First record of powdery mildew on carrots in Australia
James H. Cunnington A D , Andrew Watson B , Jose R. Liberato C and Rodney H. Jones AA Victorian Department of Primary Industries, Knoxfield Centre, Private Bag 15, Ferntree Gully Delivery Centre, Vic. 3156, Australia.
B New South Wales Department of Primary Industries, Yanco Agricultural Institute, Yanco, NSW 2703, Australia.
C Queensland Department of Primary Industries and Fisheries. Present address: Department of Primary Industry, Fisheries and Mines, Diagnostic Service Division, GPO Box 3000, Darwin, NT 0801, Australia.
D Corresponding author. Email: James.Cunnington@dpi.vic.gov.au
Australasian Plant Disease Notes 3(1) 38-41 https://doi.org/10.1071/DN08015
Submitted: 14 January 2008 Accepted: 26 March 2008 Published: 4 April 2008
Abstract
Powdery mildew is reported on carrots for the first time in Australia. The affected plants were on a property located in the Murrumbidgee Irrigation Area of New South Wales. Based on morphological data and an rDNA ITS sequence, the fungus was identified as Erysiphe heraclei. Inoculation tests showed that the fungus was aggressive on carrot, but weakly pathogenic on parsnip and parsley. Erysiphe heraclei is common on parsnip but this is the first record on carrot in Australia.
Carrots (Daucus carota) are grown in the Murrumbidgee Irrigation Area of New South Wales either for juicing or the fresh market. The crops are grown all year and watered by furrow irrigation. Parsnips (Pastinaca sativa) growing on the same properties are commonly infected by powdery mildew. Early in March 2007, powdery mildew was found affecting large areas of carrots on one farm in the region. When powdery mildew of carrots was examined on the farm that had the original outbreak, all blocks were infected and infection within the blocks was severe. The infected blocks ranged in maturity from 6 weeks after sowing to blocks that were ready for harvesting. The crops closer to maturity with the heavier canopies were more seriously infected. Spraying with sulfur was immediately carried out. The fungus showed conspicuous growth of white mycelium and conidia that covered the whole plant (Fig. 1). Morphology of the fungus was examined on microscopic mounts in lactic acid. Biometric data was obtained only from the examination of turgid structures and only mature conidia (those not attached to conidiophores) were measured. The fungus was identified as Erysiphe heraclei and its description is given below.
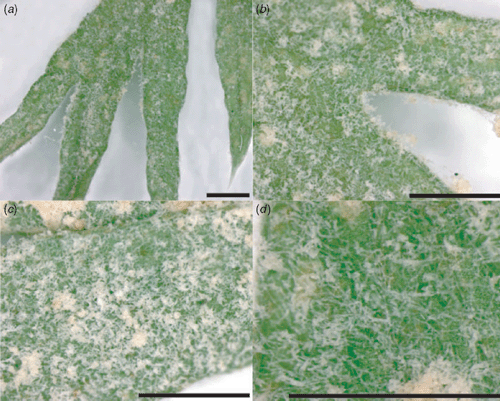
|
Anamorphic Erysiphe heraclei DC., Fl. Fr. VI: 107 (1815) on Daucus carota (Fig. 2)
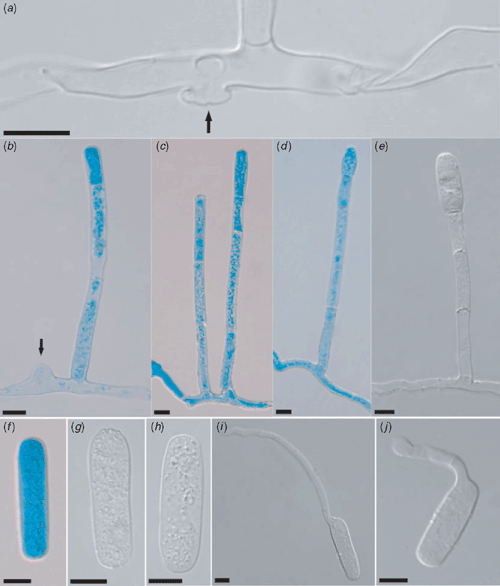
|
Mycelium amphigenous, mainly on the upper leaf surface. Hyphae 5–7.5 µm wide. Mycelial appressoria lobed. Conidiophores up to 170 µm long, sometimes two conidiophores arising from the same hyphal cell, foot-cells 25–50 × 6–7.5 µm, followed by (1–)2 cells (which can be shorter or one longer and one shorter or one shorter and one longer than the foot-cell). Conidia formed singly, most subcylindric to cylindric, 32.5–62.5 × (10–)12.5–16.5(–19) µm. Germ tube at the shoulder of conidium, up to 1.5 times the conidium length. Teleomorph not seen.
Material examined: Daucus carota, Murrumbidgee Irrigation Area, NSW, Australia, March 2007, A. Watson (BRIP 49115), 6 Aug 2007 (VPRI 41227).
To help confirm the identification, an rDNA internal transcribed spacer (ITS) sequence was obtained for the specimen VPRI 41227 according to Cunnington et al. (2003), except that total DNA was extracted from the infected plant material using a DNeasy™ Plant Mini Kit (Qiagen). The sequence was identical, or differed by up to three bases, to six E. heraclei sequences on GenBank (AB000942, AB104511, AB104514, AB104513, AB104464 and EU010381). There were two E. heraclei sequences that differed by five to six bases (AB104510 and AB104512). The next most similar sequences were from the beet pathogen Erysiphe betae. These differed by five to seven bases. Intraspecific rDNA ITS sequence similarity in powdery mildew fungi is usually ≥99% (Cunnington et al. 2003), which in this case corresponds to ≤5 bases. The sequence obtained here confirms the identity as E. heraclei and has been lodged on GenBank as accession EU371725.
To prove the pathogenicity of the fungus, diseased infected carrot plants were collected in the field and brought to a glasshouse maintained at 20–25°C where conidia were shaken over several potted healthy carrots of ~6 weeks old. As E. heraclei has been recorded from 80 genera in the Apiaceae (Braun 1987), young parsnips and parsley (Coriandrum sativum) were also inoculated. After 28 days there were only small, restricted colonies of powdery mildew on the parsnips and parsley plants, but the carrots were heavily infected (Fig. 3). This low level of infection on parsnip was surprising and may indicate that this is a new carrot specific strain of E. heraclei, rather than infection from parsnips grown in the area. Further controlled experiments using a wide range of species in the Apiaceae would be required to predict the host range of the fungus.

|
The only powdery mildew belonging to the subfamily Erysiphoideae (whose anamorphs belong to the genus Oidium) thus far reported on carrot is E. heraclei, which is distributed worldwide and can also infect numerous species of various host genera of the Apiaceae (Braun 1987). Erysiphe heraclei has been recorded on parsnips in Victoria (Cunnington 2003). Powdery mildew on parsnip has also been recorded in South Australia, Tasmania, New South Wales, Western Australia and Queensland, but these specimens were only identified as Oidium sp. (herb. DAR, VPRI and BRIP records). Many of these specimens have been examined by staff at VPRI and are all believed to be E. heraclei (I. G. Pascoe, pers. comm.).
The fungus may have been introduced on contaminated seed. But, as a range of carrot varieties from different sources were affected, we could not distinguish if a particular variety was the source. This is the first record of powdery mildew on carrots in Australia.
Acknowledgements
J.R. Liberato acknowledges the support of an Australian Biological Resources Study grant.
Braun U
(1987) A monograph of the Erysiphales (powdery mildews). Beiheft zur Nova Hedwigia 89, 1–700.

Cunnington JH,
Takamatsu S,
Lawrie AC, Pascoe IG
(2003) Molecular identification of anamorphic powdery mildew fungi. Australasian Plant Pathology 32, 421–428.
| Crossref | GoogleScholarGoogle Scholar |



