First report of Rhizoctonia solani AG 4 HG-II attacking castor bean plants (Ricinus communis) in Brazil and evaluation of two castor bean cultivars for resistance to damping-off
M. A. Basseto A , H. A. Chagas A , D. D. Rosa A B C , M. D. Zanotto A and E. L. Furtado A BA São Paulo State University, UNESP, College of Agronomic Science, FCA, Department of Plant Production, Plant Health Protection Sector, PO Box 237, Botucatu, SP 18610-307, Brazil.
B Researchship of the National Counsel of Technological and Scientific Development.
C Corresponding author. Email: ddrosa@fca.unesp.br
Australasian Plant Disease Notes 3(1) 121-123 https://doi.org/10.1071/DN08048
Submitted: 9 June 2008 Accepted: 3 September 2008 Published: 18 September 2008
Abstract
In 2006 stem rot, root rot and death of castor bean plants was widespread in parts of the São Paulo State, in Brazil. Rhizoctonia solani AG HG-II was identified as the causal agent. Two extensively grown cultivars (BRS Nordestina variety and Sara hybrid) were highly susceptible to this pathogen.
Castor bean (Ricinus communis) belongs in the Euphorbiaceae and is classified as an oleaginous species due to its great production of essential oils. It is probably native to the African continent, in current Ethiopia. This region has a tropical climate, which has favoured the adaptation of this species in Brazil. As a plant with great aptitude for essential oil production, it is grown with the aim of incorporating its oil into combustible compounds such as biodiesel. This has been the motivating factor for castor bean cultivation in Brazil (Azevedo and Lima 2001).
The resurgence of this crop and the agricultural expansion to regions with lower technological access has led to a plant population increase. Higher dissemination of phytopathogenic agents, including soil-transmitted pathogens such as Rhizoctonia spp. (Lima and Batista 1997), was found during the last few agronomic years. Consequently, properties with limited technology have not had great productivity, which has damaged the development of the Brazilian castor crop (Fornazieri Junior 1986).
Diseases caused by soil-inhabiting fungi are detected during castor bean germination or in seedlings and lead to necrosis of stem, cotyledon and young root tissues. The main injury caused is stand reduction, which needs replanting procedures, processes that elevate crop production costs (Batista et al. 1996). The aim of this study was to verify the resistance of two cultivars largely used in Brazil to Rhizoctonia spp., as well as to characterise Rhizoctonia spp. isolates that have damaged recent castor bean crops.
In March 2006, symptoms including stem rot and root rot, leading to the death of plants, were observed in castor bean plants in São Paulo State, in Brazil. White mycelium was observed in dead plants and necrotic tissue. Fragments of necrotic tissue were collected and disinfested in 70% alcohol for 30 s and 2% sodium hypochlorite for 30 s, washed in distilled water, and then plated onto PDA and incubated at 24°C in the dark for 24 h (Fenille et al. 2005). The isolated fungus showed a ramification angle of ~90°, basal constriction, a septum next to the lateral hyphae and sclerotia. This morphology is consistent with Rhizoctonia spp. (Sneh et al. 1991). A culture has been deposited in the São Paulo State University Mycology Database.
Twenty randomly selected cells per isolate were analysed, and the number of nuclei per cell was counted. Prior to examination, hyphae were stained with a DAPI technique in the vegetative cells (Kulik and Dery 1995) and the hyphae were stained with a safranin-O (0.03%) and KOH (3%) solution and then observed under brightfield microscopy at ×400 magnification, for the visualisation of the anastomosis reaction (Yamamoto and Uchida 1982). Hyphae were considered to be compatible when at least five points on each of four slides/isolate showed C2 and C3 type-reactions (Carling and Leiner 1990). Anastomosis was regarded as positive when hyphae between the castor bean isolate and AG tester made contact with each other and their walls fused, with subsequent plasmolysis of adjacent cells (Carling and Leiner 1990). Tester isolates of R. solani and yours subgroups (AG 1 IA, IB, IC; AG 2, 2 IIIB; AG 3; AG 4 HG-I, HG-II, HG-III; AG 5; AG 6; AG 7; AG 8; AG 9; AG 10; AG 11; AG 12; AG 13 and AG BI), obtained from researchers from different parts of the world, were paired with all isolates collected.
Colonies of cream-coloured mycelia grew close to the surface of the PDA and the isolate produced mostly irregular whitish brown sclerotia inside the medium. Sclerotia were an average of 4.8 mm in diameter, varying from 2.4 to 7.4 mm. Hyphae measured an average of 6.8 µm in diameter, varying from 5.7 to 7.8 µm. Clamp connections were not observed. The fungus was multi-nucleate, forming 6 to 21 nuclei per cell, providing further support for the isolate being identified as belonging to the species Rhizoctonia solani. We observed the occurrence of C2 anastomosis reaction between the castor bean isolate and the standard isolate for anastomosis group 4 HG-II, which allowed the castor bean isolate to be identified as belonging to the species Rhizoctonia solani AG 4 HG-II.
For a cultivar resistance assay, an experiment was carried out in a greenhouse. Experimental design was completely randomised, including two varieties – inoculated and non-inoculated – with five replicates, each one corresponding to one pot, which contained four plants. Evaluations were set as mean values of all plants per pot. Two cultivars were assayed: BRS Nordestina variety and Sara hybrid. Inoculation was performed according to the methodology described by Lefèvre and Souza (1993), which uses sandy-organic substrate, sterilised by autoclave at 120°C for 1 h. This substrate was subsequently inoculated with three 5-mm-diameter discs taken from the edges of isolate colonies grown in PDA for 4 days at 25°C. After inoculation the substrate was incubated at 25°C in the dark for 15 days. Then, pots containing castor bean seedlings of both cultivars were inoculated with 5% (w/v). Controls were inoculated with sandy-organic substrate without the fungus. The percentage of dead and/or symptomatic plants was estimated 15 days after inoculation.
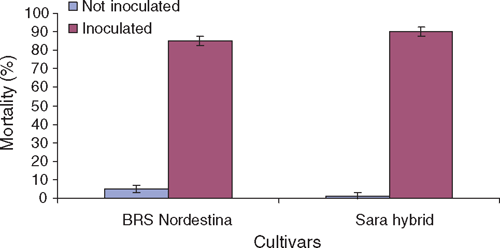
Both cultivars, BRS Nordestina variety and Sara hybrid, were highly susceptible to stem and root rot (Fig. 1). Over 80% mortality was observed (Fig. 2). These are the most cultivated commercial varieties in Brazil. Sara hybrid (90% mortality) was more susceptible than BRS Nordestina (85%) variety, which indicates that a line with a larger genetic basis may be more resistant than a hybrid line. Therefore, variety cultivation must be recommended to regions presenting historical rhizoctonia problems in Brazil.

|
|
In all experiments the pathogen was reisolated from fragments of necrotic regions of inoculated plants, and the presence of the fungus R. solani was demonstrated, thus confirming it as the causal agent.
Acknowledgements
The authors wish to thank Dr J. B. Sinclair (University of Illinois, Urbana-Champaign, USA), Dr A. Ogoshi (Hokkaido University, Sapporo, Japan), Dr L. J. Herr (Ohio State University, Wooster, USA), Dr S. Naito (Tohoku National Agricultural Experiment Station, Morioka, Japan) and Dr M.A. Cubeta (Duke University, North Carolina, USA) for supplying the tester strains of R. solani.
Carling DE, Leiner RH
(1990) Virulence of isolates of Rhizoctonia solani AG-3 collected from potato plant organs and soil. Plant Disease 74, 901–903.
| Crossref | GoogleScholarGoogle Scholar |

Fenille RC,
Ciampi MB,
Souza NL,
Nakatani AK, Kuramae EE
(2005) Binucleate Rhizoctonia sp. AG G causing root rot in yacon (Smallanthus sonchifolius) in Brazil. Plant Pathology 54, 325–330.
| Crossref | GoogleScholarGoogle Scholar |

Kulik MM, Dery PD
(1995) Use of DAPI for anastomosis group typing of strains of the fungus Rhizoctonia solani. Biotechnic & Histochemistry 70, 95–98.
| Crossref | GoogleScholarGoogle Scholar |

Lefèvre AF, Souza NL
(1993) Lethal temperature determination for Rhizoctonia solani and Sclerotium rolfsii, and solarization effect in the soil temperature. Summa Phytopathologica 19, 107–112.

Yamamoto DT, Uchida JY
(1982) Rapid nuclear staining of Rhizoctonia solani and related fungi with acridine orange and with safranin O. Mycologia 74, 145–149.
| Crossref | GoogleScholarGoogle Scholar |



