Hand decontamination: influence of common variables on hand-washing efficiency
Thomas Miller A D , Daniel Patrick B and Douglas Ormrod CA Department of Medicine, The University of Auckland, Private Bag 92019, AMC1142, Auckland, New Zealand.
B Ngā Pae o te Māramatanga, New Zealand’s Māori Centre of Research Excellence, The University of Auckland, Private Bag 92019, AMC1142, Auckland, New Zealand.
C Neurological Foundation of New Zealand, PO Box 110022, Auckland 1148, New Zealand.
D Corresponding author. Email: t.miller@auckland.ac.nz
Healthcare Infection 16(1) 18-23 https://doi.org/10.1071/HI10027
Submitted: 23 August 2010 Accepted: 2 February 2011 Published: 28 March 2011
Abstract
This study has evaluated the effects of wash time, friction and soap on the decontamination of hands seeded with Escherichia coli. In one protocol contaminated hands were held passively under running tap water. In another, contaminated hands were again held under running tap water and the fingers and palms rubbed together. In the final protocol soap and friction were used under running water. The number of contaminant E. coli transferred by touch contact to food or a surrogate item representing skin was quantified, before washing and after washing for intervals up to 20 s. Decontamination profiles were determined for each protocol. When hands were washed under running water with friction over a period of 20 s, the number of E. coli contaminating food and the skin surrogate was progressively reduced respectively to 0.18% and 0.34% of the baseline level. Running water alone was comparatively ineffective. The addition of soap showed a modest benefit. We conclude that in situations where hands are not visibly soiled, a purposeful hand wash under running water for 20 s, with friction, will deliver an effective outcome that can be improved marginally by the addition of soap.
Introduction
Infection control units consider hand hygiene, and in particular hand washing, to be the principal means of managing the transmission of infectious diseases by touch contact. Historically, the first recorded successful intervention achieved by hand washing or, more accurately, hand disinfection, was that of I. P. Semmelweis.1 Other practitioners followed his example and today the reality of the spread of infectious diseases by touch transfer is appreciated by health professionals across the spectrum of hospital infection control, food-borne diseases, childcare centre health and the containment of infection in the community.
While there is an expansive literature on hand hygiene, the majority of studies have reported on the effects of sanitation and disinfection on decontamination. Surprisingly little attention has been paid to hand washing per se and, as a result, guidelines and protocols have relied on expert opinion and intuition.2,3 Wendt2 concluded that the provision of meaningful guidelines for hand decontamination was handicapped by a paucity of data on the effectiveness of various protocols and agents under ‘in use’ conditions. Smith,4 in a recent review of hand-washing techniques, commented on the sparsity of evidence to support guidelines and the urgent need to undertake methodologically sound studies of hand-washing variables.
There is clearly a need for data that would allow informed recommendations to be made with regard to hand-hygiene protocols. The aim of this study was to evaluate the influence of time spent hand washing, hand-to-hand friction and the inclusion of plain soap on hand decontamination under running tap water. A novel method involving touch contact with a food item or surrogate skin surface was used to quantify the effect of the three variables on hand decontamination profiles.
Methods
Participating subjects
Five male and six female staff members, from within the University of Auckland and Auckland Hospital volunteered to participate in this study. The 11 subjects were drawn from clinical, technical and administrative staff. Healthy skin on forearms and hands were requirements for participation.
Ethical approval
A detailed description of the planned experiments was submitted to The University of Auckland’s Human Subjects Ethical Committee and ethical approval for the study was obtained. A detailed explanation of the purpose and nature of the study was supplied to all participants in the form of a participant information sheet. Participants were asked to give written informed consent.
Study design
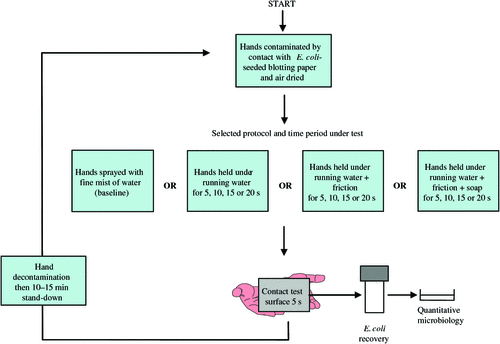
The study involved volunteers deliberately contaminating their hands by making contact with a surface impregnated with a saline suspension of a strain of Escherichia coli isolated from the subjects’ own bowel microbial flora. The experiments studied the effect of three hand-washing variables on hand decontamination. Each protocol was treated as an individual experiment that included a baseline level of hand contamination and data from samples taken after washing for 5, 10, 15 and 20 s. Prior to, and on the completion of each protocol, the subjects handled one of two surrogate surfaces representing food and skin. The numbers of contaminants translocating to the surfaces by touch contact were quantified, after elution, and the plating of aliquots of the eluate onto MacConkey agar. Bacterial numbers were counted after 24-h incubation and reported as colony forming units (cfu). At the end of each experiment the subject’s hands were decontaminated and a ‘stand down’ period of 15 min observed before any further experimentation was undertaken. Sampling involved three protocols, five time points including a baseline sampling, and two surrogate surfaces. In total, 330 samples were analysed (Fig. 1).
|
Hand contamination with E. coli
Study participants provided the investigators with a specimen of their stool on an applicator stick held in a sealed container. The stool was suspended in saline and an aliquot cultured on E. coli-selective MacConkey agar plates and incubated at 37°C for 24 h. A single lactose fermenting colony was selected and dilution streaked on MacConkey agar to ensure the presence of single colonies. After overnight incubation an individual colony was selected and transferred to horse blood agar for overnight growth and identified using an Analytical Profile Index (API) strip (bioMerieux, Marcy l’Etoile, France). These preparations were stored at 4°C and became the stock cultures for each study participant.
On the day before the test, single colonies of E. coli from the participating subject were obtained following subculture of the individual’s stock culture on to horse blood agar. An individual colony was selected and used to inoculate 9 mL of tryptone soya broth. Four such cultures were prepared. After overnight incubation at 37°C the broth cultures were centrifuged to deposit the microbial cells and the supernatant was discarded. The microbial cells were resuspended with 9 mL of sterile saline solution. The process was repeated twice. The four 9 mL washed broth cultures of E. coli were then added to 60 mL of sterile saline and mixed, using a flask shaker, to ensure a uniform bacterial suspension. A 6 mL aliquot of the bacterial suspension containing E. coli was then added to a 25 × 6 cm section of blotting paper placed in a shallow sterile plastic tray. The fingers and thumbs of both hands of participants were placed in firm contact with the contaminated blotting paper and held there for 10 s. The hands were then air-dried by swinging the arms with fingers extended backwards and forwards away from the body for 2 min. The hand contamination was carried out within a purpose-built containment unit fitted with a protective visor to prevent splashback. The unit was decontaminated after each use.
Hand-washing variables
Three variables associated with hand washing were assessed (Fig. 1).
-
Running water (RW). The subjects’ hands were held in a flow of running tap water with no hand-to-hand friction for periods of 5, 10, 15 and 20 s.
-
Running tap water plus hand-to hand friction (RW+F). The procedure was as described above, but in addition participants were instructed to wash the palms and backs of their hands and to focus on the fingers and interdigital surfaces. Hand-to-hand friction required firm contact between the palms and fingers combined with an energetic rubbing action. Participants were observed closely to ensure compliance.
-
Running water plus friction and soap (RW+F+S). Running water plus friction as above, but including the use of plain non-germicidal liquid soap. In this case the participant’s hands were lightly sprayed with tap water in a plastic container with a spray head nozzle, before 0.25 mL of liquid soap was squirted on to the hands from a dispenser. The soaped hands were then rubbed together, using the same technique as above. For the 5 s time point the soaped hands were placed immediately under running water. For the other time increments (10, 15 and 20 s) the hands were lathered for 5 s and then washing continued under the running water for the remainder of the time interval under study.
The flow of water from a standard surau neck water faucet (swan-neck, twin-lever faucet) was set to 7 L per minute and the temperature maintained at between 39°C and 41°C. The tap water used for the hand washing in all these experiments met New Zealand Ministry of Health drinking water standards and, according to company records and our own regular analyses (unpubl. data), it contained no demonstrable coliforms or other aerobic microorganisms over the period of the study.
Surrogate surfaces
Two items representing skin and food were used to determine the number of E. coli translocating on touch contact. Pieces of soft synthetic chamois, 2.5 × 2.5 cm, were washed and dried before being gas-sterilised. A strip of Black Knight Licorice (Nestle NZ Ltd, Auckland, NZ) was cut in 2.5 × 2.5 cm pieces and gas-sterilised with ethylene oxide.
Touch transfer quantification
The effect of hand-washing variables on decontamination was quantified by determining the number of E. coli translocating to the two surrogate surfaces before, and on the completion of, each hand-washing procedure. A baseline translocation level was determined for each protocol and time point. To achieve this, subjects’ hands were contaminated as previously described. After air drying at ambient temperatures for 2 min the hands were sprayed with a fine mist of water to ensure maximum transfer of contaminants.17 Samples of one of the two surrogate surfaces were picked up in each hand and the item fingered firmly for 5 s before being transferred to sterile 20 mL glass universal containers. The number of microorganisms transferred provided a baseline against which the results of the decontamination protocols were compared. After completing each protocol, subjects ‘flicked’ their hands over a basin to remove surplus water before also fingering a surrogate surface firmly for 5 s. For quantitative analysis, 9 mL of saline was added to each 20 mL glass universal container which were then vortexed for 10 s to elute contaminants. Microbiological analysis was carried out by making 10-fold dilutions of the primary sample in sterile saline. One mL aliquots of each dilution were incorporated into MacConkey agar pour plates and cfu from plates containing between 30 and 300 cfu were counted after 24 h incubation at 37°C (Fig. 1).
Hand decontamination
Subjects’ hands were decontaminated on the completion of each experimental protocol, including baseline, using a sterile, antiseptic-free, surgical scrub brush under running water for 2 min followed by thorough drying with several paper towels. Testing was conducted to ensure the removal of all E. coli contaminants.
Statistics
The effect of each hand-washing protocol on contaminant translocation to food and skin substrates has been presented as the mean and standard error of the number of cfu of E. coli translocating at each sample point. Eleven individuals participated at all time points. ‘Percentage of baseline’ was calculated by expressing the number of E. coli translocating at a given time point as a percentage of the baseline figure, that is prewash, for the protocol being tested.
Results
Effect of the time spent washing, friction and soap on decontamination rates
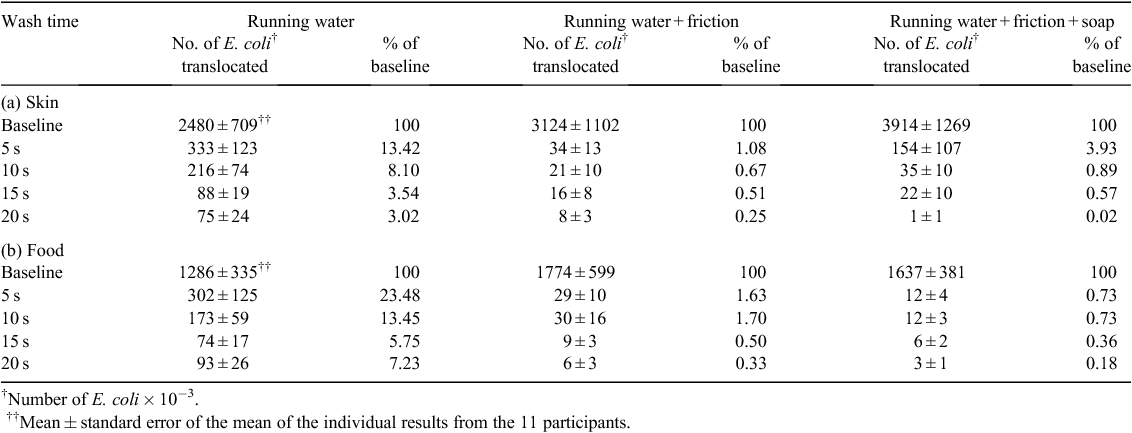
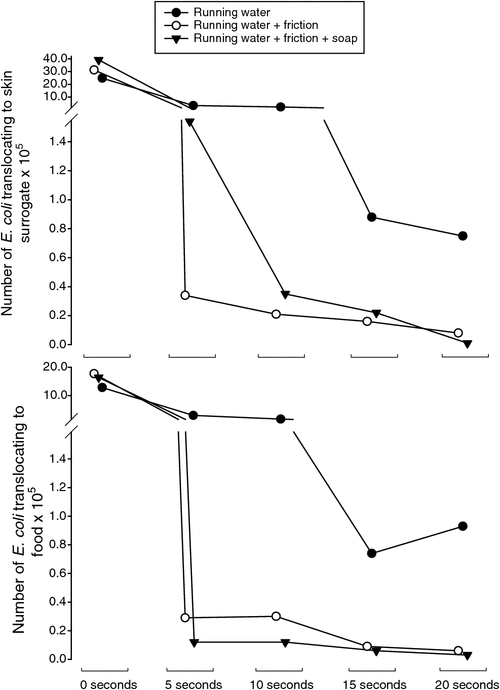
The time spent washing was an important determinant of the effectiveness of the three protocols. A substantial level of decontamination was achieved when a wash time of 10 s was used with running water plus friction and running water plus friction and soap. Extending the wash time to 20 s markedly improved the effectiveness of all three protocols. Touch contact transfer of E. coli contaminants to skin-surrogate was reduced to 3.02%, 0.25% and 0.02% of baseline levels for running water, running water plus friction and running water plus friction and soap, respectively and to 7.23%, 0.33% and 0.18% in the case of food (Table 1, Fig. 2).
|
|
The addition of hand-to-hand friction markedly improved the level of decontamination. Passively holding the hands under running tap water was a comparatively ineffective process with 3.02% and 7.23% of the baseline contaminants translocating to skin and food, respectively after 20 s. When the hands were purposefully rubbed together under running water, contaminants remaining were reduced to 0.25% and 0.33% of baseline, respectively.
The addition of soap initially delayed the decontamination process when skin-surrogate was used as the contact surface, but conferred a modest benefit at the 20 s sampling time point. In the case of food, decontamination was marginally improved at all time points by the addition of soap.
Discussion
In view of the considerable literature on hand-washing practices, one might question the need for a further investigation. Smith4 has recently drawn attention to the alarming lack of robust evidence to support current hand-washing protocols. The reality, which has been commented on by experienced researchers, is that few studies have addressed the effects of common variables affecting hand-washing efficacy and outcomes.2,3,5 In the current investigation the effects of three such variables on hand decontamination were assessed. Of the variables evaluated, the time spent under running tap water and hand-to-hand friction during washing had the most influence on the decontamination profile of hands deliberately contaminated with E. coli. The numbers of E. coli translocating to a representative surface with touch contact were substantially reduced by a 5–10 s wash but a 20 s wash was required to reduce translocating contaminants to an acceptable level (0.2% to 0.3% of the baseline figure). Holding contaminated hands passively under running water was ineffective. However, the decontamination rate was markedly enhanced when friction, that is energetically rubbing the hands together while washing, was added to the protocol. The addition of soap led to a delay in decontamination during the initial 5–10 s of washing but this differential was not evident at the 15–20 s time points where marginal benefits were found.
Realism was added to the protocol through the use of a novel touch contact method, coupled with microbial recovery, to quantify the decontamination process. Both Wendt2 and McDonald6 have commented on the need for some methodological innovation that would allow the results of hand hygiene studies to align more closely with actual events. The concept of using touch-contact microbial transfer to surrogate surfaces to quantify the effect of a hand-hygiene protocol on decontamination goes some way to fulfilling this requirement, as it closely mimics everyday situations.
The degree to which friction during hand washing enhanced the decontamination profile was surprising. Hand hygiene guidelines suggest rubbing the hands together3 but there is no indication that the recommendations are evidence-based. The role of friction in hand hygiene clearly needs emphasising as the contribution of an ‘active’ hand washing technique to decontamination is not widely appreciated.
Assessment of the effect of soap on hand decontamination also produced seemingly unexpected results. However, on reflection the observation that decontamination was delayed by the inclusion of soap could have been expected. The explanation we favour is that soap acted as a lubricant and initially reduced the frictional component of the wash. This is supported by the observation that the decontamination rate was restored at a time when the soap would have been washed off the hands. Because the primary action of soap is to solubilise fats and oils and suspend macro-soiling, the contribution of soap is likely to be more evident when these substrates and their contaminants are present on the hands. In the current study the hands of participating subjects were contaminated with a saline suspension of E. coli where the use of soap would not have been expected to affect the decontamination profile. Indeed a criticism of the study could be that the contaminating microorganisms were presented in a saline suspension rather than an organic milieu. However, the choice was a considered one given that in most situations where hand hygiene is practiced, the hands would not be obviously ‘soiled’; that is, presenting with visible surface organic material. In an environment where overt soiling is common the use of soap would be mandatory.
Prevention of the spread of infectious disease by touch contact is the aim of health professionals and educationalists in several hand hygiene-sensitive areas. One hundred years after Semmelweiss initiated his interventions in an Austrian hospital, nosocomial infections are still recognised as a major clinical problem.7 Evidence for an association between incidents of hospital-acquired infection and the carriage of pathogens on the hands of healthcare workers has been steadily accumulating,8,9 as has confirmation that such infections are reduced when hand-washing guidelines are adhered to.10,11 The positive effect of hand washing in reducing the incidence of infection has also been reported in studies involving elementary school pupils,12,13 and the wider community.14 Nonetheless the limitations of hand washing as a decontamination procedure also need to be recognised as it is likely that some transient contaminants remain on the hands even when an approved protocol has been used. Evidence for the persistence of ‘cadaverous particles’ following hand washing led Semmelweiss to add chloride of lime to his hand hygiene protocol.1 Microbial persistence was also the basis of the effectiveness of the soap and water wash being questioned.15,16 The disclosure that moisture on the hands acts as a ‘microbial mobiliser’ relates directly to this issue and has added a new dimension to hand-hygiene principles.17 A recent community-based study demonstrated that microbial transfer following touch contact can be reduced by as much as 99.8% if the hands are carefully dried after washing.18 Emphasis now needs to be placed on the maxim that effective hand hygiene is a dual process and indeed as much attention should be paid to hand drying as to the decontamination wash.
Although alcoholic hand rubs continue to be promoted as an alternative to hand washing, their use is a matter of convenience rather than efficacy. Sickbert-Bennet et al.19 tested the comparative efficacy of hand-hygiene practices, including hand washing and alcoholic rubs, on the removal of Seratia marcesens and the non-enveloped MS2 virus from contaminated hands. Decontamination was best achieved with a non-medicated soap and water wash. In a similar study Grayson et al.20 compared the ability of a soap and water wash with an alcohol-based rub to decontaminate the hands of volunteers seeded with live H1N1 virus. Soap and water proved to be more effective than the alcoholic rub. While alcoholic rubs are becoming increasingly accepted in the clinical environment, their value outside this setting has yet to be established.
Conflict of interest
None reported.
Acknowledgement
The authors are grateful to ALSCO/NZTS and Auckland Regional Public Health Services for their financial contributions in support of this project.
References
[1] Newsom SW. Pioneers in infection control. Ignaz Philipp Semmelweis. J Hosp Infect 1993; 23 175–87.| Pioneers in infection control. Ignaz Philipp Semmelweis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3nsVSmsA%3D%3D&md5=7fb40d70dda89b0081ee42d988b15e34CAS | 8099092PubMed |
[2] Wendt C. Hand hygiene–comparison of international recommendations. J Hosp Infect 2001; 48(Suppl. A) S23–8.
| 11759020PubMed |
[3] Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control 1995; 23 251–69.
| APIC guideline for handwashing and hand antisepsis in health care settings.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28%2Fis1KgtA%3D%3D&md5=bcf8fe2384365e6ce13b00dd4e801afaCAS | 7503437PubMed |
[4] Smith SMS. A review of hand-washing techniques in primary care and community settings. J Clin Nurs 2009; 18 786–90.
| A review of hand-washing techniques in primary care and community settings.Crossref | GoogleScholarGoogle Scholar | 19239662PubMed |
[5] Boyce JM, Pittet D. Healthcare Infection Control Practices Advisory Committee. Society for Healthcare Epidemiology of America. Association for Professionals in Infection Control. Infectious Diseases Society of America. Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Cont Hosp Ep 2002; 23 S3–40.
[6] McDonald LC. Hand hygiene in the new millennium: drawing the distinction between efficacy and effectiveness. Infect Control Hosp Epidemiol 2003; 24 157–9.
| Hand hygiene in the new millennium: drawing the distinction between efficacy and effectiveness.Crossref | GoogleScholarGoogle Scholar | 12683504PubMed |
[7] Burke JP. Infection control – a problem for patient safety. N Engl J Med 2003; 348 651–6.
| Infection control – a problem for patient safety.Crossref | GoogleScholarGoogle Scholar | 12584377PubMed |
[8] Larson E. A causal link between handwashing and risk of infection? Examination of the evidence. Infect Control Hosp Epidemiol 1988; 9 28–36.
| A causal link between handwashing and risk of infection? Examination of the evidence.Crossref | GoogleScholarGoogle Scholar | 19722934PubMed |
[9] Pessoa-Silva CL, Dharan S, Hugonnet S, Touveneau S, Posfay-Barbe K, Pfister R, et al Dynamics of bacterial hand contamination during routine neonatal care. Infect Control Hosp Epidemiol 2004; 25 192–7.
| Dynamics of bacterial hand contamination during routine neonatal care.Crossref | GoogleScholarGoogle Scholar | 15061408PubMed |
[10] Larson EL, Early E, Cloonan P, Sugrue S, Parides M. An organizational climate intervention associated with increased handwashing and decreased nosocomial infections. Behav Med 2000; 26 14–22.
| An organizational climate intervention associated with increased handwashing and decreased nosocomial infections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FlsFaktw%3D%3D&md5=abb5452369caed784c0beb353a022293CAS | 10971880PubMed |
[11] Won SP, Chou HC, Hsieh WS, Chen CY, Huang SM, Tsou KI, et al Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 2004; 25 742–6.
| Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit.Crossref | GoogleScholarGoogle Scholar | 15484798PubMed |
[12] Nandrup-Bus I. Mandatory handwashing in elementary schools reduces absenteeism due to infectious illness among pupils: a pilot intervention study. Am J Infect Control 2009; 37 820–6.
| Mandatory handwashing in elementary schools reduces absenteeism due to infectious illness among pupils: a pilot intervention study.Crossref | GoogleScholarGoogle Scholar | 19850374PubMed |
[13] Pönkä A, Poussa T, Laosmaa M. The effect of enhanced hygiene practices on absences due to infectious diseases among children in day care centers in Helsinki. Infection 2004; 32 2–7.
| The effect of enhanced hygiene practices on absences due to infectious diseases among children in day care centers in Helsinki.Crossref | GoogleScholarGoogle Scholar | 15007735PubMed |
[14] Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer WL, Hoekstra RM. Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA 2004; 291 2547–54.
| Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkslWgtr8%3D&md5=c9c689ead9f53ba0022730fc5b7ebfbdCAS | 15173145PubMed |
[15] Ehrenkranz NJ. Bland soap handwash or hand antisepsis? The pressing need for clarity. Infect Control Hosp Epidemiol 1992; 13 299–301.
| Bland soap handwash or hand antisepsis? The pressing need for clarity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK383ns1Olsw%3D%3D&md5=d2a73fc104783aeefe4ccc3c2523fbdcCAS | 1593113PubMed |
[16] Kjølen H, Andersen BM. Handwashing and disinfection of heavily contaminated hands–effective or ineffective? J Hosp Infect 1992; 21 61–70.
| Handwashing and disinfection of heavily contaminated hands–effective or ineffective?Crossref | GoogleScholarGoogle Scholar | 1351497PubMed |
[17] Patrick DR, Findon G, Miller TE. Residual moisture determines the level of touch-contact-associated bacterial transfer following hand washing. Epidemiol Infect 1997; 119 319–25.
| Residual moisture determines the level of touch-contact-associated bacterial transfer following hand washing.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7gtFertw%3D%3D&md5=988462f6cb11a707822820898fb6f62eCAS | 9440435PubMed |
[18] Patrick D, Miller TE, Ormrod D. Reduction of microbial transmission in childcare using an improved hand drying protocol. Healthc Infect 2010; 15 15–9.
| Reduction of microbial transmission in childcare using an improved hand drying protocol.Crossref | GoogleScholarGoogle Scholar |
[19] Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Sobsey MD, Samsa GP, Rutala WA. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control 2005; 33 67–77.
| Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses.Crossref | GoogleScholarGoogle Scholar | 15761405PubMed |
[20] Grayson ML, Melvani S, Druce J, Barr IG, Ballard SA, Johnson PD, et al Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis 2009; 48 285–91.
| Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivVSlsb4%3D&md5=4ef398f40079060389d856df32a87f73CAS | 19115974PubMed |

