First molecular identification of a begomovirus associated with yellow vein net disease in grain amaranth (Amaranthus cruentus L.) in India
S. K. Raj A C , S. K. Snehi A , M. S. Khan A , P. Chandra B and R. M. Pandey BA Plant Molecular Virology, National Botanical Research Institute (NBRI), Lucknow, UP 226001, India.
B Cytogenetics Laboratory, National Botanical Research Institute (NBRI), Lucknow, UP 226001, India.
C Corresponding author. Email: skraj2@rediffmail.com.
Australasian Plant Disease Notes 3(1) 129-131 https://doi.org/10.1071/DN08050
Submitted: 2 April 2008 Accepted: 8 September 2008 Published: 22 September 2008
Abstract
The association of a distinct begomovirus with yellow vein net disease in grain amaranths in India was detected by polymerase chain reaction using begomovirus-specific primers. The begomovirus possessed highest identities and closest relationship with Ageratum enation virus.
Grain amaranth (Amaranthus cruentus L., family Amaranthaceae) is one of the most important subsidiary food crops in the tropical and subtropical highlands of Asia and South Americas (Sauer 1950). The crop is recognised worldwide as an excellent source of high quality and easily digestible protein. A yellow vein net disease affecting 10–15% of plants was observed during the winter of 2007–08 on grain amaranth grown in breeding plots at the National Botanical Research Institute, Lucknow, India. Naturally infected plants exhibited yellow vein net, swelling of veins, reduction of leaf lamina and stunting of the whole plant (Fig. 1). The causal pathogen was suspected to be a begomovirus due to the large population of whitefly (Bemisia tabaci) observed in the area and the similarity of the yellow net symptoms to those caused by begomoviruses.

|
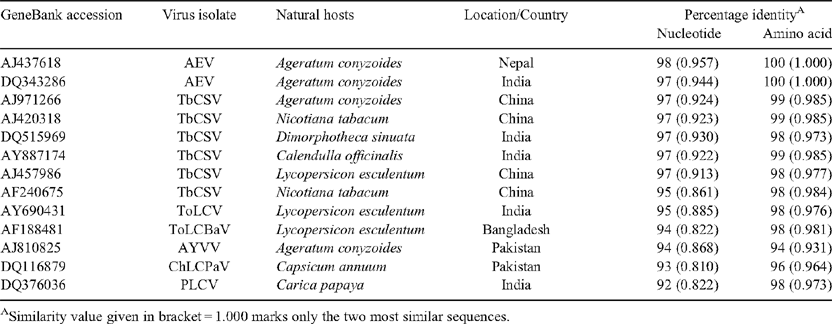
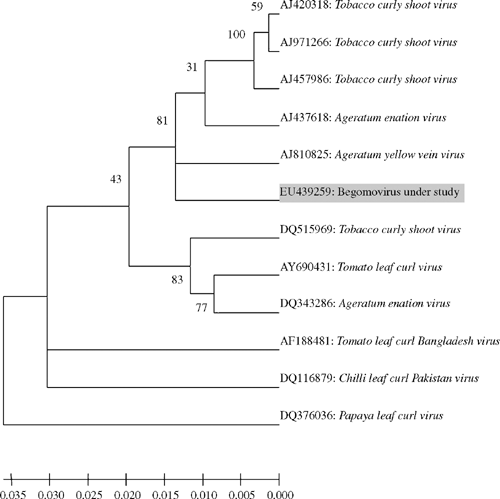
To check for the presence of a begomovirus infection, total DNA was isolated from leaf tissues of five symptomatic and two asymptomatic plants using a published method (Dellaporta et al. 1983). The DNA samples were subjected to polymerase chain reaction (PCR) with a set of primers designed from the coat protein region of begomovirus (GenBank accessions AM180920 and AM180921). As expected, amplicons of ~800 bp were obtained from all five symptomatic but not from the two asymptomatic plant samples. Amplicons obtained from each plant sample were sequenced in both orientations and the consensus sequence was deposited in GenBank database (GenBank accession. EU439259). Basic local alignment search tool (BLAST) search analysis of the sequence data showed highest sequence identity (98%) with Ageratum enation virus (AEV) isolates (GenBank accessions AJ437618, DQ343286) and 97% with several isolates of Tobacco curly shoot virus (TbCSV, GenBank accessions DQ515969, AJ420318, AJ971266, AJ457986). Genomatix DiAlign analysis with selected begomovirus sequences also showed highest identities of 97–98% at the nucleotide and 100% at the amino acid levels with AEV. The identities of the coat protein region (EU439259) with that of TbCSV isolates were 95–97% and 98–99% at nucleotide and amino acid levels, respectively (Table 1). Ageratum yellow vein virus (AYVV) showed 94% identities at nucleotide and the amino acid levels. Phylogenetic analysis of the selected begomovirus isolates using molecular evolutionary genetics analysis (MEGA) 4.0 tool (Tamura et al. 2007) showed the closest relationships with AYVV, AEV and TbCSV isolates and more distant relationships with other begomovirus isolates (Fig. 2). The closest relative of the begomovirus under study could not be determined using the phylogentic tree as the distinction between AEV, AYVV and TbCSV was not well supported (i.e. a bootstrap value of only 31). The virus isolate is, therefore, proposed to be a begomovirus closely related to AEV based on the highest (98%) sequence identity, and in accordance with the latest report of International Committee on Taxonomy of Viruses (Fauquet et al. 2008).
|
Tomato yellow leaf curl virus has been detected by nucleic acid tissue blot hybridisation in Amaranthus sp. (Abou-Jawdah et al. 1999). Amaranthus spinosus and A. viridis weeds are reported as hosts of begomoviruses (Arnaud et al. 2007). Several species of Amaranthus have also been found to be the natural hosts of other groups of viruses (Horvath 1991; Raj et al. 1997). However, natural occurrence of a begomovirus (closely related to Ageratum enation virus) in A. cruentus is a first report from India. Amaranthus cruentus may be considered as an alternate host of AEV which might be playing a key role in its dissemination to other important crop species.
Acknowledgement
The authors are thankful to the director, NBRI, Lucknow for facilities and Uttar Pradesh Council of Science and Technology, Lucknow for financial support.
Abou-Jawdah Y,
Maalouf R,
Shebaro W, Soubra K
(1999) Comparison of the reaction of tomato lines to infection by tomato yellow leaf curl begomovirus in Lebanon. Plant Pathology 48, 727–734.
| Crossref | GoogleScholarGoogle Scholar |

Arnaud LSEP,
Santos CDG, Lima JAA
(2007) Predominance of begomovirus on tomato in chapada da Ibiapaba, Ceará, and its natural detection on weeds plants. Fitopatologia Brasileira 32, 241–246.
| Crossref | GoogleScholarGoogle Scholar |

Dellaporta SL,
Wood J, Hicks JB
(1983) A plant DNA minipreparation: Version III. Plant Molecular Biology Reporter 1, 19–21.
| Crossref | GoogleScholarGoogle Scholar |

Fauquet CM,
Briddon RW,
Brown JK,
Moriones E,
Stanley J,
Zerbini M, Zhou X
(2008) Geminivirus strain demarcation and nomenclature. Archives of Virology 153, 783–821.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Horvath J
(1991) Amaranthus species (Family Amaranthaceae) as host of plant viruses: a review. Acta Phytopathologica et Entomologica Hungarica 26, 385–422.

Raj SK,
Aminuddin
,
Singh BP, Pal M
(1997) Characterization of a cucumber mosaic virus isolate causing leaf crinkle and severe mosaic of Amaranthus in India. Canadian Journal of Plant Pathology 19, 97–100.

Sauer JD
(1950) The grain amaranths: A survey of their History and Classification. Annals of the Missouri Botanical Garden 37, 561–632.
| Crossref | GoogleScholarGoogle Scholar |

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar | PubMed |