Two viruses detected in table grapes imported into Australia from California
N. Habili A B and J. W. Randles AA Waite Diagnostics, School of Agriculture, Food and Wine, University of Adelaide, PMB1, Glen Osmond, SA 5064, Australia.
B Corresponding author. Email: nuredin.habili@adelaide.edu.au
Australasian Plant Disease Notes 3(1) 63-64 https://doi.org/10.1071/DN08025
Submitted: 29 February 2008 Accepted: 15 April 2008 Published: 1 May 2008
Abstract
Bunches of two table grape varieties, white Thompson Seedless and black Gem Seedless, imported from California were tested for 12 viruses, for phytoplasmas and for Xylella fastidiosa, the causal agent of Pierce’s disease. Reverse transcription–polymerase chain reaction (RT-PCR) of the extracts of pedicels and berry skin revealed the presence of one virus, Rupestris stem pitting associated virus (RSPaV) in Thompson Seedless, while in Gem Seedless both RSPaV and Grapevine leafroll-associated virus 4 were detected. This is the first report of the use of bunches for monitoring the movement of intracellular pathogens across quarantine borders.
Australia is a continent which is still free of most harmful plant pathogens, especially viruses. Quarantine measures are required to prevent the entry of plant viruses by all potential routes and detection methods must be specific, reliable, quick and sensitive. Increasing international trade in fresh foods presents new hazards to plant health. An import risk analysis by the Australian Department of Agriculture, Fisheries and Forestry on table grapes imported from California, USA, led to the granting of permits which were subject to a series of phytosanitary measures against the prokaryotic quarantinable agent of Pierce’s disease (PD), Xylella fastidiosa (http://www.daff.gov.au/__data/assets/pdf_file/0009/82962/2006_32.pdf). The measures are directed against introducing the insect vector of PD, but do not specifically preclude the entry of PD-infected grape bunches into Australia from California in boxes of table grapes.
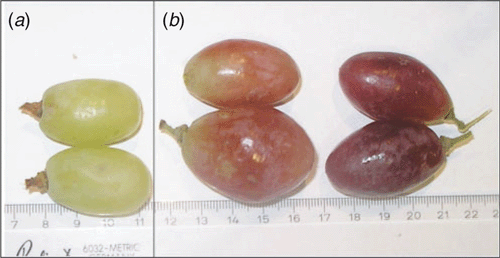
As a risk assessment exercise we have applied RT-PCR analysis to grapevine bunch samples purchased from local supermarkets in Adelaide, South Australia during October 2007. The bunches were from two varieties of table grapes, Vitis vinifera cv. Thompson Seedless (syn. Sultana) and cv. Gem Seedless (Fig. 1) imported from California, USA. Total nucleic acids were prepared from both pedicels and the skins of berries by guanidine hydrochloride–silica matrix extraction followed by elution in TE buffer based on MacKenzie et al. (1997). The RT-PCR diagnosis was conducted for 12 different grapevine viruses (Habili and Randles 2002; Rowhani and Osman 2006), including Grapevine leafroll-associated virus (GLRaV) types 1, 2, 3, 4, 5, and 9, Rupestris stem pitting associated virus (RSPaV), the vitiviruses Grapevine virus A and B, Grapevine fleck virus, Grapevine fanleaf virus (a quarantinable virus) and the Red Globe strain of GLRaV-2 (Table 1). PCR assays included a specific test for X. fastidiosa (Rowhani and Osman 2006) and a generic nested test for phytoplasmas (Constable et al. 2007). Total DNA from the grapevine was extracted following a cetyl trimethyl ammonium bromide (CTAB) procedure (Doyle and Doyle 1987).
|
The primers VV23Sr-F: GAA GGG TGA AAA GAA CCC CCA and VV23Sr-R: CGG TGG GTT CCC TTA ACC AA developed from the complete sequence of Vitis vinifera 23 S rRNA gene (Accession number AF263517) with an amplicon size of 151 bp were used in PCR as an internal control to evaluate the quality of nucleic acid extracts.
All samples tested were negative for X. fastidiosa and for phytoplasmas. In the screening of 12 viruses, only RSPaV was detected in Thompson Seedless, while RSPaV as well as GLRaV-4 were detected in Gem Seedless (Table 1). Little is known about the epidemiology of these two viruses except that RSPaV is present in over 80% of Australian vines and GLRaV-4 is rare in Australia and it is not considered to be a major risk as it is latent under field conditions and synergistic effects with other viruses have not been reported. We conclude that our method would be useful for evaluating import risks as it can be used for random testing of any imported produce obtained directly from fresh food markets. This is the first report of the detection of plant pathogens in pedicels and in berry skins of imported unprocessed grape bunches by PCR and RT-PCR analysis and further illustrates the scope for pathogen detection by sequence-based methods.
Acknowledgements
We wish to thank Mrs. Sara G. Habili for obtaining tagged grapevine bunches from fruit suppliers.
Constable FE,
Joyce PA, Rodoni BC
(2007) A survey of key Australian pome fruit growing districts for exotic and endemic pathogens. Australasian Plant Pathology 36, 165–172.
| Crossref | GoogleScholarGoogle Scholar |

Doyle JJ, Doyle JL
(1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin 19, 11–15.

Habili N, Randles JW
(2002) Developing a standardised sampling protocol for consistent detection of grapevine viruses by the PCR assay. The Australian & New Zealand Grapegrower & Winemaker 464, 88–92.

MacKenzie DJ,
McLean MA,
Mukerji S, Green M
(1997) Improved RNA extraction from woody plants for the detection of viral pathogens by reverse transcription–polymerase chain reaction. Plant Disease 81, 222–226.
| Crossref | GoogleScholarGoogle Scholar |

Rowhani A, Osman F
(2006) Application of a spotting sample preparation technique for the detection of pathogens in woody plants by RT-PCR and real-time PCR (TaqMan). Journal of Virological Methods 133, 130–136.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



