Interactions between plant nutrition and symptom expression in mandarin trees infected with the disease huanglongbing
A. B. Pustika A B , S. Subandiyah B , P. Holford C F , G. A. C. Beattie C , T. Iwanami D and Y. Masaoka EA Yogyakarta Assessment Institute of Agricultural Technology, Karangsari, Wedomartani, Ngemplak Sleman, Yogyakarta 55010, Indonesia.
B Graduate School, Faculty of Agriculture, Gadjah Mada University, Bulak Sumur, Yogyakarta 55281, Indonesia.
C Centre for Plant and Food Science, University of Western Sydney, Locked Bag 1797, Penrith South DC, NSW 1797, Australia.
D National Institute of Fruit Tree Science, Fujimoto 2-1, Tsukuba, Ibaraki 305-8605, Japan.
E Graduate School of Biosphere Science, Hiroshima University, Kagamiyama 1-4-4, Higashi-Hiroshima, Hiroshima 739-8528, Japan.
F Corresponding author. Email: p.holford@uws.edu.au
Australasian Plant Disease Notes 3(1) 112-115 https://doi.org/10.1071/DN08045
Submitted: 27 May 2008 Accepted: 29 July 2008 Published: 6 August 2008
Abstract
A survey of Siem mandarin trees (Citrus reticulata Blanco) grown in different soil types and given different fertiliser regimes showed that applications of a foliar fertiliser reduced the symptom expression of trees infected with huanglongbing by ~40%. In contrast, infected trees growing in a sandy soil, or in a clay–loam, did not differ in their level of symptom expression, despite differences in the fertility of the two soils. These data suggest that infection restricts either nutrient uptake or transport and, therefore, that foliar applied minerals may prolong tree life and reduce yield losses.
Huanglongbing (HLB, citrus greening) is the most devastating disease of citrus in many parts of the world and is caused by non-culturable, phloem-limited bacteria. In Indonesia, the causal pathogen is Candidatus Liberibacter asiaticus (Subandiyah et al. 2000; S. Subandiyah, pers. comm.). Symptoms of HLB are variable, the most noticeable being leaf chlorosis that resembles the deficiency symptoms of Mg, Fe or Zn (Fig. 1). Work by Koen and Langenegger (1970), using an unnamed citrus species infected with the African form of the disease (Ca. Liberibacter africanus), found that concentrations of potassium were higher in infected plants, whilst calcium and magnesium were lower. Aubert (1979) showed that infected plants in Réunion contained lower concentrations of Ca, Mn and Zn. However, the causal organism was not identified, the species of citrus tested was not stated, and no statistical inference made.

|
Despite the similarity of the symptoms with mineral deficiencies, other than the two studies outlined above, there has been little work on the interactions between the disease, edaphic factors and fertiliser applications. To address this deficit, during the rainy season (August) of 2005, citrus orchards were surveyed at three sites in Indonesia: S1, Panjatan District, Kulonprogo Regency, Yogyakarta Special Province (coastal, 5 m above sea level (asl)); S2, Ngombol District, Purworejo Regency, Central Java Province (50 m asl); and S3, Candisari District, Purworejo Regency, Central Java Province (50 m asl). Each site contained 5-year-old trees of Siem mandarin (Citrus reticulata Blanco) budded on to Japanese citron (yuzu: C. × junos Seibold ex Tanaka) rootstocks that were produced at Kutoarjo, Central Java. Soil conditions and fertiliser program varied at each site. S1 had a sandy soil and plants received soil applications of farmyard manure and NPK fertilisers. S2 had a clay–loam soil and plants received soil applications of farmyard manure and NPK fertilisers plus additional foliar fertiliser applications. S3 had a clay–loam soil and plants received soil applications of farmyard manure and NPK fertilisers. Farm yard manure and NPK fertilisers were applied to the soil twice per year, at the start and end of the rainy season (October to April). The farmyard manure was applied at the rate of 20 kg per tree per year, while N, P, and K were applied to the soil at 460 g, 45 g, and 210 g per tree per year, respectively. The foliar fertiliser contained: NH4-N 330 mg/L, NO3-N 24 mg/L, K 759 mg/L, Ca 145 mg/L, Mg 153 mg/L, Fe 277 mg/L, Mn 180 mg/L, Zn 135 mg/L, Cu 95 mg/L. Five mL of fertiliser solution was diluted in 1 L of water and applied at the rate of 1 L of the diluted fertiliser mix per tree during fruit ripening and again after harvest.
Approximately 200 leaves were randomly collected from the four quadrants (north, south, east and west) of each tree for mineral analysis. The leaves were collected at least 3 months after the last spray application from 2-month-old foliage on non-fruiting branches located ~1.5 m above the ground. The leaf samples were washed once with tap water followed by three washes with distilled water. Soil samples were collected at a depth of 300–500 mm from beneath the tree canopy. Leaf and soil samples were taken from five infected (expressing symptoms and PCR positive) and five healthy (PCR negative) trees at each site.
Leaf samples used for mineral analysis were tested for the presence of Ca. Liberibacter asiaticus using the polymerase chain reaction (PCR). Total DNA was extracted from ~0.2 g of leaf midribs (Murray and Thompson 1980) and used to amplify a portion of nusG-rplKAJL gene cluster (Okuda et al. 2005) using primers MHO353 and MHO354 (Hoy et al. 2001).
The P content of soil samples was determined by the Bray I extraction protocol (Bray and Kurtz 1945) and by spectrophotometry at 693 nm. K, Mg, Fe and Zn in soil samples were extracted using HNO3−HClO4 and from leaves using digestion with HNO3−HClO4. Concentrations of K were determined by flame photometry whilst atomic absorbance spectrophotometry was used to determine concentrations of Mg, Fe and Zn. A micro-Kjehdahl method was used to determine the N content of leaf and soil samples (Ma and Zuazaga 1942).
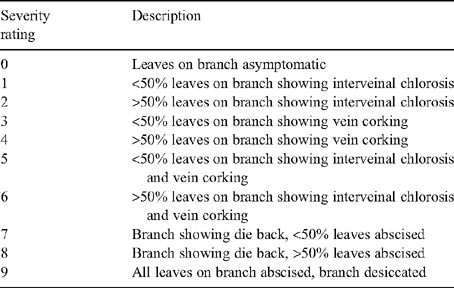
The symptom severity of HLB on each tree was determined on 16 branches of each tree as follows. The foliage of each tree was divided into four quadrants (north, west, south and east) and then the symptoms on a randomly chosen branch from the left top, right top, left basal and right basal branch of each quadrant assessed. Branches were assessed for interveinal chlorosis, vein corking, leaf death and branch dieback (Table 1). Symptom severity for each tree was calculated using the formula described by McKinney (1923).
|
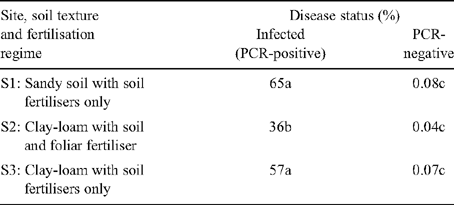
In this study, the pathogen status of trees was determined using PCR. However, the absence of an amplicon cannot be taken as evidence that the bacterium was not present within the tree. Therefore, trees failing to produce an amplicon were referred to as PCR-negative. The symptom severity of PCR-negative trees at all three sites was low (0.04–0.07%) and these scores may have reflected nutrient deficiencies rather than disease (Table 2). In contrast, the average disease severity of PCR-positive trees varied from 36–65% (Table 2). Hence, there was a good agreement between the visual assessment of symptoms and identification of the pathogen status of the trees by PCR. Therefore, if PCR-negative trees do contain the causal organism, their titres must have been below detectable levels.
|
Vein corking symptoms were observed during this survey and are regularly found associated with trees infected with HLB in Indonesia, where the disease is known as citrus vein-phloem degeneration disease (Tirtawidjaja et al. 1964). Vein corking is also known to be associated with other diseases, e.g. infection with Citrus tristeza virus (Broadbent et al. 1996), and for this reason is not considered a reliable characteristic for disease identification.
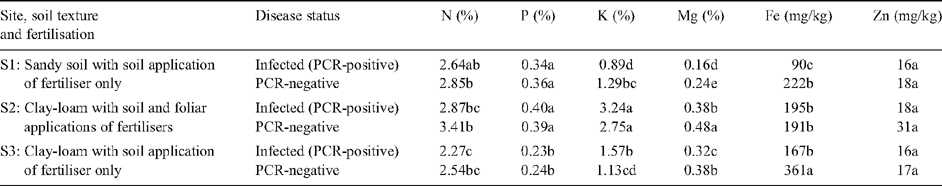
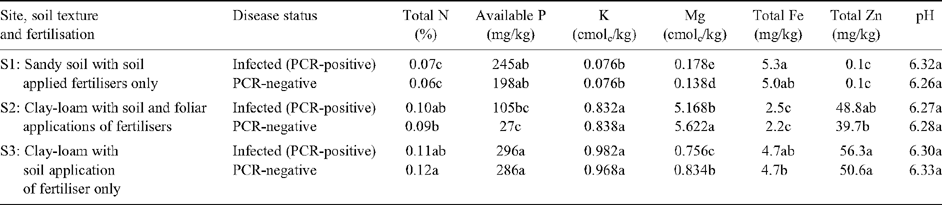
Infection of mandarin plants with HLB affected their foliar mineral status (Table 3). On average, the foliar concentrations of N, Mg and Fe were, respectively, 12, 21 and 42% lower in infected trees compared with those that were PCR-negative (P < 0.001 for all elements). In contrast, no differences were found in foliar concentrations of K, P and Zn. These results suggest that if spray residues remained on leaf surfaces, their concentrations were minimal. Soil analysis showed no difference between samples taken from under the tree canopies of infected or PCR-negative trees (Table 4). Therefore, it is unlikely that the differences in foliar mineral concentrations between infected and PCR-negative trees at each site were due to differences in the soil.
Differences were also found in the foliar nutrient status of trees grown on different soil types or given different fertiliser treatments. For all elements except Zn, concentrations in the leaves were higher in plants given foliar fertiliser applications (Site 2) compared with those only given soil applied fertilisers: foliar Zn concentrations did not differ between fertiliser treatments nor between sites. With the exception of P, foliar concentrations of minerals were higher in plants grown in clay–loam soil (Site 3) compared with those grown in sand (Site 1). P concentrations were low in the clay soils at Site 2. The pH of the soils at all three sites was similar (Table 4) and was within the range considered ideal for the growth of citrus trees (Revelant et al. 2005).
Symptom severity was affected by fertiliser treatments. At Site 2, where the plants were given applications of foliar fertilisers, infected plants had a severity score of 36%, ~40% less than trees given soil-applied fertilisers only. No difference was observed in symptom severity between the other two sites (Site 1, 65%; Site 3, 57%) despite the large difference in the nutrient status of the soils. Correlation analysis was performed to look for relationships between symptom severity and the mineral composition of the leaves or soil. Significant negative relationships existed between the symptom severity of infected plants and foliar concentrations of K, Mg and Fe (r = −0.71, −0.78 and −0.75; P < 0.01 in all cases). When the data from the trees given the foliar application of fertiliser was excluded, the only significant correlation was with Fe (r = −0.67, P = 0.048). In addition, significant negative relationships also existed between the symptom severity of infected plants and concentrations of N (r = −0.55, P = 0.03) and Mg (r = −0.80, P < 0.001) in the soil. However, a positive relationship existed with soil Fe content (r = 0.84, P < 0.001). This was surprising as, in this study, a negative correlation was found between symptom severity and foliar Fe content and a previous study has also shown that Fe is low in the leaves of HLB-infected plants (A. B. Pustika, unpubl. data).
In this study, there was little difference between the severity of disease symptoms of mandarins grown on the sandy compared with clay loam soils, despite the large difference in the fertility of the two soil types. It is likely that HLB-infected plants may have poor root development or that the transport of minerals between the roots and leaves is disrupted. If this is the case, it appears that foliar applications of minerals can correct any foliar nutrient deficiency in infected plants as, in this study, the applications of a foliar fertiliser significantly reduced the severity of disease symptoms. However, further studies need to be made to determine if foliar applied minerals can reduce symptom expression in other citrus cultivars and species and under other growing conditions, and whether foliar fertiliser applications can prolong tree life and reduce yield losses.
Acknowledgements
This work was partially funded through a grant (CS2/2000/043) from the Australian Centre for International Agricultural Research. The authors would also like to thank Paul Milham (NSW Department of Primary Industries) and Pat Barkley for their comments on the manuscript.
Aubert B
(1979) Progrès accompli dans la lutte contre le greening des citrus à la Réunion. Revue Agricole et Sucrière 58, 53–56.
[Verified 31 July 2008]
Subandiyah S,
Nikoh N,
Tsuyumu S,
Somowiyarjo S, Fukatsu T
(2000) Complex endosymbiotic microbiota of the citrus psyllid Diaphorina citri (Homoptera: Psylloidea). Zoological Science 17, 983–989.
| Crossref | GoogleScholarGoogle Scholar |