First report of phytoplasma ‘Candidatus phytoplasma asteris’ (16SrI) from Parthenium hysterophorus L. showing symptoms of virescence and witches’-broom in India
S. K. Raj A C , M. S. Khan A , S. K. Snehi A , S. Kumar A , S. Mall B and G. P. Rao BA Plant Molecular Virology, National Botanical Research Institute, Lucknow 226 001, India.
B Sugarcane Research Station, Kunraghat, Gorakhpur 273 008, UP, India.
C Corresponding author: Email: skraj2@rediffmail.com
Australasian Plant Disease Notes 3(1) 44-45 https://doi.org/10.1071/DN08017
Submitted: 28 February 2008 Accepted: 28 March 2008 Published: 9 April 2008
Abstract
Phytoplasma was detected in Parthenium hysterophorus displaying symptoms of witches’-broom disease by direct and nested polymerase chain reaction using universal primers specific to 16S rRNA gene of phytoplasma. Sequence identities of 99% were found to several isolates of ‘Candidatus phytoplasma asteris’ (aster yellows, 16SrI group).
Parthenium hysterophorus L. (family Asteraceae) is one of the 10 worst weeds in the world. It is an herbaceous plant and a native of tropical America. In India, parthenium is locally called ‘Congress grass’. It is believed that it entered India accidentally in the mid-1956 and is now considered as one of the most feared noxious weed species (Rao 1956). Parthenium is also known to cause asthma, bronchitis, dermatitis and hay-fever in man and livestock. The natural occurrence of virescence and witches’-broom was noticed on ~10–15% of P. hysterophorus plants growing wildly along the road side in Bahraich and Gorakhpur districts of Uttar Pradesh, India during summer of 2006. The infected plants showed excessive green, tiny narrow leaves, shortening of internodes and witches’-broom-like symptoms.
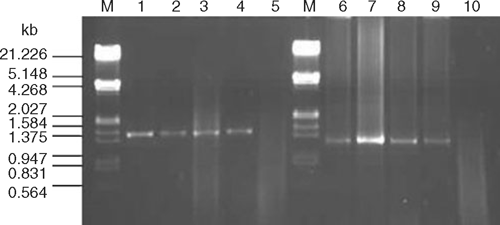
Two symptomatic and one asymptomatic P. hysterophorus plants were collected from both a Bahraich and a Gorakhpur site. Total DNA was extracted from ~100 mg of leaf tissue employing a phytoplasma enrichment procedure (Ahrens and Seemüller 1992). Direct polymerase chain reaction (PCR) using P1/P6 (Deng and Hiruki 1991) universal primers specific to the 16S rRNA gene of phytoplasmas resulted in the expected size bands of ~1.5 kb from symptomatic (4/4) but not from healthy (0/1) samples (Fig. 1, lanes: 1–5). The nested PCR using 1 : 10 diluted first stage (P1/P6) products and R16F2n/R16R2 primers (Gundersen and Lee 1996) resulted in ~1.2 kb bands from all the infected samples (Fig. 1, lanes: 6–9), suggesting the association of a phytoplasma with the disease.
|
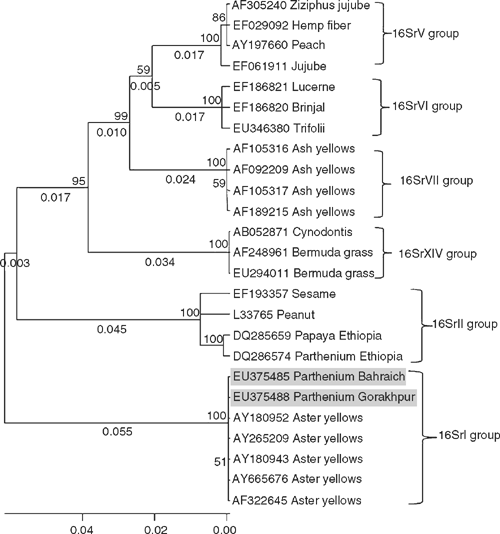
The amplicons of ~1.2 kb obtained by nested PCR from each sample from both locations were sequenced and data deposited in GenBank (Accessions: EU375485, Bahraich and EU375488, Gorakhpur). BLAST search analysis of the phytoplasma isolates EU375485 and EU375488 showed that both the isolates were identical. These sequences had 99% identity to the members of ‘Candidatus phytoplasma asteris’ (16SrI) group, i.e. sugarcane yellows phytoplasma (EU423900); periwinkle little leaf phytoplasma (EU375834, DQ381535); onion yellows phytoplasma (DQ321822, D12569, AB292849); carrot phytoplasma (EU215426, EU215425); silene nicaeensis virescence phytoplasma (EF570134, AY744070); hydrangea phyllody phytoplasma (AY265219, AY265207) and several strains of aster yellows phytoplasma (EF489024, AY665676, AY265209, AY180952, AY180943 and AF322645). Phylogenetic analysis of the phytoplasma isolates using molecular evolutionary genetics analysis (MEGA) 4.0 tool (Tamura et al. 2007) also showed a close relationship with isolates of aster yellows ‘Candidatus phytoplasma asteris’ (16SrI) group (Fig. 2) but not to P. hysterophorus phytoplasma (DQ286574) of Ethiopia which belongs to peanut witches’-broom (16Sr II) group.
|
Based on highest sequence identity and close relationships, the phytoplasma obtained from P. hysterophorus plants showing virescence and witches’-broom symptoms at Bahraich and Gorakhpur were identified as isolates of aster yellows 16SrI group phytoplasma (‘Candidatus phytoplasma asteris’). There are reports of phyllody disease of P. hysterophorus weed in Ethiopia (Waller 1996; Taye et al. 2004). Association of a phytoplasma was determined with this disease using PCR with the P1/P7 primers followed by AluI-digestion of the PCR amplicons (Janke et al. 2007). Sequence information on P. hysterophorus phytoplasma of Ethiopia which belongs to the peanut witches’-broom (16Sr II) group (‘Ca. phytoplasma aurantifolia’) is also available in GenBank database (DQ286574). Association in India of a ‘Candidatus phytoplasma asteris’ (16SrI) group member with virescence and witches’-broom on P. hysterophorus is a first report.
Ahrens U, Seemüller E
(1992) Detection of DNA of plant pathogenic mycoplasma-like organism by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 82, 828–832.
| Crossref | GoogleScholarGoogle Scholar |

Deng S, Hiruki C
(1991) Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. Journal of Microbiological Methods 14, 53–61.
| Crossref | GoogleScholarGoogle Scholar |

Gundersen DE, Lee IM
(1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathologia Mediterranea 35, 144–151.

Rao RS
(1956) Parthenium: a new record for India. Journal of the Bombay Natural History Society 54, 218–220.

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Taye T,
Obermeier C,
Einhorn G,
Seemüller E, Büttner C
(2004) Phyllody Disease of Parthenium Weed in Ethiopia. Pest Management Journal of Ethiopia 8, 39–50.



