IB CMV. The high sequence identities observed between Indian isolates of CMV compared with isolates from other localities indicate that periwinkle isolate of CMV originated locally.
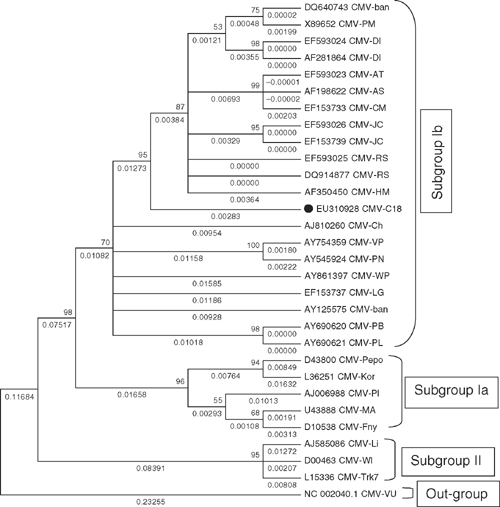
|
Acknowledgements
The authors are thankful to Dr S.P.S. Khanuja (Director, CIMAP) for encouragement and providing necessary facilities. Thanks also to Dr AK Gupta, National Gene Bank of Medicinal and Aromatic Plants, CIMAP, Lucknow for providing the germplasm of periwinkle (C. roseus) for this study.
Aglave BA,
Krishnareddy M,
Patil FS, Andhale MS
(2007) Molecular Identification of a Virus Causing Banana Chlorosis Disease from Marathwada Region. International Journal of Biotechnology and Biochemistry 3, 13–23.

Chatzivassiliou EK,
Weekes R,
Morris J,
Wood KR,
Barker I, Katis NI
(2000) Tomato spotted wilt virus (TSWV) in Greece: its incidence following the expansion of Frankliniella occidentalis, and characterisation of isolates collected from various hosts. The Annals of Applied Biology 137, 127–144.
| Crossref | GoogleScholarGoogle Scholar |

Huang CH, Chang YC
(2005) Identification and molecular characterization of Zantedeschia mild mosaic virus (ZaMMV), a new calla lily-infecting potyvirus. Archives of Virology 150, 1221–1230.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Madhubala R,
Bhadramurthy V,
Bhat AI,
Hareesh PS,
Pretheesh ST, Bhai RS
(2005) Occurrence of Cucumber mosaic virus on vanilla (Vanilla planifolia Andrews) in India. Journal of Biosciences 30, 339–350.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Palukaitis P,
Roossinck MJ,
Dietzgen RG, Francki RIB
(1992) Cucumber mosaic virus. Advances in Virus Research 41, 281–348.
| PubMed |

Prince WC
(1934) Isolation and study of some yellow strains of cucumber mosaic virus. Phytopathology 24, 743–761.

Raj SK,
Srivastava A,
Chandra G, Singh BP
(2002) Characterisation of Cucumber mosaic virus isolate infecting Gladiolus cultivars and comparative analysis of serological and molecular methods for sensitive diagnosis. Current Science 83, 1132–1136.

Raj SK,
Kumar S,
Pratap D,
Vishnoi R, Snehi SK
(2007) Natural Occurrence of Cucumber mosaic virus on Rauvolfia serpentina, a new record. Plant Disease 91, 322.
| Crossref | GoogleScholarGoogle Scholar |

Raj SK,
Kumar S, Snehi SK
(2008) First Report of Cucumber mosaic virus on Jatropha curcas in India. Plant Disease 92, 171.
| Crossref | GoogleScholarGoogle Scholar |

Salazar LF,
Muller G,
Querci M,
Zapata JL, Owens RA
(2000) Potato yellow vein virus: its host range, distribution in South America and identification as a crinivirus transmitted by Trialeurodes vaporariorum. The Annals of Applied Biology 137, 7–19.
| Crossref | GoogleScholarGoogle Scholar |

Samad A,
Raj SK,
Srivastava A,
Chandra G,
Ajayakumar PV,
Zaim M, Singh BP
(2000) Characterization of a cucumber mosaic virus isolates infecting Egyptian henbane (Hyoscyamus muticus L.) in India. Acta Virologica. English Ed. 44, 131–136.

Singh HP,
Hallan V,
Raikhy G,
Kulshrestha S,
Sharma ML,
Ram R,
Garg ID, Zaidi AA
(2005) Characterization of an Indian isolate of carnation mottle virus infecting carnations. Current Science 88, 594–601.

Srivastava A,
Chandra G, Raj SK
(2004) Molecular characterization of a strain of cucumber mosaic virus based on coat protein and movement protein genes. Acta Virologica. English Ed. 48, 229–239.

Sudhakar N,
Nagendra-Prasad D,
Mohan N, Murugesan K
(2006) First Report of Cucumber mosaic virus Subgroup II Infecting Lycopersicon esculentum in India. Plant Disease 90, 1457.
| Crossref | GoogleScholarGoogle Scholar |

Terami F,
Fukumoto F, Handa K
(2004) Cucumber mosaic virus isolated from Amazon lily (Eucharis grandiflora). Journal of General Plant Pathology 70, 192–193.
| Crossref | GoogleScholarGoogle Scholar |

Verma N,
Mahinghara BK,
Ram R, Zaidi AA
(2006) Coat protein sequence shows that Cucumber mosaic virus isolate from geraniums (Pelargonium spp.) belongs to subgroup II. Journal of Biosciences 31, 47–56.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zaim M, Khan MMAA
(1988) Green mosaic of Hyoscyamus niger L. and Rauvolfia serpentina Benth. Indian Journal of Plant Pathology 6, 152–157.