Frequency of use and activation of safety-engineered sharps devices: a sharps container audit in five Australian capital cities
Terry GrimmondGrimmond and Associates, Hamilton 3216, New Zealand. Email: terry@terrygrimmond.com
Healthcare Infection 19(3) 95-100 https://doi.org/10.1071/HI14009
Submitted: 10 March 2014 Accepted: 9 May 2014 Published: 10 June 2014
Journal Compilation © Australasian College for Infection Prevention and Control 2014
Abstract
Introduction: Sharps injuries (SI) among healthcare personnel (HCP) in Australia are of such concern the matter was brought before Parliament in 2013. Many SI from safety-engineered devices (SED) are due to non-activation. Monitoring of activation is recommended. This paper outlines a sharps container (SC) contents audit conducted in Australian capital cities.
Methods: Reusable, 22 L SC (Sharpsmart, Daniels Corporation, Melbourne) were randomly selected from random healthcare facilities (HCF) in five cities. Wearing protective apparel, the operator opened and decanted SC and sorted hollow-bore needles (HBN) into: capped v. uncapped non-SED, and activated or non-fully activated SED. Volumes and weights were recorded for inter-study comparisons. WinPepi v2.78 was used to calculate probability (significance set at ≤ 0.05), relative-risk and 95% confidence limits.
Results: 1212 L of sharps (167.9 kg) from 102 SC from 27 hospitals were audited. Many devices were blood-contaminated. Of the 9651 HBN, 30.4% were SED and 19.4% of the SED were not, or partially, activated. Of the 6718 non-SED, 30.6% were capped needles or capped needle-syringes. City averages for capped or naked sharps ranged from 64.2% (Sydney) to 97.8% (Adelaide) while hospital averages ranged from 32.6 to 100%. Overall, 54.2% of devices were discarded ‘sharp’.
Conclusions: It is disturbing that 75.5% of hollow-bore needles were capped or naked, indicating a high proportion of Australian HCP are unnecessarily at risk of SI while handling sharps. The high non-use of SED and non-activation of SED needs researching. Widespread SED evaluation and adoption (automatic and semi-automatic SED where feasible), repetitive competency training and safety-ownership are needed. Legislation may be indicated.
Implications
-
Sharps injuries among Australian hospital personnel are unacceptably and inexplicably high when compared internationally.
-
This national audit of disposed sharps found only 30% were safety-engineered devices of which 20% were not activated.
-
The findings offer some explanation as to the high injury-incidence and support urgent change, including possible legislation.
Introduction
A recent USA study of 125 hospitals found the incidence of sharps injuries among healthcare professionals (HCP) was 1.9 per 100 full time equivalent staff (FTE).1 There are no Australian national databases from which the total number of SI among Australian HCP can be calculated, however a recent estimate placed the figure at 19 355 reported SI per year.2 With 329 000 HCP employed in Australian hospitals,3 the incidence of reported SI among hospital staff is thus estimated at 5.9 per 100 FTE. This higher incidence over that of the U.S is of great concern2 and was brought to the Australian Parliament’s attention in July 2013.4
Sharps injuries significantly decrease with the use of safety-engineered sharps devices (SED).5 However, with increasing use of SED, the proportion of SI sustained from SED (over non-SED) increases6 and the majority of ‘after-use’ SI are due to non- or incomplete activation of SED.6 From an early time, the activation of SED has been recognised as a key component in SED efficacy, and monitoring of activation rates is recommended.7 Monitoring of SED activation rates can only effectively be achieved by decanting SC contents and counting activated and non-activated devices under controlled environments.8 No published studies of Australian SC contents audits were found in the literature and this paper presents the first national study of SC contents.
Methods
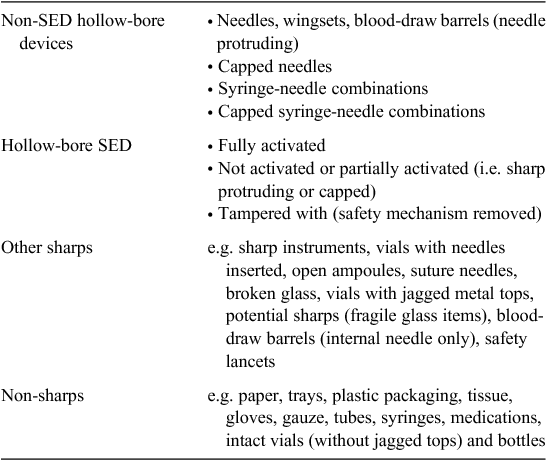
The study was conducted between November 2012 and July 2013. An area was set aside at a regulated medical waste factory in each city and patient-room, 22 L reusable sharps containers (Sharpsmart, Daniels Corporation, Melbourne Australia) were randomly selected from large transporters arriving from randomly selected large healthcare facilities (HCF) in Sydney, Melbourne, Perth, Brisbane and Adelaide. The names of HCF were unknown to the operator. Depending on factory deliveries, between 3 and 10 HCF were sampled from each city. Between 3 and 6 SC were chosen from each facility. Sharps containers were confined to the 22 L size commonly used for patient rooms so as to exclude laboratory and operating room sharps waste. Eye and face protection, long-sleeve gown, covered leather shoes and heavy-duty gloves were worn and tongs were used, and each SC was opened and the contents gently decanted onto a large plastic-lined bench. The contents of each SC were sorted item-by-item into the categories depicted in Table 1. Only hollow-bore needle (HBN) devices were counted as these have the greatest risk of bloodborne pathogen (BBP) transmission6 and are more likely to have SED available than ‘other sharps’ such as ampoules, broken glass, etc. Safety-engineered devices connected by tubing to a non-SED HBN were classified as ‘non-SED’ devices. Volume and weight of sharps sampled were recorded for reader comparison with other published audits. All categories were weighed to nearest gram on electronic kitchen scales and the volume of each category recorded. Upon completion of the audit, all sharps waste was returned to the factory system for destruction and disposal. WinPepi v2.78 (JH Abramson, Hebrew University, Jerusalem, Israel) was used to calculate probability (significance set at ≤0.05), relative-risk and 95% confidence limits.
|
Results
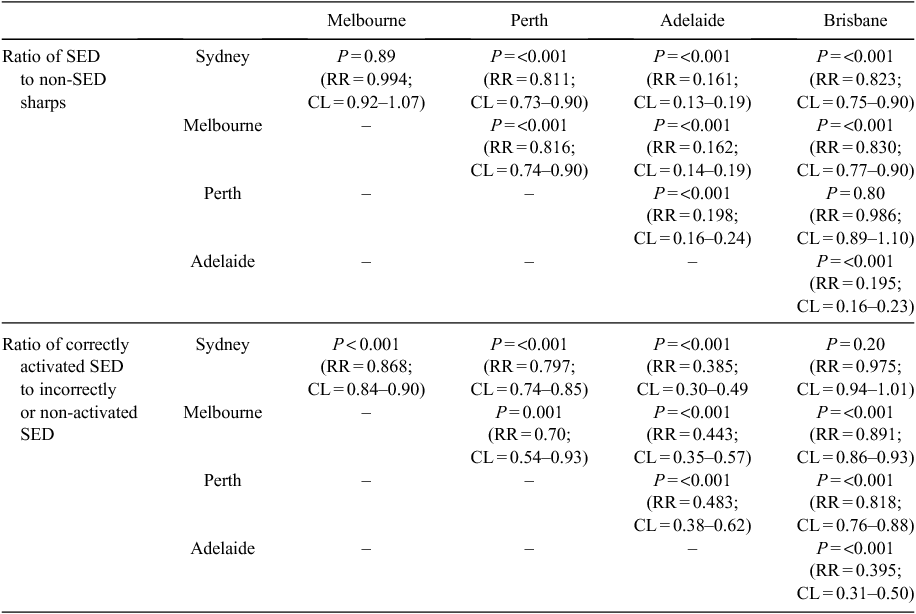
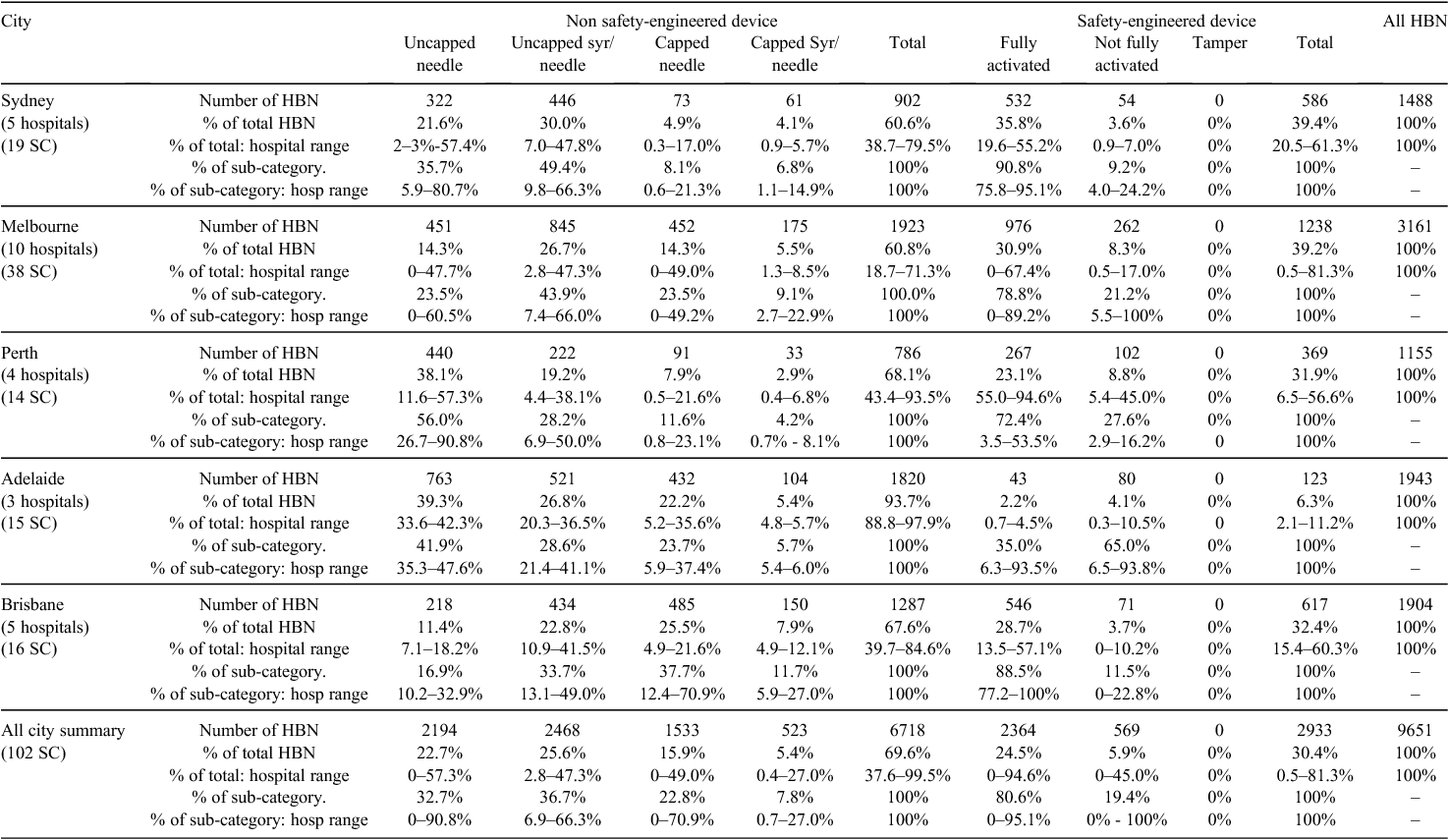
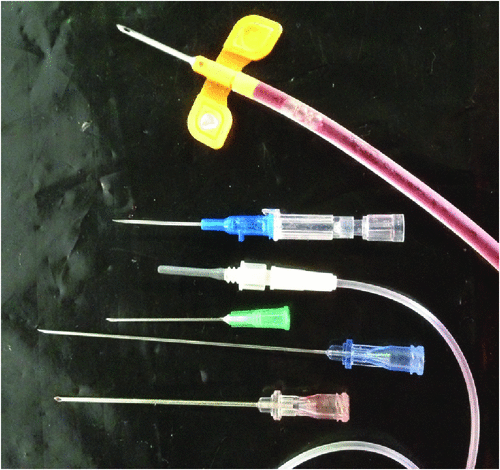
A total of 102 sharps containers were sampled from 27 hospitals in five Australian capital cities. A total volume of 1212 L of sharps (167.9 kg) was audited and 9651 HBN categorised and enumerated. Data on device numbers and percentages within major categories and their ranges are shown in Table 2. No SED had evidence of tampering or removal of the safety mechanism. Examples of non-activated SED are shown in Fig. 1 and examples of non-SED are shown in Fig. 2. Statistically significant differences were found between the 5 capital cities in both the ratio of SED to non-SED and the percentage of SED correctly activated (Table 3).
|
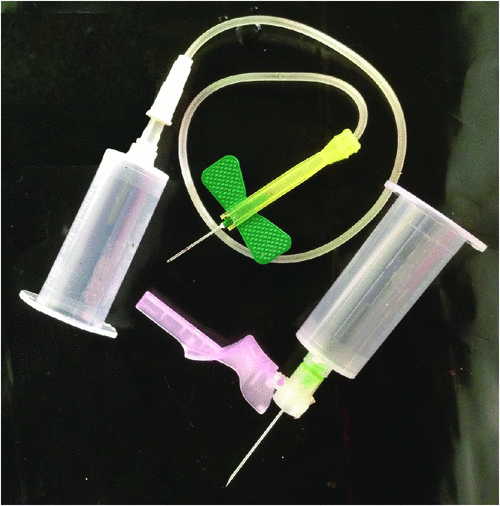
|
|
Discussion
In this study, 70% of HBN were non-SED and 30% were SED. Among the 27 HCF the proportion of HBN being SED varied from 0.5% to 81.3% and city averages varied from 6.3% to 39.4% (Table 2). Of the SED discarded, the percent correctly activated among the 27 HCF ranged from 0% to 100% and city averages ranged from 35.0% to 90.8% (Table 2). The level of non-SED use is higher than that found in a recent USA sampling9 and is surprising given that commercial SED are available for most sharps procedures in Australia. Several sharp procedures do not have SED available, for example, some biopsies, but of the 6718 non-SED, few were biopsy needles. In a 2013 unpublished survey conducted by the author among 200 USA occupational health managers, more than 80% of all sharps procedures are conducted with SED – considerably higher than the level of SED use found in this study.
Of the non-SED present, 31% were capped but whether these were recapped or unused could not be determined; however, Stringer found persistent recapping was also an issue in Canada.10 Overall, 54% of HBN were discarded as a ‘naked’ sharp. Included in the latter category (as well as non-activated SED) were numerous non-SED needles, syringe-needle combinations, non-SED phlebotomy blood-draw barrels, non-SED wingsets, and non-SED dialysis fistula. Recapping is disallowed in Australian guidelines and uncapped syringe-needles must be discarded ‘as is’.11 However, it is alarming that 22.7% of discarded HBN were uncapped needles, as it indicates these uncapped needles may have been removed from syringes – possibly manually – a practice disallowed in Australia.11 That 54% of sharps were naked at disposal also confirms the continued need for ergonomically sited, safety-engineered sharps containers.12,13
It is disturbing that 75% of HBN (7287 of 9651) were either capped or naked, indicating that a high proportion of HCP are unnecessarily placing themselves at risk of SI while handling sharps after use. While it was not part of this study to record the presence of blood, many naked-needle phlebotomy devices were visibly blood-contaminated.
In several HCF sampled in this study and many SC, it was obvious to the author that SED were not available or not compulsorily required under the hospital’s policy. The significant differences in SED use and activation between capital cities (Table 3) may indicate a city- or state-specific culture and hopefully this study may provoke discussion in low SED use cities and facilities. The reasons for the lack of widespread use of SED in Australia is puzzling – it cannot be due to commercial non-availability of SED. Further research into SI-prevention strategies and cultures in high and low-use SED hospitals is needed.
Unlike USA, France, Spain, most Canadian provinces and now all EU member countries including the UK, Australia has not enacted SED-specific legislation. Nevertheless all Australian states have enacted broad occupational health legislation which requires employers to ensure staff-injury risk is minimised. The incidence of SI in Australia, and the results of this study, indicate that Australia’s current legislation and SI safety culture is suboptimal.
That the use of SED can significantly reduce SI is incontestable.14 Legislated SED use in the USA reduced SI by 38% the year the legislation took effect.15 Following SED legislation in Canada the non-SED syringe proportion was shown to drop from 56 to 7%.8,10 However, SED-specific legislation is not the complete answer. This is illustrated by the fact that following the immediate reduction in SI with the SED law in the USA,15 the incidence of SI has failed to fall further in the decade since,1 and a similar pattern was documented immediately following SED legislation in British Columbia.10
The experience of countries with SED legislation tells us that not only is widespread use of SED required, but continued education in SED use and activation is paramount. Of the SED present in this study, 19% were not activated correctly and Black et al. state that up to a third of total SI could be prevented if SED were activated after use.6 The reasons for non- or partial activation of SED are reported to be ease of use, device preference, perception of patient adverse event, and training.16 Prior to U.S SED legislation, the non-activation rate of SED was found to be 30–40%,16,17 but with SED familiarity, high activation rates were achieved pre-legislation.18 Based on BBP transmission risk, Stringer and Haines proposed that an ‘acceptable’ phlebotomy and IV SED activation rate should be 100% or close to it, whereas syringe SED activation rates should be 90% or greater.8 Such activation levels were not evident in this study. Accepting that not all procedures have SED available, and that education is essential in SI prevention, the dependence on manual activation of SED plays a major part in SI. HCF need to examine a greater use of automatic (passive) and semi-automatic (e.g. push-button) devices wherever possible.19,20
We cannot rest in our quest for zero SI. This study indicates that Australian hospitals need to urgently adopt SED more universally. The author believes that finding funds for SED uptake is a limiting factor in Australia and the nation may need legislation to overcome this obstacle as is the case in the U.S where it is unlawful to use cost as the reason for non-adoption of SED.14 To provoke action on SI incidence reduction and SED uptake in Australia, the Alliance for Sharps Safety and Needlestick Prevention in Healthcare was established in 200921 and this matter was raised in Parliament by Dr Mal Washer for the first time in June 2013.4
The study concludes that the results give an indication of the possible reasons for the high incidence of SI among Australian hospital workers: an unacceptable proportion of sharps are non-SED and an unacceptable proportion of SED are not activated. We must find new vigour to protect our HCP – it must encompass a more widespread use of SED and needs encompass more regular, competency-based education, staff ownership of their safety, thorough user-evaluation of SED, and use of SED less dependent on human behaviour. As stated by Murphy, the situation in Australia is serious and change is urgent.2,22
This study’s limitations are: that audits were conducted on one day in each capital city and the results may not be representative of the HCF’s average sharps usage; a small number of SC were sampled from each HCF and may not be representative of the HCF as a whole; blind selection of SC did not allow hospital size or teaching status to be ascertained; unattached caps were evident in the waste so it was not possible to determine if some uncapped needles had lost their caps in the decanting process; with some capped needles and capped syringe-needles, it was not possible to tell if they had been discarded unused – the same dilemma applied with some capped non-activated SED; further division into finer sub-categories of sharps was considered too cumbersome for this preliminary study; and it was not possible to know whether HCF risk assessment or clinical assessment dictated that SED were not required, not appropriate or not available in certain procedures. Strengths of the study were: in the number of SC sampled overall; the number of hospitals sampled; the detailed recording of numbers, weight and volumes of each device category; and that five state capital cities were sampled.
Conflicts of interest
The author is a consultant to the medical waste and healthcare staff safety industries internationally and declares no conflict of interest in this study. No commercial entity influenced the initiation, intent, study methodology, results or manuscript content of this project.
Acknowledgements
The author thanks SteriHealth Limited for their generosity in allocating space in their factories for this work to be conducted and for the supply of personal protective equipment. No specific funding was received from any source for this project.
References
[1] Grimmond T, Good L. EXPO-S.T.O.P.: a national survey and estimate of sharps injuries and mucocutaneous blood exposures among healthcare workers in USA. J Assoc Occ Hlth Prof 2013; 33 31–6.[2] Murphy CL. The serious and ongoing issue of needlestick in Australian healthcare settings. Collegian 2013;
| The serious and ongoing issue of needlestick in Australian healthcare settings.Crossref | GoogleScholarGoogle Scholar |
[3] Australian Institute of Health and Welfare. Australia’s hospitals 2011–12 at a glance. Health services series no. 49. Cat. no. HSE 133. Canberra: AIHW; 2013. Available from: http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129543143 [verified March 2014].
[4] Parliament debates sharps and needlestick injuries, 2013. Alliance for Sharps Safety and Needlestick Prevention in Health-care. Available from: http://www.allianceforsharpssafety.org/wp-content/uploads/2010/08/Alliance-media-Private-Member-Motion.pdf [verified March 2014].
[5] Phillips EK, Conaway M, Parker G, Perry J, Jagger J. Issues in understanding the impact of the needlestick safety and prevention act on hospital sharps injuries. Infect Control Hosp Epidemiol 2013; 34 935–9.
| Issues in understanding the impact of the needlestick safety and prevention act on hospital sharps injuries.Crossref | GoogleScholarGoogle Scholar | 23917907PubMed |
[6] Black L, Parker G, Jagger J. Chinks in the armor: activation patterns of hollow-bore safety-engineered sharp devices. Infect Control Hosp Epidemiol 2012; 33 842–4.
| Chinks in the armor: activation patterns of hollow-bore safety-engineered sharp devices.Crossref | GoogleScholarGoogle Scholar | 22759553PubMed |
[7] Jagger J , Bentley M. Disposal-related sharp object injuries. Adv Exp Prev 1995; 1: 1,2,6,7,11.
[8] Stringer B, Haines T. Ongoing Use of Conventional Devices and Safety Device Activation Rates in Hospitals in Ontario, Canada. J Occup Environ Hyg 2011; 8 154–60.
| Ongoing Use of Conventional Devices and Safety Device Activation Rates in Hospitals in Ontario, Canada.Crossref | GoogleScholarGoogle Scholar | 21347957PubMed |
[9] Grimmond T. Use and activation of safety engineered sharps devices in a sample of 5 Florida healthcare facilities. J Assoc Occ Hlth Prof 2014; 34 13–5.
[10] Stringer B, Astrakianakis G, Haines T, Kamsteeg K, Danyluk Q, Tang T, et al Conventional and sharp safety devices in 6 hospitals in British Columbia, Canada. Am J Infect Control 2011; 39 738–45.
| Conventional and sharp safety devices in 6 hospitals in British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar | 21696858PubMed |
[11] NHMRC. Australian guidelines for the prevention and control of infection in healthcare. Commonwealth of Australia; 2010. Available from: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cd33_infection_control_healthcare.pdf [verified April 2014].
[12] Jagger J Bentley MB Safe disposal of safety devices. Adv Expo Prev 1999 ; 4 2 13,17.
[13] Grimmond T, Bylund S, Anglea C, Beeke L, Callahan A, Christiansen E, et al Sharps injury reduction using a sharps container with enhanced engineering: a 28 hospital nonrandomized intervention and cohort study. Am J Infect Control 2010; 38 799–805.
| Sharps injury reduction using a sharps container with enhanced engineering: a 28 hospital nonrandomized intervention and cohort study.Crossref | GoogleScholarGoogle Scholar | 21093697PubMed |
[14] Jagger J, Perry J, Gomaa A, Phillips EK. The impact of U.S. policies to protect healthcare workers from bloodborne pathogens: the critical role of safety engineered devices. J Infect Public Health 2008; 1 62–71.
| The impact of U.S. policies to protect healthcare workers from bloodborne pathogens: the critical role of safety engineered devices.Crossref | GoogleScholarGoogle Scholar | 20701847PubMed |
[15] Phillips EK, Conaway MR, Jagger JC. Percutaneous injuries before and after the Needlestick Safety and Prevention Act. N Engl J Med 2012; 366 670–1.
| Percutaneous injuries before and after the Needlestick Safety and Prevention Act.Crossref | GoogleScholarGoogle Scholar | 22335760PubMed |
[16] Alvarado-Ramy F, Beltrami EM, Short L, Srivastava PU, Henry K, Mendelson M, et al A comprehensive approach to percutaneous injury prevention during phlebotomy: results of a comprehensive study, 1993–1995. Infect Control Hosp Epidemiol 2003; 24 97–104.
| 12602691PubMed |
[17] Mulherin S, Rickman L, Jackson M. Initial worker evaluation of a new safety syringe. Infect Control Hosp Epidemiol 1996; 17 593–4.
| Initial worker evaluation of a new safety syringe.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FjsVGktA%3D%3D&md5=86e4571def0084ab3c783a795d47886cCAS | 8880232PubMed |
[18] Mendelson MH, Lin-Chen BY, Solomon R, Bailey E, Kogan G, Goldbold J. Evaluation of a safety resheathable winged steel needle for prevention of percutaneous injuries associated with intravascular-access procedures among healthcare workers. Infect Control Hosp Epidemiol 2003; 24 105–12.
| Evaluation of a safety resheathable winged steel needle for prevention of percutaneous injuries associated with intravascular-access procedures among healthcare workers.Crossref | GoogleScholarGoogle Scholar | 12602692PubMed |
[19] Tosini W, Ciotti C, Goyer F, Lolom I, L’Hériteau F, Abiteboul D, et al Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study. Infect Control Hosp Epidemiol 2010; 31 402–07.
| Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study.Crossref | GoogleScholarGoogle Scholar | 20175681PubMed |
[20] Black L. Chinks in the armor: percutaneous injuries from hollow bore safety-engineered sharps devices. Am J Infect Control 2013; 41 427–32.
| Chinks in the armor: percutaneous injuries from hollow bore safety-engineered sharps devices.Crossref | GoogleScholarGoogle Scholar | 23044172PubMed |
[21] Alliance for Sharps Safety and Needlestick Prevention in Health-care. Available from: http://www.allianceforsharpssafety.org/about-2/ [verified March 2014].
[22] Murphy C. Improved surveillance and mandated use of sharps with engineered sharp injury protections: a national call to action. Healthc Infect 2008; 13 33–7.
| Improved surveillance and mandated use of sharps with engineered sharp injury protections: a national call to action.Crossref | GoogleScholarGoogle Scholar |