Molecular evidence for existence of a New World begomovirus associated with yellow mosaic disease of Corchorus capsularis in India
R. Ghosh A , S. Paul A , S. Das A , P. Palit A , S. Acharyya A , A. Das A , J. I. Mir A , S. K. Ghosh A and A. Roy A BA Plant Virus Laboratory & Biotechnology Unit, Division of Crop Protection, Central Research Institute for Jute and Allied Fibres, Kolkata 700120, India.
B Corresponding author. Email: anirbanroy75@yahoo.com
Australasian Plant Disease Notes 3(1) 59-62 https://doi.org/10.1071/DN08024
Submitted: 25 January 2008 Accepted: 15 April 2008 Published: 1 May 2008
Abstract
Yellow mosaic disease of jute (Corchorus capsularis) was found to be associated with a whitefly-vectored begomovirus. Sequencing of part of the begomovirus DNA-A indicated the presence of a New World begomovirus which has been detected for the first time in India.
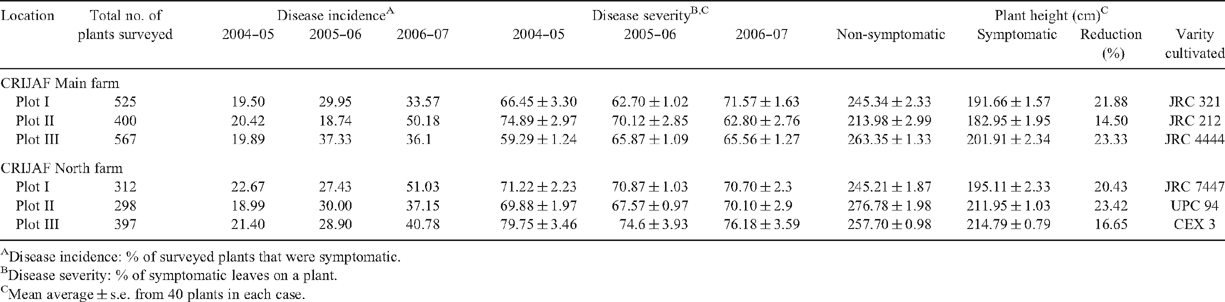
Jute (Corchorus capsularis L. and Corchorus olitorius L.), belonging to the family Tiliaceae, is one of the most important fibre crops of India. It has an average production of over 1 620 000 tonnes of jute goods per annum with an average export of 200 000 tonnes/year earning of Rs. 7500 million/year. (http://pib.nic.in/release/release.asp?relid=9038). Almost 80% of India’s raw jute is produced in West Bengal. Apart from making hessians, sacking, gunny bags, carpets, mat and rope, jute is also used for making many and diverse products for domestic consumption. Jute plants in India are infected by many diseases (Ghosh and Som 1998). A whitefly-vectored yellow mosaic disease is known to have infected C. capsularis since 1975 (Ahmed 1978). The disease is characterised by symptoms such as small yellow flakes on the lamina during the initial infection stage which gradually increase in size to form green and chlorotic intermingled patches producing a yellow mosaic appearance (Fig. 1). Over the last 4 years we have conducted a survey on the disease within different jute growing regions of India. The results of this survey indicate that the incidence of the disease has increased from nearly 20% in 2004 to above 40% in 2007 (Table 1). The incidence of the disease has been found to be around 50% on some of the leading C. capsularis cultivars such as JRC 7447 and JRC 212. It was also observed from the survey that infection reduces plant height to the extent of 20% and thus adversely affects the yield of the fibre.

|
|
The diseased jute plants showing typical yellow mosaic symptoms, collected from the experimental fields of Central Research Institute for Jute and Allied Fibres, Barrackpore, Kolkata, India were used as initial source of virus inoculum. For virus transmission studies, a virus free stock of whitefly (Bemisia tabaci Genn.) was reared on healthy tobacco plants (Nicotiana tabacum) in insect-proof wooden cages. The adult whiteflies that emerged from nymphs grown on the N. tabacum were used for transmission. Healthy jute plants (C. capsularis cv. JRC 7447 and JRC 212) were raised under glasshouse conditions with five seedlings per pot. Sets of 10 healthy whiteflies were collected with an aspirator into polyvinylchloride (PVC) bottles containing a leaf of a jute plant showing typical yellow mosaic symptom and allowed to feed for 24 h. After virus acquisition, each set of viruliferous whiteflies was released onto 30 healthy jute seedlings (five seedlings per pot) and was contained by a small plastic cylindrical cage (7.5 × 2.5 cm). After a 12-h transmission period, the plants were sprayed with 0.2% dimethoate (Rogor) and kept in an insect proof rectangular cage until symptom development. The experiment was repeated three times with 30 plants in each case. The same numbers of healthy plants were also inoculated with non-viruliferous whitefly as controls in each replication and no symptom expression was observed in these plants. Typical yellow mosaic symptoms of the disease were observed on glasshouse-grown healthy plants of cv. JRC 7447 and JRC 212 after 10 days of viruliferous whitefly transmission. It has been observed that when 10 whiteflies were used for transmission the efficiency was 60%, but the transmission efficiency was as low as 20% when only three viruliferous whiteflies were used. Back-inoculation to a new set of healthy plants produced similar yellow mosaic symptoms and confirmed the whitefly transmissibility of the disease. The whitefly transmissibility and the type of symptomatology highlighted the probable association of a whitefly-vectored begomovirus with the disease.
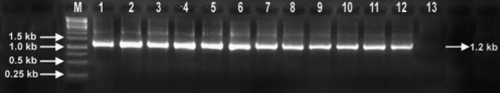
To confirm the association of a begomovirus with the yellow mosaic disease of jute, total nucleic acid was isolated and purified following the method of Doyle and Doyle (1987) from the leaves that showed distinctive yellow mosaic symptoms under glasshouse conditions (nine samples) and field conditions (three samples). To remove the mucilaginous substances, the isolated total nucleic acid was further purified through a DNeasy Mini Spin Column (Qiagen). Begomovirus-specific primers (Rojas et al. 1993) were used to amplify the corresponding genomic fragments of the virus. The primers PAL1v1978 and PAR1c496 amplified the expected 1.2-kb segment of DNA-A from all the 12 samples tested (Fig. 2). Gel-eluted amplicons (eight amplicons from glasshouse samples and two amplicons from field samples) were cloned into the pJET1 positive selection vector using the GeneJET™ PCR Cloning Kit (Fermentas) following the manufacturer’s protocol and competent Escherichia coli cells (strain DH5-α) were transformed following standard molecular biology procedures. Sequencing of a representative clone from each of the 10 amplicons revealed that all the inserted fragments were 1263 nucleotides in length and identical in sequence except for two clones in which only two nucleotides were found to vary. These sequence variations may be due PCR or sequencing error. One representative sequence was deposited in the GenBank database under the accession number EU047706.
|
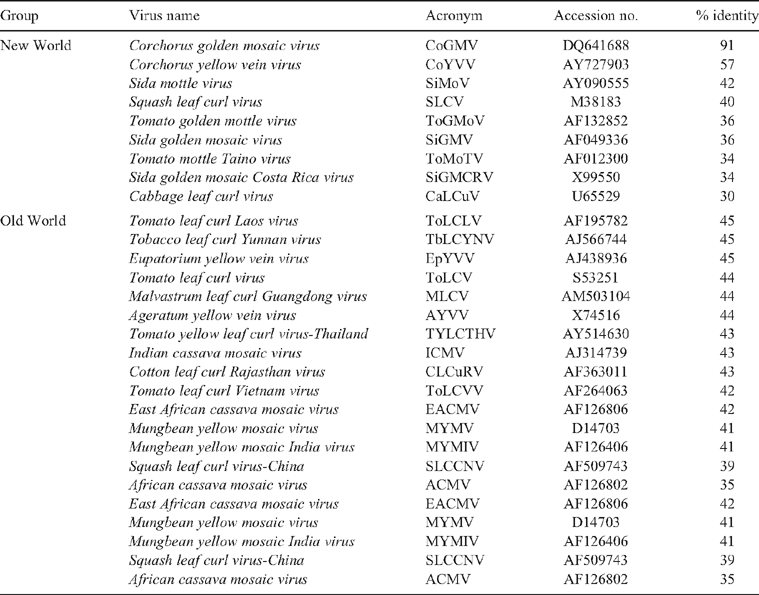
Sequence analysis of accession number EU047706 revealed that it has a 91.2% nucleotide identity with Corchorus golden mosaic virus (DQ641688), a New World begomovirus that was recently reported from Vietnam (Ha et al. 2008) (Table 2). Interestingly, the nonanucleotide sequence at the origin of replication was CATTATTAC in the jute isolate instead of the conventional TAATATTAC which has previously been determined as the most conserved feature within the Geminiviridae family (Roberts and Stanley 1994). This unique change was consistently observed in all of the 10 clones, obtained both from the glasshouse samples (eight clones, Lanes 1–8 of Fig. 2) and field samples (two clones, Lanes 10–11 of Fig. 2). We observed that in Corchorus golden mosaic virus reported from Vietnam, the nonanucleotide sequence also has the modified nucleotides but in this case it is TATTATTAC and Ha and his co-workers did not comment on this observation.
|
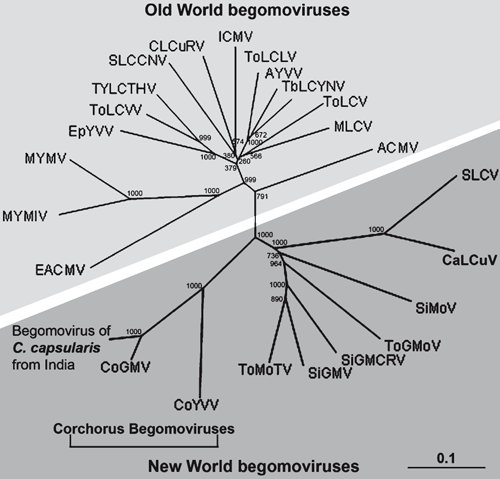
To assess the relationships with other begomoviruses, the corresponding genomic regions of nine New World and 13 Old World begomoviruses were obtained from GenBank and a phylogenetic tree was constructed and bootstrapped after multiple alignment using the Neighbour Joining algorithm of Clustal X (version 1.8) software (Thompson et al. 1997) and viewed by Treeview software. Sequence identity matrix was generated for comparison using BioEdit Sequence Alignment Editor (version 5.0.9) (Hall 1999). Phylogenetic analysis revealed that this jute begomovirus isolate from India grouped with other begomoviruses reported to be associated with Corchorus sp. and clustered with the New World begomoviruses (Fig. 3). Corchorus golden mosaic virus was reported earlier from Vietnam (Ha et al. 2008), where this virus was shown to be a member of New World begomovirus group and this proposition has been approved by the International Committee on Taxonomy of Viruses. As the segment of sequence we report here shares the highest sequence identity with Corchorus golden mosaic virus, we propose that this Indian isolate of Corchorus golden mosaic virus is also a member of the New World begomoviruses. The present investigation thus constitutes the first report of the association of a begomovirus, related to New World begomoviruses, with yellow mosaic disease of C. capsularis in India.
|
Acknowledgements
We acknowledge Dr H.S. Sen, Director, Central Research Institute for Jute and Allied Fibres for providing the infrastructural support for this investigation. The first author is also grateful to Indian Council of Agricultural Research, New Delhi for providing financial assistance during the tenure of this work.
Ahmed M
(1978) A whitefly vectored yellow mosaic of jute. FAO Plant Protection Bulletin 26, 169–171.

Doyle JJ, Doyle JL
(1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19, 11–15.

Ha C,
Coombs S,
Revill P,
Harding R,
Vu M, Dale J
(2008) Molecular characterization of begomoviruses and DNA satellites from Vietnam: additional evidence that the New World geminiviruses were present in the Old World prior to continental separation. Journal of General Virology 89, 312–326.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hall TA
(1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41, 95–98.

Roberts S, Stanley J
(1994) Lethal mutations within the conserved stem-loop of African cassava mosaic virus DNA are rapidly corrected by genomic recombination. Journal of General Virology 75, 3203–3209.
| PubMed |

Rojas MR,
Gilbertson RL,
Russell DR, Maxwell DP
(1993) Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminiviruses. Plant Disease 77, 340–347.

Thompson JD,
Gibson TJ,
Plewniak F,
Jeanmougin F, Higgins DG
(1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



