Bursaphelenchus hunanensis associated with dying Pinus species in Victoria, Australia
D. I. Smith A , M. Hodda B D , I. W. Smith A , L. Nambiar C , I. G. Pascoe C and R. Aldaoud CA School of Forest and Ecosystem Science, The University of Melbourne, 500 Yarra Boulevard, Richmond, Vic. 3121, Australia.
B CSIRO Entomology, GPO Box 1700, Canberra, ACT 2601, Australia.
C Department of Primary Industries, Knoxfield Centre, Private Bag 15, Ferntree Gully Delivery Centre, Vic. 3156, Australia.
D Corresponding author. Email: mike.hodda@csiro.au
Australasian Plant Disease Notes 3(1) 93-95 https://doi.org/10.1071/DN08037
Submitted: 3 June 2008 Accepted: 3 July 2008 Published: 18 July 2008
Abstract
Bursaphelenchus hunanensis (Nematoda: Aphelenchida: Parasitaphelenchidae), identified morphologically in 2001, was associated with dying trees from several species of the genus Pinus at several locations around Melbourne, Victoria. This is the first record for Australia of this nematode and the first detection of this species outside China. The nematode was apparently eradicated through a targeted campaign and perhaps poor vectors. This success is significant because it demonstrates that eradication may be a viable strategy for dealing with incursions of exotic nematodes.
Pine wilt disease is a devastating disorder of pine trees in the Northern Hemisphere, caused by the pinewood nematode Bursaphelenchus xylophilus. Following infection of susceptible pine species through an insect vector and under favourable environmental conditions, the nematode can rapidly multiply within the xylem, where it feeds on epithelial cells and resin ducts. This causes vascular dysfunction leading to rapid wilting, yellowing of needles and tree death within 4–6 weeks (Mamiya 1983; Cram and Hanson 2004). Wilting may appear simultaneously throughout the whole canopy, or it may appear initially on only one branch (‘flag’) and later spread (Malek and Appleby 1984). Because of the rapid, death needles are retained on the tree.
Some other species of the genus Bursaphelenchus are pathogenic to pine trees in some circumstances, and there is debate over the pathogenicity of several species, including B. xylophilus, B. mucronatus, B. sexdentati, B leoni and B. hellenicus (Giblin-Davis, pers. comm.; Coroppo et al. 2000; Skarmoutsos and Michalopoulos-Skarmoutsos 2000; Kruglik 2001; Kulinich 2004; Michalopoulos-Skarmoutsos et al. 2004). However, many species in the genus have never been tested experimentally for pathogenicity, and thus there is considerable, and justified, concern over any appearances of any species of Bursaphelenchus in Australia (Mireku and Simpson 2002).
In Australia, this concern is especially great because many extensively planted species of the genus Pinus are susceptible to pine wilt (Hodda 2006), and environmental conditions that favour disease development (dry with summer temperature >20°C) are common in many local pine-growing regions (Rutherford and Webster 1987; Cram and Hanson 2004). Further complicating the uncertain pathogenicity of Bursaphelenchus species is the absence of molecular information on most species.
Nematode species of the genus Bursaphelenchus are predominantly transmitted from tree to tree by wood-boring insects, mostly beetles. Potentially pathogenic species are principally vectored by pine sawyer beetles (a group of longicorn beetles, Family Cerambycidae). However, some bark beetles that are present in Australia have also been shown to carry Bursaphelenchus species (Stone 1991; Hodda 2003). Most feed only on dead or dying trees, which makes them an unlikely cause of pine wilt disease because the disease only occurs when nematodes are transmitted to healthy trees by the insects’ feeding.
In February 2000, wood samples taken from a dying Pinus halepensis in Williamstown, a suburb of Melbourne, Australia, near extensive docks, were submitted to Crop Health Services of the Victorian Department of Primary Industries, Knoxfield. This tree was reported as having declined rapidly over a 4–6-week period, commencing in early summer, with the needles turning yellow to brown and the twigs becoming dry and brittle and with the needles retained on the tree.
The wood samples were taken by cutting wood chips with an axe from the bole and branches of different sizes, and nematodes of several species were extracted from them using Whitehead trays (Hooper 1986). Most were from taxonomic groups regarded as non-pathogenic. However, one of the species recovered was identified morphologically as probably belonging to the species Bursaphelenchus hunanensis, which had only previously been recorded from dead Pinus massoniana in China in 1984 (Yin et al. 1988). This identification remained indefinite because positive identification requires adults of both sexes, and adult males were not observed. Attempts to culture the nematode using methods used for B. xylophilus were unsuccessful (Hodda et al. 2008). There are also taxonomic issues regarding the definitions of the Bursaphelenchus genera, with some of the characters supposedly diagnostic for the genus also appearing in other genera such as Aphelenchoides or Laimaphelenchus, and some of the characters supposedly uniting Bursaphelenchus, not appearing in some species (Hodda 2009). The nematode was definitely neither B. xylophilus nor B. mucronatus. The disease was reported as caused by an undescribed species, which was distributed widely across Melbourne and the immediate vicinity by Ridley et al. (2001).
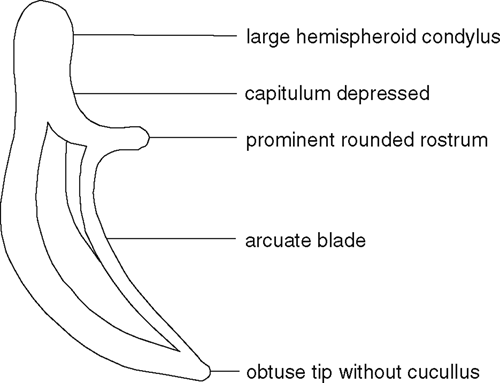
The distinguishing attributes of the adult females extracted from the wood include small body length (500 to 800 µm), long stylet (20 µm), posterior vulva position (morphometric ratio V between 76 and 80), absence of a vulval flap, and long conoid tail. Adult males of B. hunanensis should have spicules with a shape distinctive within the genus (Fig. 1). However, the shape of the spicules could not be confirmed due to the lack of males in the sample. The nematodes definitely did not belong to any of the species of Bursaphelenchus reported as pathogenic. The Australian specimens are in the Australian National Insect Collection Nematode Collection, Canberra.
|
Following national consultation within government and stakeholders, an eradication campaign was instigated in Melbourne which ran from 2000 to 2003. Wood samples were collected from trees identified as showing any symptoms in aerial surveillance, drive-through surveys or reports from the public. Bark was removed from the base of these trees, then pieces of wood taken from the stem and out of exposed roots. The wood samples were returned to the laboratory and nematodes were extracted using Whitehead trays. Further surveillance activity was also carried out across Victoria and Australia to delineate the outbreak.
The nematode was isolated from 35 out of 450 trees sampled that had died rapidly across Victoria. Further samples of ~50 dying pines sent from across Australia tested negative for the nematode. Bursaphelenchus hunanensis was confined to Melbourne and the metropolitan area mainly within the eastern suburbs. Trees that died in Melbourne with B. hunanensis present were generally older trees (estimated to be over 40 years of age) that were also infected with other pathogens such as Diplodia and Armillaria, and affected by abiotic factors such as drought and high salinity. Many were also being attacked by bark beetles and the longicorn beetle Arhopalus rusticus, which was also a new record for Australia. As a precautionary measure, all trees that tested positive for B. hunanensis were removed under quarantine conditions and disposed of by either burning or deep burial.
Since the last trees were removed in January 2004, Pinus species have still been dying across Melbourne. Subsequent deaths have not been associated with B. hunanensis but with extended drought conditions: the deaths have generally been slower after signs of stress appear, and symptoms have been less typical of pine wilt disease. The eradication of this nematode appears to have been successful as no more specimens have been found (Hodda et al. 2008).
It is not known whether B. hunanensis is a primary or secondary pathogen of Pinus species. Attempts to experimentally demonstrate pathogenicity were hampered by inability to culture the nematode. Attempts to induce pathogenicity through direct infection of seedlings by contact with infested wood did not succeed. Bursaphelenchus hunanensis was originally isolated from a dying pine tree, but whether it was the primary cause of death, part of a pathogenic complex, or merely a secondary associate of dying trees remained unresolved.
Whether local or exotic insects were the vectors of B. hunanensis in Australia is unknown. Extensive light and pheromone trapping found two potential vectors: Ips grandicollis and A. rusticus. A. rusticus also emerged 12 months after tree removal from billets cut from several of the trees infested with B. hunanensis and placed in mesh covered drums. A.rusticus is also exotic to Australia and is a new incursion record. No nematodes were found on the beetles that emerged from the billets. Larvae of A. rusticus were found within several dead trees during the surveys in Melbourne suburbs. Bursaphelenchus hunanensis were detected in some of these trees, but not all. A. rusticus is a vector of B. xylophilus and I. grandicollis a potential vector (Hodda 2006), but they usually only attack dying trees so while they can transmit the nematode, they do not cause pine wilt disease.
Long distance spread of pine wilt disease can occur through the movement of infested wood, but there was no evidence of dispersal by this means during this study. Nevertheless, it does indicate that firewood should not be taken from potentially infested trees as subsequent feeding on the infested wood by insects could spread the nematode.
The nematode may have entered Australia through the Port of Melbourne in infested wood and spread throughout Melbourne by either a single flight of vectors which subsequently failed to establish, or by the incursion of an inefficient vector that could not transmit the nematode in sufficient numbers to suitable trees. This hypothesis is supported by the apparent lack of adult male nematodes and distribution of trees where the nematode was found, which was generally in a plume shape in the direction of prevailing winds from the port and had a length approximating that of the flight distance of potential vectors. A complete analysis of the distribution and characteristics of trees affected, together with the adequacy and cost-effectiveness of the response are presented by Hodda et al. (2008).
Coroppo S,
Cavalli M,
Coniglio D, Ambrogioni L
(2000) Pathogenicity studies with various Bursaphelenchus populations on conifer seedlings under controlled and open air conditions. Redia (Firenze) 83, 61–75.

Hodda M
(2009) Ptychaphelenchus eucalypticola gen. nov., sp. nov. (Nematoda: Aphelenchida: Aphelenchoidoididae) from wood and bark of Eucalyptus macrorhyncha in Australia, with a key to genera of Aphelenchoididae and Parasitaphelenchidae. Transactions of the Royal Society of South Australia in press. 133,

Kruglik IA
(2001) The pathogenicity of Far Eastern isolate of Bursaphelenchus mucronatus. Vestnik Dal’nevostochnogo Otdeleniya Rossiiskoi Akademii Nauk 1, 72–76.

Kulinich O
(2004) Surveys for the pine wood nematode in Russia. Nematology Monographs and Perspectives 1, 65–75.

Malek RB, Appleby JE
(1984) Epidemiology of pine wilt in Illinois. Plant Disease 68, 180–186.
| Crossref | GoogleScholarGoogle Scholar |

Mamiya Y
(1983) Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annual Review of Phytopathology 21, 201–220.
| Crossref | GoogleScholarGoogle Scholar |

Michalopoulos-Skarmoutsos H,
Skarmoutsos G,
Kalapanida M, Karageorgos A
(2004) Surveying and recording of nematodes of the genus Bursaphelenchus in conifer forests in Greece and pathogenicity of the most important species. Nematology Monographs and Perspectives 1, 113–126.

Mireku E, Simpson JA
(2002) Fungal and nematode threats to Australian forests and amenity trees from importation of wood and wood products. Canadian Journal of Plant Pathology 24, 117–124.

Ridley G,
Bain J, Dick M
(2001) Exotic nematode found in pine trees in Melbourne, Victoria. New Zealand Journal of Forestry 46, 41–42.

Rutherford TA, Webster J
(1987) Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Canadian Journal of Forest Research 17, 1050–1059.
| Crossref | GoogleScholarGoogle Scholar |

Skarmoutsos G, Michalopoulos-Skarmoutsos H
(2000) Pathogenicity of Bursaphelenchus sexdentati, Bursaphelenchus leoni and Bursaphelenchus hellenicus on European pine seedlings. Forest Pathology 30, 149–156.
| Crossref | GoogleScholarGoogle Scholar |

Stone C
(1991) Parasitic and phoretic nematodes associated with Ips grandicollis (Coleoptera: Scolytidae) in New South Wales, Australia. Nematologica 37, 478–480.

Yin K,
Fang Y, Tarjan AC
(1988) A key to species in the Genus Bursaphelenchus with a description of Bursaphelenchus hunanensis sp. n. (Nematoda: Aphelenchoididae) found in pine wood in Hunan Province, China. Proceedings of the Helminthological Society of Washington 55, 1–11.



