Leaf and pseudobulb diseases on Bifrenaria harrisoniae (Orchidaceae) caused by Phyllosticta capitalensis in Brazil
M. Silva A , O. L. Pereira A C , I. F. Braga B and S. M. Lelis BA Departamento de Fitopatologia, Universidade Federal de Viçosa, 36570-000, Viçosa, M.G., Brazil.
B Departamento de Biologia Vegetal, Universidade Federal de Viçosa, 36570-000, Viçosa, M.G., Brazil.
C Corresponding author. Email: oliparini@ufv.br
Australasian Plant Disease Notes 3(1) 53-56 https://doi.org/10.1071/DN08022
Submitted: 20 February 2008 Accepted: 8 April 2008 Published: 17 April 2008
Abstract
New leaf spot and pseudobulb diseases caused by Phyllosticta capitalensis are reported for the first time on the orchid Bifrenaria harrisoniae.
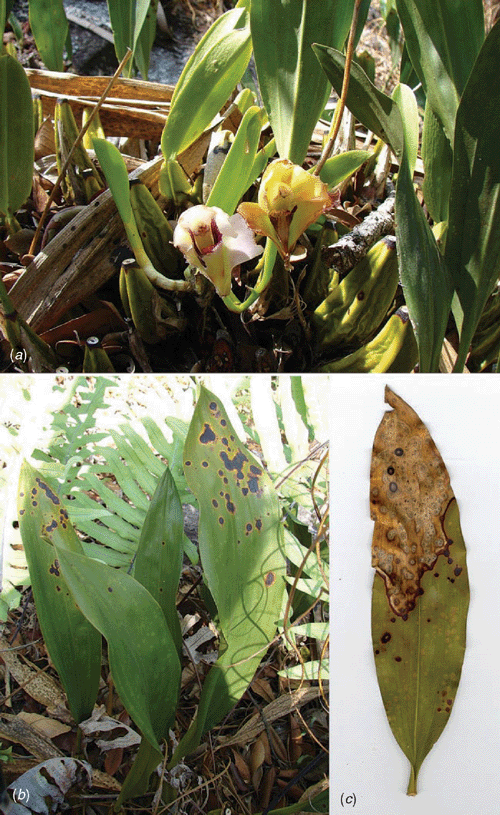
Bifrenaria harrisoniae (Hook.) Rchb. f. is an orchid species with terrestrial habitat, characteristic of the Brazilian ‘campos rupestres’. Due to its high ornamental potential, this species has been collected from its natural habitat and in conjunction with the vulnerability of its habitat, in some Brazilian states it is considered to be a threatened species. Recently, this species has been propagated in vitro by some Brazilian orchid growers for commercial purposes. In June 2006, an exploratory project surveying and describing the phytopathogenic and endophytic mycodiversity associated with the family Orchidaceae in the state of Minas Gerais was initiated. In a survey of the campos rupestres of Serra de Ouro Branco (Fig. 1), at Ouro Branco city in Minas Gerais State, Brazil, samples of the orchid B. harrisoniae with severe leaf spotting symptoms were collected (Fig. 2). Spots were initially chlorotic and circular in shape, became necrotic and black with a chlorotic halo, and coalesced to cover the entire width of the leaves, leading to leaf death. Pseudobulbs were also diseased in severely infected plants (Fig. 3).

|
|

|
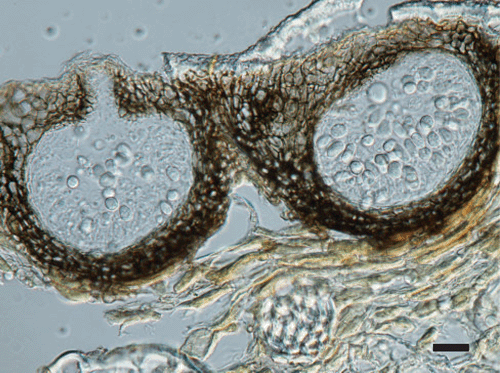
Morphology of the fungus on host tissue included, pycnidia immersed, solitary or aggregated, subepidermal, variable in shape, black, glabrous, with an apical ostiole (Fig. 4); wall stromatic, composed of several layers of dark brown, thick walled cells; conidiogenous cells aseptate, discrete, hyaline, pyriform to cylindrical, invested in mucus; conidia 1-celled, hyaline, obovate to pyriform, 9–14 × 5–7 µm, smooth-walled, guttulate, surrounded by a thick mucilaginous coat, with a hyaline apical appendage, 6–12 µm long, in all the isolates. Teleomorph not observed.
|
Material examined: VIC 30556, on leaves of Bifrenaria harrisoniae, Gerdau Açominas RPPN, 1186 m, Serra de Ouro Branco, Ouro Branco, State of Minas Gerais, Brazil, S.M. Lelis & I.F. Braga, 06 Nov 2007.
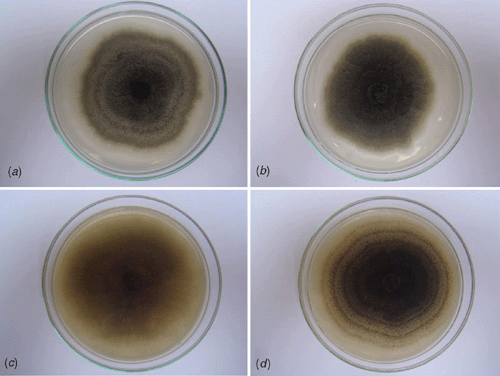
Seventy isolates were obtained by surface sterilising selected leaf fragments in 2% sodium hypochlorite. Thirty isolates were obtained from faint chlorotic lesions, 30 from the periphery of necrotic lesions and 10 from pseudobulbs. The isolates were obtained from lesions of different leaves and pseudobulbs from different plants and presented very similar colonies. To describe the colony morphology of the fungus, pure cultures were grown in Petri dishes containing potato dextrose agar (PDA) and 2% malt extract agar (MEA) for 8 days at 27°C in the dark and under near UV light with a 12-h photoperiod (Fig. 5). In culture: 7.0–7.5 cm after 8 days. On PDA, colonies grayish to dark grey with white aerial mycelium border, showing concentric haloes when grown in the dark, flocculose when grown under near UV light with a 12 h photoperiod, sporulation absent to scarce. On MEA, colonies brown to dark brown with no white aerial mycelium border, presence of concentric haloes and abundant sporulation only when grown under near UV light with a 12-h photoperiod (Fig. 5). In culture the apical ostiolar regions of pycnidia were a little longer than observed on host tissue, but other morphological characteristics presented on host tissue were the same as in culture.
|
The fungus fits the description of Phyllosticta capitalensis P. Henn., a well known fungal pathogen of the family Orchidaceae (Cash and Watson 1955; Uchida 1994; van der Aa and Vanev 2002). Mendes et al. (1998) did not record the occurrence of P. capitalensis in Brazil. However, in this country, P. capitalensis has been previously reported on the orchid genus Stanhopea, a host in which the type species was described (Nag Raj 1993; van der Aa and Vanev 2002). Its teleomorph, Guignardia endophyllicola Okane, Nakagiri & Tad. Ito, a species previously known only as an endophyte of ericaceous plants (Okane et al. 2001), was recently reported for the first time on orchids (Silva and Pereira 2007).
Mycelial plugs containing reproductive structures of the fungus were taken from an 8-day-old culture growing on 1% V8 juice-agar and placed on healthy young and mature leaves and pseudobulbs of B. harrisoniae. The inoculated plants were maintained moistened in plastic bags for two days and then in a greenhouse at 25°C. Faint chlorotic symptoms, similar to those previously observed, were detected after 10 days on young and mature leaves and after 25 days in pseudobulbs. The fungus was reisolated from infected plant tissue. The control leaves, on which V8 juice-agar plugs were placed, remained healthy.
This is the first report of P. capitalensis causing leaf spot disease on the orchid B. harrisoniae. Despite the fact that this disease has been observed only on plants growing on its natural habitat, it could become a serious problem for some Brazilian orchid growers, due to its high damage to B. harrisoniae and the lack of registered chemical products for this pathogen on orchids.
Acknowledgements
This work is part of an ongoing program of surveying and describing the phytopathogenic and the endophytic mycodiversity associated with the family Orchidaceae in the state of Minas Gerais supported by grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG (project 279/07).
Cash EK, Watson AJ
(1955) Some fungi on Orchidaceae. Mycologia 47, 729–747.
| Crossref | GoogleScholarGoogle Scholar |

Okane I,
Nakagiri A, Ito T
(2001) Identity of Guignardia sp. inhabiting ericaceous plants. Canadian Journal of Botany 79, 101–109.
| Crossref | GoogleScholarGoogle Scholar |

Silva M, Pereira OL
(2007) First report of Guignardia endophyllicola leaf blight on Cymbidium (Orchidaceae) in Brazil. Australasian Plant Disease Notes 2, 31–32.
| Crossref | GoogleScholarGoogle Scholar |

Uchida JY
(1994) Diseases of orchids in Hawaii. Plant Disease 78, 220–224.



