High incidence of Sugarcane yellow leaf virus (SCYLV) in sugar plantations and germplasm collections in Thailand
A. T. Lehrer A B , A. Kusalwong C and E. Komor A DA Pflanzenphysiologie, Universität Bayreuth, Bayreuth, Germany.
B Hawaii Agriculture Research Center, Aiea, HI, USA.
C Plant Pathology Division, Department of Agriculture, Jatuchak, Bangkok, Thailand.
D Corresponding author. Email: ewald.komor@uni-bayreuth.de
Australasian Plant Disease Notes 3(1) 89-92 https://doi.org/10.1071/DN08036
Submitted: 25 July 2007 Accepted: 3 July 2008 Published: 17 July 2008
Abstract
Sugarcane plantations and germplasm collections from across Thailand were tested in two surveys within the years 2000–2003 for Sugarcane yellow leaf virus infection. Twenty-five to 100% of cultivars tested at each plantation/germplasm collection were infected, among them those which had been imported from international breeding stations. Plantation management based on resistant cultivars or virus-free seed cane plantation practices is proposed.
The new sugarcane disease Yellow Leaf Syndrome (YLS) was described in the 1990s in Hawaii (Schenck 1990), Brazil (Vega 1994), Florida (Comstock et al. 1994) and Africa (Bailey et al. 1996). The luteovirus Sugarcane yellow leaf virus (SCYLV) was identified as causal agent of the disease (Scagliusi and Lockhart 2000). Further tests for SCYLV by tissue blot immunoassays and/or PCR (Schenck et al. 1997; Korimbocus et al. 2002) revealed that SCYLV also occurred worldwide. The worldwide distribution most likely proceeded through germplasm exchange and it depended very much on whether the imported germplasm was susceptible to and infected by SCYLV. Spread of SCYLV usually occurs by vegetative propagation of infected stem pieces (so-called seed pieces). It can also be facilitated through the vector activity of viruliferous black sugarcane aphid (Melanaphis sacchari), but infection rates are generally slow (Lehrer et al. 2007). SCYLV-infected plants may be free of obvious symptoms (Lehrer and Komor 2008); thus infections and subsequent spread of SCYLV may fail to be noted, resulting in underestimation of the problem. Sugarcane is an important commodity for Thailand and disease control of sugarcane is, therefore, of vital economic interest. This report shows that SCYLV is widespread in Thai plantations and germplasm collections, and that SCYLV-free management of cane fields for seed pieces and of plantation fields is recommended.
The surveys were conducted from October to February in the years 2000–01 and 2002–03. Samples were taken from the uppermost, fully expanded leaf from 6–12-month-old sugarcane plants (Saccharum spp. hybrids). Leaf pieces of ~15 cm length were collected in a plastic bag with damp tissue enclosed. Within 5 h of sampling, the blade was stripped from the midrib and a freshly cut cross-section of the midrib was pressed onto a nitrocellulose membrane (Biorad TransBlot membrane, 0.2 µm pore size). Three prints were made from each midrib, each from a freshly cut surface. This tissue blot immunoassay occasionally failed to detect SCYLV infections in extremely chlorotic leaf samples (data not shown) and in these instances tissue prints from root sections were used. The membranes with the prints were stored until subsequent processing (Fitch et al. 2001). The leaf sample was considered as infected by SCYLV when at least one bundle of the cross-section showed colour deposits in each of the three prints.
Leaf samples from more than 300 sugarcane cultivars from plantations and germplasm collections in the main sugarcane areas of Thailand were tested for SCYLV in the years 2000–01 and 2002–03. SCYLV was found in all sugarcane regions and on average 27% of the tested cultivars were infected. (Table 1). A second survey (2002–03) collected samples from cultivars which expressed YLS-like symptoms on a regular basis in the fields of sugar mills. Nearly all of these cultivars showed SCYLV-infection (Table 1). Many of the tested cultivars had been imported from breeding stations outside Thailand. Imports from Fiji exhibited a lower proportion of infected cultivars (17%) than cultivars from Canal Point, FL, USA and Kantalai, Sri Lanka (84–100%).
A positive immunoreaction of the tissue blot immunoassay confirms the presence of the virus. However, due to low virus titre, particularly in older leaves, some infections may fall below the sensitivity threshold of the immunoassay (Lehrer and Komor 2008). Here we confirm that positive detection of SCYLV by tissue blot immunoassay depended on the leaf age. Whereas all samples from young source leaves (#1 to #3) showed infection, only one to two-thirds of the tissue prints from older leaves were positive, although the SCYLV-infection was most likely present in the phloem of all leaves and some of these leaves even expressed YLS-symptoms (Table 2).
The survey showed that plantations and breeding stations in all sugarcane areas of Thailand are infected by SCYLV (Fig. 1). Although YLS does not destroy the entire harvest, it reduces yield up to 30%, even when the plants are asymptomatic (Lehrer et al. 2001). Yields are further decreased when plants are infected by SCYLV in combination with phytoplasma (Aljanabi et al. 2001). Two combined strategies are proposed to confine SCYLV-infection to a low level. One is to identify and deploy resistant varieties (Schenck and Lehrer 2000). UT 91-2-633 appears to be a Thai resistance candidate according to our survey. No infection was noted in eight samples of this cultivar, but further work would be required to confirm this observation (0/8 samples from one site only).
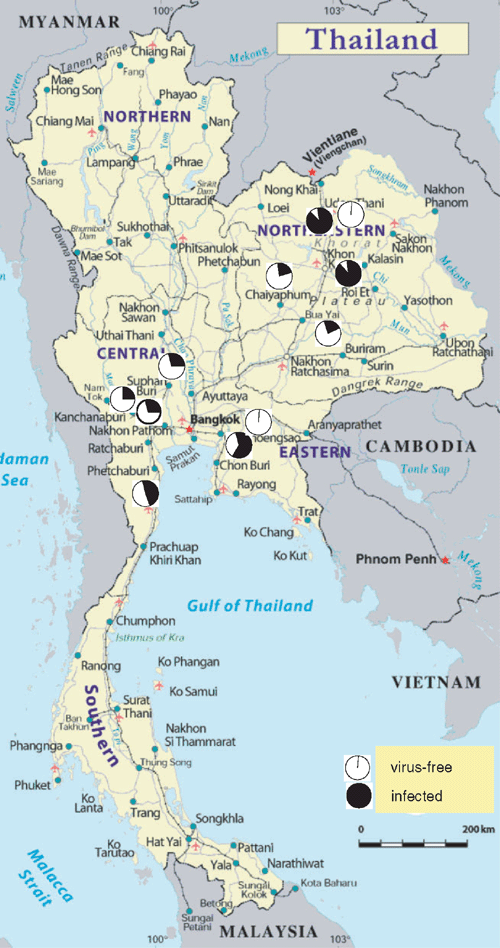
|
The other strategy is a cultivation scheme in which virus-free cane plants, generated by meristem tip culture, are grown for seed piece production in fields remote from commercial sugarcane fields. It has been shown that a sugarcane-free gap of a few hundred metres, or a boundary of resistant cane of a similar distance, was sufficient to prevent de novo infection by aphids (Lehrer et al. 2007). The necessary distance needed for the plantations in Thailand would have to be determined. Whether SCYLV is spreading further in Thailand or has already reached a steady-state of infection would have to be determined by further surveys.
Acknowledgements
We would like to thank the Thai Sugar Trading Board, several Thai sugar companies and the Thai Phytopathological Society, which supported A. Lehrer for travel. This study would not have been possible without the support of numerous helping hands from Thai Department of Agriculture staff and personnel at the various germplasm collections. We are also thankful to staff from Mitr Phol, Kasetphol and Kumphawapi sugar companies. Last but not least we want to thank Dr Susan Schenck and the Hawaii Agriculture Research Center for fruitful collaboration, and the Deutsche Forschungsgemeinschaft for supporting the research project in Hawaii.
Aljanabi SM,
Parmessur Y,
Moutia Y,
Saumtally S, Dookun A
(2001) Further evidence of the association of a phytoplasma and a virus with yellow leaf syndrome in sugarcane. Plant Pathology 50, 628–636.
| Crossref | GoogleScholarGoogle Scholar |

Bailey RA,
Bechet GR, Cronje CPR
(1996) Notes on the occurrence of yellow leaf syndrome of sugarcane in southern Africa. South African Sugar Technology Association Proceedings 70, 3–6.

Fitch MMM,
Lehrer AT,
Komor E, Moore PH
(2001) Elimination of Sugarcane Yellow Leaf Virus from infected sugarcane plants by meristem tip culture visualized by tissue blot immunoassay. Plant Pathology 50, 676–680.
| Crossref | GoogleScholarGoogle Scholar |

Korimbocus J,
Coates D,
Barker I, Boonham N
(2002) Improved detection of Sugarcane yellow leaf virus using a real-time fluorescent (TaqMan) RT-PCR assay. Journal of Virological Methods 103, 109–120.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lehrer AT, Komor E
(2008) Symptom expression of yellow leaf disease in sugarcane cultivars with different degrees of infection by Sugarcane yellow leaf virus.
Plant Pathology 57, 178–189.
| Crossref | GoogleScholarGoogle Scholar |

Lehrer AT,
Schenck S,
Yan S-L, Komor E
(2007) Movement of aphid-transmitted Sugarcane yellow leaf virus (SCYLV) within and between sugarcane plants. Plant Pathology 56, 711–717.
| Crossref | GoogleScholarGoogle Scholar |

Scagliusi SM, Lockhart BEL
(2000) Transmission, characterization, and serology of a luteovirus associated with yellow leaf syndrome of sugarcane. Phytopathology 90, 120–124.
| Crossref | GoogleScholarGoogle Scholar |

Schenck S, Lehrer AT
(2000) Factors affecting the transmission and spread of Sugarcane yellow leaf virus.
Plant Disease 84, 1085–1088.
| Crossref | GoogleScholarGoogle Scholar |

Schenck S,
Hu JS, Lockhart BEL
(1997) Use of a tissue blot immunoassay to determine the distribution of Sugarcane yellow leaf virus in Hawaii. Sugar Cane 4, 5–8.

Vega J
(1994) Sugarcane leaf yellowing syndrome: Evidence for association with virus. Summa Phytopathologica 20, 50–
.



