How quickly do hospital surfaces become contaminated after detergent cleaning?
Alexandra Bogusz A , Munro Stewart A , Jennifer Hunter B , Brigitte Yip A , Damien Reid A , Chris Robertson C D E and Stephanie J. Dancer B FA Department of Care of the Elderly, Hairmyres Hospital, NHS Lanarkshire, UK.
B Department of Microbiology, Hairmyres Hospital, NHS Lanarkshire G75 8RG, UK.
C Department of Mathematics and Statistics, University of Strathclyde, Glasgow, UK.
D Health Protection Scotland, Glasgow, UK.
E International Prevention Research Institute, Lyon, France.
F Corresponding author. Email: stephanie.dancer@lanarkshire.scot.nhs.uk
Healthcare Infection 18(1) 3-9 https://doi.org/10.1071/HI12063
Submitted: 25 December 2012 Accepted: 22 January 2013 Published: 27 February 2013
Journal Compilation © Australasian College for Infection Prevention and Control 2013
Abstract
Background: Hospital cleanliness is important for controlling infection. This study aimed to determine the effect of detergent-based cleaning on microbial load at near-patient sites on one ward over a 48 h period.
Methods: Lockers, left and right bedrails and overbed tables in 30 bed spaces were screened for aerobic colony counts (ACC) and staphylococci (methicillin-susceptible and methicillin-resistant Staphylococcus aureus: MSSA/MRSA) before detergent-based cleaning. Sites were rescreened at: 1, 2, 4, 8, 12, 24 and 48 h after cleaning. Microbial growth was quantified as number of ACC/cm2 and presence of MSSA/MRSA at each site. The study was repeated 3 times at monthly intervals.
Results: There was a significant reduction in average ACC (360 sites) from a pre-clean level of 6.72 ACC/cm2 to 3.46 ACC/cm2 at 4 hours after detergent-based cleaning (P < 0.0001). Average counts increased to 4.89 ACC/cm2 at 24 h and 5.27 ACC/cm2 at 48 h for all sites. Levels on bed rails and lockers, but not overbed tables, fell below a proposed standard (5 cfu/cm2) for 24 h after cleaning. MSSA/MRSA decreased 2–4 h after cleaning (P = 0.014) before increasing but failed to reach pre-clean levels.
Conclusion: Detergent cleaning reduces ACC at near-patient sites on a hospital ward. S. aureus (including MRSA) was not completely eliminated but showed a similar pattern of decrease. Microbial burden at high-risk sites beside the patient could potentially be controlled by daily cleaning with single-use detergent wipes.
Additional keywords: cleaning standards, detergents, environmental contamination, hospital-acquired infection, hospital cleaning, MRSA.
Implications
-
Hospital cleaning is the ‘Cinderella’ of infection control because difficulties with measuring its impact mean that there is little scientific evidence for it.
-
This paper presents the microbiological effect of using detergent wipes to clean patients’ beds and furniture on one 30-bedded ward.
-
Once-daily cleaning with detergent wipes appears to control the level of microbes at sites beside the patient, including S.aureus and MRSA.
Introduction
Previous work has shown that hospital pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) can persist in the healthcare environment for months.1 The most important reservoirs are hand-touch sites right beside the patient, especially the bedside locker, overbed table and bed frame.2–5 Higher levels of microbes on these surfaces are associated with increased risk of finding S. aureus and MRSA.5
Current UK cleaning regimens specify once-daily detergent-based cleaning for near-patient furniture and beds, usually delivered by auxiliary (or clinical support) nurses whilst the bed is occupied, and/or by domestic staff when the bed is free. It is possible that these items may not receive sufficient cleaning if the ward is busy.6 Even if they are cleaned regularly, they may be rapidly repopulated by air- or hand-borne organisms, some of which pose an infection risk to bed-bound patients. Contamination of near-patient sites is important because genotyping of MRSA, for example, has demonstrated indistinguishable strains isolated from both these sites and patients within varying time periods, with presumed transmission in both directions.7
We aimed to screen high-risk sites beside the patients on one ward in order to determine baseline microbial load. Study personnel then subjected the sites to a comprehensive detergent-based clean. Surfaces were rescreened at varying intervals after cleaning, first to gauge the immediate effect of the cleaning process, and then to monitor the rate of recontamination at these sites over time.
Materials and methods
One care-of-the-elderly assessment and rehabilitation ward in a 450 bed National Health Service (NHS) hospital was chosen as the study ward. The 30 bed ward runs at 100% bed occupancy and patients tend to stay for longer than patients on acute wards. There are six ensuite single rooms and four bays each containing six beds. Whilst patients of either sex can reside in the single rooms, three of the four bays accommodated female patients during the study.
Four sites (bedside locker, left bedrail, overbed table and right bedrail) were screened using previously validated microbiological methods for assessing surface level cleanliness.8 After screening, each site was cleaned using a fresh disposable detergent wipe. (Tuffie detergent wipes, Vernacare, Bolton, UK). These wipes contain a mixture of non-ionic constituents at neutral pH. Whilst cleaning bed-frame components posed few practical problems, gaining access to the bedside lockers and overbed tables was difficult due to the quantity of patient belongings. These had to be removed to enable comprehensive cleaning. They were then replaced but organised in such a way as to permit later placement of dipslides. The cleaning process was systematically repeated for each of the four sites within every bed space, starting from Bed 1 to Bed 30, and performed on three separate occasions over a time period of 3 months.
The quality of cleaning was standardised by preliminary training and assessment using microbiological methods. Cleaning was performed by the leader of the study on each occasion, assisted by one of a team of three physicians. Both screening and cleaning personnel wore freshly laundered overalls and washed and dried their hands before the study began; hands were also washed with soap and water and dried between screening and/or cleaning of each bed space. Disinfectant gels were not used by any of the study personnel during the course of the study.
Each site was rescreened at the following intervals after cleaning: 1 h, 2h, 4h, 8h, 12h, 24 h and 48 h. The pre-clean screen began at 8a.m. on a Saturday morning, followed by the cleaning intervention, with post-cleaning screening performed throughout Saturday, once at 8 a.m. on Sunday, and the final screen at 8a.m. on Monday morning. Normal ward care for patients continued throughout all 3 phases of the study, including routine cleaning delivered by domestic staff. No further cleaning of the sampled sites, usually cleaned by nurses, took place until after the 48 h screen other than attention to spillages. The protocol was discussed with the domestic supervisor and senior nurses on the ward in order to coordinate the study with routine cleaning practices. Ethical exemption was obtained from the NHS Lanarkshire Research & Development department.
Microbiology
Screening was performed using dipslides (Hygiena Int., Watford, UK), coated with nutrient and staphylococcal selective (Baird Parker) agars.5,7 After sampling each of the four sites around all 30 beds, dipslides were incubated for 48–72 h according to laboratory protocol. Placing slides at each site was performed in a pre-determined systematic fashion, so that slides did not sample areas previously screened.
Growth on nutrient agar supplied total aerobic colony counts (ACC) per cm2 which were classified within the following categories: no growth (NG); scanty growth (SG) <2.5 cfu/cm2; light growth (LG) 2.5–12 cfu/cm2; moderate growth (MG) 12–40 cfu/cm2; and heavy growth (>40 cfu/cm2) as previously defined.5,7 Selective agar highlighted potential coagulase-positive staphylococci, which were sub-cultured onto blood agar and identified as methicillin-susceptible or resistant according to standard laboratory protocol. Hygiene standards have been proposed whereby ACC >5 cfu/cm2 and/or presence of MSSA/MRSA at any hand-touch site suggests increased infection risk to patients.8,9
Statistical analyses
All data were subjected to statistical analyses. Each of the four sites around 30 beds at time t = 0, 1, 2, 4, 8, 12, 24 and 48 h supplied an ACC categorised as indicated, along with data for presence or not of MSSA/MRSA. Each study phase provided a series of results for 30 × 4 sites, ultimately giving data from 360 sites. We compared total mean ACC against time in order to investigate the recontamination rate after cleaning. MSSA and MRSA were also calculated and plotted over time. Data were analysed for side-rooms vs multiple patient bays.
This was an observational study and analysis of variance methods were used to assess the importance of the time from cleaning, site and phase on total ACC over the four sites near all 30 beds in the ward. The main investigation centred on modelling the trends in growth over time. A normal distribution for total growth was assumed and the assumptions of the model were validated through residual plots. Two-way interactions were tested, using F tests, at the pre-specified 1% significance level, as these were of secondary importance, while the main effects were tested at the 5% level. Poisson regression was employed for analysing numbers of MSSA and MRSA detected, with a chi-square deviance test used.
Results
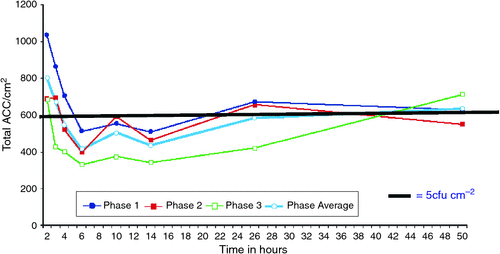
There was an overall reduction in ACC at all sites following the detergent-based clean (Table 1). Fig. 1 shows average and total ACC (cfu/cm2) for 120 sites from each study phase. The decline began gradually for phases 1 and 3, but was delayed by an hour for phase 2, possibly because sites were still moist after cleaning and permitted capture of more environmental microbes. The greatest reduction in counts occurred during the period 4–12 h after cleaning, with an average reduction, compared with 1 h before cleaning, of 97.7 (SE 13.3) ACC/cm2 at 4 h and 91.4 (SE 13.3) ACC/cm2 at 12 h. Total counts for phase 2 were lower than for phase 1 by an average of 30.2 (SE 8.71) ACC/cm2, and phase 3 counts were lower than phase 1 by an average of 65.6 (SE 8.71) ACC/cm2. This successive decline may have been due to a Hawthorne effect caused by better cleaning from nurses following the first phase.10
|
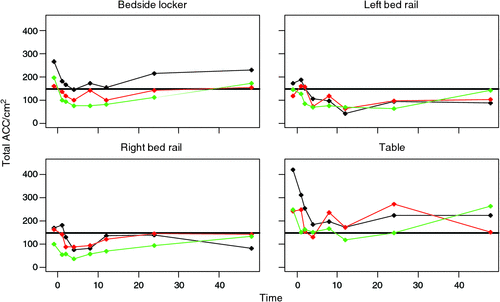
The reduction in average ACC occurred for all four sites following cleaning (Table 1; Fig. 2a–d). There was an unexpected resurgence of microbial growth at 8 h, particularly from overbed tables, lockers and left bedrails, which then declined again at 12 h (also seen in Fig. 1). Microbial recovery was higher from tables than lockers by 62.9 (SE 9.4) ACC/cm2 and lower from the bedrails. Analysis of variance showed that there were significant interactions between phase, site and phase by time period and a clear outlier in phase 1 before cleaning (Table 1). These interactions are caused by the different patterns in microbial recovery at baseline and 48 h as seen by crossing of the lines in Fig. 2 (a–d). Omitting these two times from the analysis simplifies the statistical models and leaves a model with no significant interactions (Phase by Site, P = 0.056; Phase by Time, P = 0.044; Time by Site, P = 0.013). There are significant differences among the phases, times and sites (all P < 0.0001).
|
Both Figures show a superimposed cleanliness standard (5 cfu/cm2). Counts rarely go below this limit from the overbed tables and lockers in phase 1 (Fig. 2). For the other sites and before cleaning, ACC initially exceeded this level for all three phases, falling beneath within 1 h (phase 3); before 2 h (phase 2); and before 3h (phase 1). Counts from all three phases remained beneath this level until 18 h (phase 1); 20 h (phase 2); and 40 h (phase 3) post-cleaning. When the ward was ‘cleaner’ initially, there was a longer period before microbial contamination reached the designated standard.
The pattern of reduction of viable MSSA/MRSA was similar to that seen for ACC (Table 2). There were differences in MSSA/MRSA over the phases, P < 0.0001, with 76.9% (95% CI 44.9, 89.4) fewer MRSA/MSSA found in phases 2 and 3 compared with phase 1. MSSA/MRSA decreased from a pre-cleaning high to a low level at 2–4 h before increasing (P = 0.014), but did not regain pre-clean levels. There were differences in MRSA/MSSA contamination between the four sites (P = 0.016). The site most likely to be contaminated with MSSA/MRSA was the overbed table (17 of 44 isolates), followed by the bedside locker (15 isolates). Both bedrail sites together provided 12 of 44 isolates.
The data were examined for differences in ACC and MSSA/MRSA recovery between sites in six single rooms as opposed to those in the four six-bedded bays (Tables 1 and 2). The side-rooms had proportionately slightly less MSSA/MRSA (6 isolates: 1 MSSA; 5 MRSA) than sites in multiple-patient bays (37 isolates; Table 2). In contrast, side-room sites yielded proportionately greater total ACC compared with multiple-patient bay sites (56–70% of total ACC) (Table 1). It is possible that isolation may protect patients from MRSA, but it is also possible that side-rooms do not receive the cleaning attention delivered to sites in multiple-bedded bays.
During the 3 months in which this project took place, there were no outbreaks of hospital-acquired infection nor patient clusters of either MSSA or MRSA in the study ward. No patients were known to be colonised or infected with MRSA during the three 48 h periods in which screening took place. Prevalence of MSSA and MRSA among patients on this ward was low throughout the study period.
Discussion
This study sought first, to demonstrate the usual levels of microbial burden at high-risk near-patient sites; and second, to determine the effect of a comprehensive detergent-based clean on these levels. The final objective was to ascertain how quickly levels accumulate after this type of cleaning. The results have provided data that could be used to plan cleaning specifications in hospitals. Detergent cleaning reduced ACC at near-patient sites on a ward to a level below that of a proposed standard (5 cfu/cm2).8 The average ACC then remained below this standard for 24 h before exceeding it at 48 h. If the 5 cfu/cm2 standard is accepted as a benchmark for infection risk, then near-patient sites should be cleaned daily in order to keep microbial contamination beneath this standard. It has already been established that higher ACC are associated with increased risk of finding MSSA and MRSA.5
There are some limitations to this study. Cleaning two of the sites was not as straightforward as it should have been, owing to the large amount of patient belongings and foodstuffs on the bedside locker and overbed table. These all had to be removed before cleaning could take place. This took time, and in one instance (phase 2), the post-clean screening team caught up with the cleaning team, which may explain the delayed reduction of counts already mentioned at 1 h after cleaning. Difficulties in cleaning the overbed table are also reflected by the results, as higher ACC were consistently recovered from this item (Fig. 2). Whilst we would recommend daily cleaning for the other sites as a routine practice, the table probably requires cleaning on a more frequent basis, preferably after every meal.
The objectives of this study were solely to examine the effect of detergent cleaning on normal environmental flora on a hospital ward. We did not attempt to identify specific pathogens (e.g. Clostridium difficile) other than coagulase-positive staphylococci, nor model environmental data against clinical outcome. Previous studies have tried to address this but further work is required to ultimately identify microbiological standards for healthcare environments that accurately indicate clinical risk.7–9
Attaway et al. investigated microbial bioburden at near-patient sites before and after cleaning.11 There are some important differences between this study and the one reported here. These authors screened at half-hour intervals up to a total period of 7 h after cleaning, used two different disinfectants and not detergent, six intensive therapy unit (ITU) beds were screened on six occasions, the chosen site was bedrails only, and microbial growth was quantified as cfu/100 cm2 whereas we used ACC/cm2. Mean bacterial concentration on bedrails (n = 36) before cleaning was 4756 cfu/100 cm2; in our study, mean microbial concentration on bedrails, locker and table (n = 360) was 6.72 ACC/cm2. This may be explained by different sampling methods, since bioburden recovery would be expected to vary according to the sensitivity of the method used. In addition, the bedrails in ITU were habitually exposed to disinfectants whereas our sites were cleaned with detergent only. Routine cleaning methods may impact on pre-existing environmental bioburden in ways that we do not yet fully understand.
The Attaway study found that pre-cleaning levels of bacteria were reached at 3 h after disinfection.11 Another ITU cleaning study, also using disinfectants, found MRSA recontamination at hand-touch sites from 1–7 h after cleaning.12 There is a suspicion of resurgence at 2.5 h in the Attaway study, followed by a decline at 4.5 h. Our study showed resurgence of growth at 8 h, followed by a decline at 12 h. This resurgence, which was seen for every study phase, may have been a microbiological phenomenon representing damage inflicted on environmental microbes from physical impact of vigorous cleaning. Damaged organisms, whilst uncultivable at 4 h, may have regained viability at 8 h. Another explanation is a temporary inhibitory effect by one or more components in the detergent wipes. These fluctuations reflect expected patterns of microbial flora re-establishing itself after removal, although different patterns occur at varying times after cleaning depending upon which cleaning agents and methods are used.
This study illustrates the impact on microbial burden following one-step detergent-based cleaning. There have been several other papers recently that suggest that physical removal of bioburden is an important feature of the cleaning process.13–17 Physical removal may be just as effective as using disinfectants for controlling environmental microbes.14–17 This is partially, but not fully, explained by the fact that the microbiocidal activity of a disinfectant is inversely proportional to the degree of organic soiling of a surface.18 More work is required to clarify this, because aside from cost issues, detergents are less toxic to both the environment and staff and less likely to encourage spread of tolerant or resistant hospital-associated strains.18
In conclusion, detergent-based cleaning appears to offer effective physical removal of bioburden without the expense and toxicity associated with disinfectants. Hand-touch sites around a patient’s bed in acute wards should be systematically cleaned once a day, since the time period before contamination exceeds the proposed cleanliness standard is ~24 h. Overbed tables require greater frequency of cleaning. Comprehensive daily cleaning of hand-touch sites around the patients’ beds would also help maintain low levels of MSSA/MRSA.
Conflicts of interest
None reported by any of the authors.
Funding
Laboratory consumables (dipslides) were purchased using a research grant originally received from UNISON, the UK healthcare workers’ union.
Acknowledgements
We wish to acknowledge all Ward 16 staff and microbiology laboratory staff at Hairmyres hospital for their support, interest and assistance.
References
[1] Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009; 73 378–85.| The role of environmental cleaning in the control of hospital-acquired infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MjmsFOqsQ%3D%3D&md5=89acbc17a0be9228b386dd194d448998CAS |
[2] Bhalla A, Pultz NJ, Gries DM, Ray AJ, Eckstein EC, Aron DC, et al Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalised patients. Infect Control Hosp Epidemiol 2004; 25 164–7.
| Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalised patients.Crossref | GoogleScholarGoogle Scholar |
[3] Lloyd-Hughes R, Talbot S, Jumaa P. Bedside Bibles, notes trolleys and other forgotten sites for cleaning. J Hosp Infect 2008; 69 200–1.
| Bedside Bibles, notes trolleys and other forgotten sites for cleaning.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1czjvVWrsQ%3D%3D&md5=ff9fc7bdde07020ac37299f966c13b2dCAS |
[4] Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. A quantitative approach to defining ‘‘High-Touch’’ surfaces in hospitals. Infect Control Hosp Epidemiol 2010; 31 850–3.
| A quantitative approach to defining ‘‘High-Touch’’ surfaces in hospitals.Crossref | GoogleScholarGoogle Scholar |
[5] Dancer SJ, White L, Robertson C. Monitoring environmental cleanliness on two surgical wards. Int J Environ Health Res 2008; 18 357–64.
[6] Eckmanns T, Rath A, Ruden H, Gastmeier P, Daschner F. Outbreak of Enterobacter cloacae related to understaffing, overcrowding, and poor hygiene practices. Infect Control Hosp Epidemiol 2000; 21 305–8.
| Outbreak of Enterobacter cloacae related to understaffing, overcrowding, and poor hygiene practices.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FgsVyntg%3D%3D&md5=3be13f0e285d1c240c64d79bfd6e1401CAS |
[7] Dancer SJ, White LF, Lamb J, Girvan EK, Robertson C. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC Med 2009; 7 28
| Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study.Crossref | GoogleScholarGoogle Scholar |
[8] Dancer SJ. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hosp Infect 2004; 56 10–5.
| How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c%2FhtVWjtw%3D%3D&md5=a54f2cdbae6c78d857515c2c71b59780CAS |
[9] White L, Dancer SJ, Robertson C, MacDonald J. Are hygiene standards useful in assessing infection risk? Am J Infect Control 2008; 36 381–4.
| Are hygiene standards useful in assessing infection risk?Crossref | GoogleScholarGoogle Scholar |
[10] Hayden MK, Bonten MJ, Blom DW, Lyle EA, van de Vijver DA, Weinstein RA. Reduction in acquisition of vancomycin-resistant Enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006; 42 1552–60.
| Reduction in acquisition of vancomycin-resistant Enterococcus after enforcement of routine environmental cleaning measures.Crossref | GoogleScholarGoogle Scholar |
[11] Attaway HH, Fairey S, Steed LL, Salgado CD, Michels HT, Schmidt MG. Intrinsic bacterial burden associated with intensive care unit hospital beds: Effects of disinfection on population recovery and mitigation of potential infection risk. Am J Infect Control 2012; 40 907–12.
| Intrinsic bacterial burden associated with intensive care unit hospital beds: Effects of disinfection on population recovery and mitigation of potential infection risk.Crossref | GoogleScholarGoogle Scholar |
[12] Aldeyab MA, McElnay J, Elshibly SM, Hughes CM, McDowell DA, McMahon MA, et al Evaluation of the efficacy of a conventional cleaning regimen in removing methicillin-resistant Staphylococcus aureus from contaminated surfaces in an intensive care unit. Infect Control Hosp Epidemiol 2009; 30 304–6.
| Evaluation of the efficacy of a conventional cleaning regimen in removing methicillin-resistant Staphylococcus aureus from contaminated surfaces in an intensive care unit.Crossref | GoogleScholarGoogle Scholar |
[13] Schmidt MG, Anderson T, Attaway HH, Fairey S, Kennedy C, Salgado CD. Patient environment microbial burden reduction: a pilot study comparison of 2 terminal cleaning methods. Am J Infect Control 2012; 40 559–61.
| Patient environment microbial burden reduction: a pilot study comparison of 2 terminal cleaning methods.Crossref | GoogleScholarGoogle Scholar |
[14] Gillespie EE, Scott C, Wilson J, Stuart R. Pilot study to measure cleaning effectiveness in healthcare. Am J Infect Control 2012; 40 477–8.
| Pilot study to measure cleaning effectiveness in healthcare.Crossref | GoogleScholarGoogle Scholar |
[15] Gillespie EE, Wilson J, Lovegrove A, Scott C, Abernethy M, Kotsanas D, et al Environment cleaning without chemicals in clinical settings. Am J Infect Control 2013; in press.
| Environment cleaning without chemicals in clinical settings.Crossref | GoogleScholarGoogle Scholar |
[16] Berendt AE, Turnbull L, Spady D, Rennie R, Forgie SE. Three swipes and you’re out: How many swipes are needed to decontaminate plastic with disposable wipes? Am J Infect Control 2011; 39 442–3.
| Three swipes and you’re out: How many swipes are needed to decontaminate plastic with disposable wipes?Crossref | GoogleScholarGoogle Scholar |
[17] Rutala WA, Gergen MF, Weber DJ. Efficacy of different cleaning and disinfection methods against Clostridium difficile spores: Importance of physical removal versus sporicidal inactivation. Infect Control Hosp Epidemiol 2012; 33 1255–8.
| Efficacy of different cleaning and disinfection methods against Clostridium difficile spores: Importance of physical removal versus sporicidal inactivation.Crossref | GoogleScholarGoogle Scholar |
[18] Sattar S. Promises and pitfalls of recent advances in chemical means of preventing the spread of nosocomial infections by environmental surfaces. Am J Infect Control 2010; 38 S34–40.
| Promises and pitfalls of recent advances in chemical means of preventing the spread of nosocomial infections by environmental surfaces.Crossref | GoogleScholarGoogle Scholar |



