Characterisation of an antinematicidal compound from Leucaena leucocephala
O. K. Adekunle A C and M. A. Aderogba BA Department of Crop Production and Protection, Obafemi Awolowo University, Ile-Ife, Nigeria.
B Department of Chemistry, Obafemi Awolowo University, Ile-Ife, Nigeria.
C Corresponding author. Email: kolaade2002@yahoo.co.uk
Australasian Plant Disease Notes 3(1) 168-170 https://doi.org/10.1071/DN08066
Submitted: 29 April 2008 Accepted: 15 November 2008 Published: 1 December 2008
Abstract
Leucaena leucocephala is a small tropical tree used for a variety of purposes in agriculture, land management and homeopathic medicine. Quercetin, a flavonoid, was isolated and characterised from extracts of leaves of L. leucocephala and its effects on egg hatching and juvenile mortality of Meloidogyne incognita were investigated at 0.8, 0.4 and 0.2% in vitro. The compound was highly toxic to eggs and juveniles of the nematode at the three rates tested.
Additional keywords: phenolic compound, root-knot nematode.
Introduction
Globally, the most important plant-parasitic nematodes are the root-knot nematodes, Meloidogyne spp., since they infect the majority of the economically important species of plants in the world (Trudgill and Blok 2001). This genus contains at least 80 species (Karssen 2002) reported to cause an estimated US$100 billion loss per year worldwide (Oka et al. 2000).
Over the last 30–40 years, agricultural chemicals have been the main control method for plant-parasitic nematodes in intensive agriculture (Talavera and Mizukubo 2005). However, increasing public and governmental concerns over the harmful effects of some chemical pesticides have led to their withdrawal or reduced use. Therefore, there is a need for alternative control methods to manage plant-parasitic nematodes with the aim of maintaining the current standards of quality and production.
Crude extracts of roots and leaves of Leucaena leucocephala have been found to be toxic to eggs and juveniles of Meloidogyne incognita (Adekunle and Akinlua 2007). Also, reduced populations of M. incognita, Pratylenchus spp., Paratylenchus spp. and Hoplolaimus spp. were recorded when okra varieties were planted in alleys of L. leucocephala (Adekunle 2008). Leucaena leucocephala is not a host of M. incognita and M. javanica (Stirling et al. 1992). In this study, we report the isolation and structural elucidation of a phenolic compound (flavonoid) – quercetin, an antinematicidal agent from the ethyl acetate fraction of a 20% aqueous methanol extract of leaves of L. leucocephala – and the toxicity of the isolated compound to M. incognita.
Materials and methods
Cultures of Meloidogyne incognita and extraction of eggs
Pure cultures of M. incognita obtained from nematode culture plots of Obafemi Awolowo University, Ile-Ife, Nigeria were maintained on Celosia argentea cv. TLV8 in the greenhouse. M. incognita eggs were extracted by shaking infected celosia root pieces with 0.5% sodium hypochlorite (Hussey and Barker 1973). Some of the extracted eggs were incubated using extraction trays to obtain second-stage juveniles (J2).
Collection of plant materials and extraction
The leaves of L. leucocephala were collected from the Obafemi Awolowo University Teaching and Research Farm, Ile-Ife, Nigeria. The leaves were air-dried for 2 weeks and powdered. The powdered leaves (500 g) were extracted with 20% aqueous methanol (6 L) at room temperature for 24 h and filtered. The crude extract was concentrated in vacuo at 40°C and reduced to about one third of the original volume. This was extracted with n-hexane, dichloromethane, ethyl acetate and finally n-butanol. Four solvent fractions were obtained and concentrated to dryness in vacuo.
General
All thin-layer chromatography analyses were performed at room temperature using pre-coated plates (MERCK, silica gel 60 F254 0.2 thickness). Nuclear magnetic resonance (NMR) 1H (300 MHz) and 13C (75 MHz) spectra were recorded on a Bruker UltraSheild spectrometer. The electron spray ionisation (ESI) mass spectrum was recorded on a Finnigan LCQ deca spectrometer.
Isolation of an antinematicidal agent from the ethyl acetate fraction of the crude extract
Column chromatography of the ethyl acetate fraction (10 g) on silica gel (70–230 mesh) using a mixture of hexane and ethyl acetate followed by an increasing gradient of methanol up to 20% afforded four fractions (A1–D1), as determined by analysis using a silica gel TLC plate and 9.5 : 0.5 CH2Cl2 : MeOH as the solvent system. Repeated purification of fraction D1 (0.5 g) on a Sephadex LH 20 column using a mixture of CH2Cl2 : MeOH and EtOAc : MeOH led to isolation of compound 1 (7 mg) after analysis of the different fractions collected on a TLC plate using CH2Cl2 : MeOH (7 : 2) as the solvent system.
Egg-hatch and juvenile mortality tests
In total, 4 mL aliquots of 300 eggs or juveniles of M. incognita in water suspension were transferred separately into Petri dishes (60 mm diameter). Compound 1 was introduced into the Petri dishes at concentrations of 0.8%, 0.4% or 0.2%. Petri dishes containing nematode eggs or juveniles in water suspension without compound 1 served as controls. Petri dishes were incubated in the laboratory at ambient temperature. The numbers of hatched or immobilised juveniles were counted every 24 h for 14 days. Nematodes were confirmed as being dead when they remained immobile and failed to respond to touch when transferring them by picking brush into distilled water. Each treatment was replicated four times while the egg-hatch and juvenile mortality trials were performed twice.
Statistics
Egg hatch data were subjected to statistical analysis using the SAS statistical package (1985, SAS Institute Inc. Cary, NC, USA). Differences between means were tested using Fisher’s least significant difference at P = 0.05.
Results
Identification of the antinematicidal compound
The isolated compound was identified based on the following spectral characteristics. 1H NMR (300 MHz, MeOD): δ 6.20 (1H, d, J = 2.1 Hz, H-6), 6.41 (1H, d, 2.1 Hz, H-8) meta related ring A hydrogen atoms, 6.91 (1H, d, J = 8.4 Hz, H-5′), 7.66 (1H, dd, J = 2.1 and 8.4 Hz, H-6′), 7.75 (1H, d, J = 2.1 Hz, H-2′), showing meta related H-2′ and H-6′ in addition to ortho related H-5′ and H-6′ of ring B. 13C NMR spectroscopic data are in good agreement with the literature (Harborne and Mabry 1982; Markham 1982). The molecular formula of compound 1 is C15H10O7 from the interpretation of NMR spectra. This corresponds to a molecular weight of 302. The ESI mass spectrum (negative mode) showed a molecular ion [M+-H] corresponding to the molecular mass of the compound at m/z = 301.1 as the base peak. The structure of compound 1 is presented in Fig. 1 and identified as quercetin.
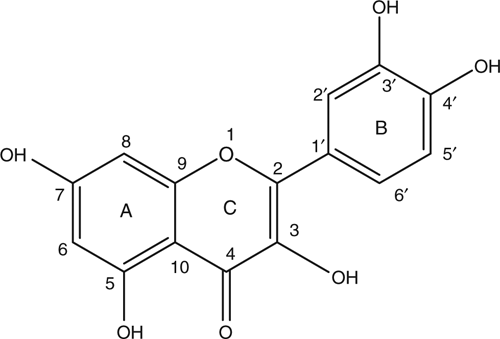
|
Egg-hatch and juvenile mortality tests
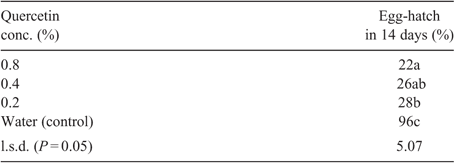
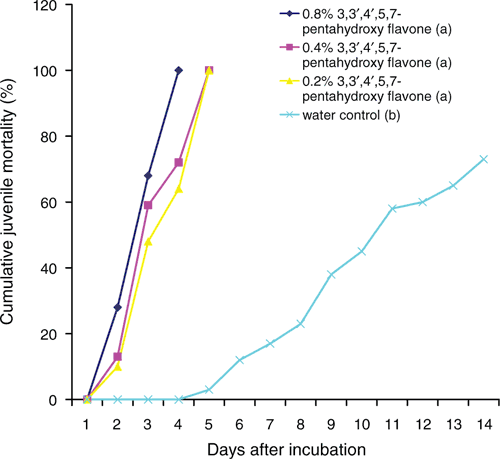
Toxicity of quercetin to eggs of M. incognita is presented in Table 1. The phenolic compound tested was highly toxic to eggs of the nematode at 0.8%, 0.4% and 0.2%, with only 22–28% of the eggs hatching into juveniles over a period of 14 days. Also, juveniles of M. incognita in 0.8% quercetin were all killed within 4 days while in 0.4% and 0.2% quercetin all juveniles were killed in 5 days (Fig. 2). In the water (control), however, only 73% of the juveniles were killed by 14 days.
|
|
Discussion
Egg hatching and the number of active living juveniles were reduced in the presence of quercetin isolated from L. leucocephala, when compared with eggs and juveniles kept in water. The findings of the current study corroborate those of a recent study, which reported that crude extracts of L. leucocephala exhibited antinematicidal properties (Adekunle and Akinlua 2007).
The effect of quercetin on Caenorhabditis elegans was a reduction in the reactive oxygen species accumulation at thermal stress (Kampkotter et al. 2007) indicating that quercetin may act as an antioxidant as well as a modulator of cellular signalling processes to exert inhibitory effects on microorganisms. Several studies have demonstrated antithelminthic effects of extracts of L. leucocephala in animals (Bamualim et al. 1984; Pearce et al. 1984; Ademola et al. 2005) and crude extracts of its bark and roots have broad uses medicinally in Latin America (Duke 1983).
Given increasing public and governmental concerns over the use of synthetic nematicides, antinematicidal compounds of plant origin could be used for nematode management, complementing synthetic nematicides to reduce the quantities of synthetic chemicals used in agriculture. Testing the isolated compound on root-knot nematodes infecting plants under greenhouse conditions is recommended. The results of this study suggest that concerted efforts should be made to further cultivate L. leucocephala with the aim of extracting antinematicidal compounds from it for management of phytonematodes.
Acknowledgements
The authors are grateful to Network for Analytical and Bioassay Services in Africa (NABSA), Department of Chemistry, University of Botswana, Gaborone for running NMR and Mass spectra of the isolated compound. Mrs. Abimbola Oluwaranti is thanked for running Statistical Analysis and drawing the curves.
Adekunle OK
(2008) Population dynamics of Meloidogyne incognita and three other phytonematodes on okra varieties planted in alleys of Leucaena leucocephala and Gliricidia sepium. Unpublished data ,
[Verified 19 November 2008]
Hussey RS, Barker KR
(1973) A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter 57, 1025–1028.

Kampkotter A,
Nkwonkam CG,
Zurawski RF,
Timpel C,
Chovolou Y,
Watjen W, Kahl R
(2007) Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 234, 113–123.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oka Y,
Koltai H, Bar-Eyal M
(2000) New strategies for the control of plant-parasitic nematodes. Pest Management Science 56, 983–988.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Pearce FL,
Befus AD, Bienenstock J
(1984) Mucosal mast cells. III. Effect of quercetin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. The Journal of Allergy and Clinical Immunology 73, 819–823.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Stirling GR,
Stanton JM,
Brandon N, O’Donnell WE
(1992) Reaction of Leucaena to Australian populations of root-knot nematode (Meloidogyne spp.). Australasian Plant Pathology 21, 143–146.
| Crossref | GoogleScholarGoogle Scholar |

Talavera M, Mizukubo T
(2005) Effects of DL–methionine on hatching and activity of Meloidogyne incognita eggs and juveniles. Pest Management Science 61, 413–416.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Trudgill DL, Blok VC
(2001) Apomitic polyphagous root knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annual Review of Phytopathology 39, 53–77.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |



