A nurse-led antimicrobial stewardship intervention in two residential aged care facilities
Rhonda L. Stuart A B C D , Elizabeth Orr B , Despina Kotsanas A and Elizabeth E. Gillespie BA Monash Infectious Diseases, Monash Health, Clayton, Vic. 3168, Australia.
B Infection Prevention and Epidemiology, Monash Health, Clayton, Vic. 3168, Australia.
C Department of Medicine, Monash University, Clayton, Vic. 3168, Australia.
D Corresponding author. Email: rhonda.stuart@monashhealth.org
Healthcare Infection 20(1) 4-6 https://doi.org/10.1071/HI14016
Submitted: 29 May 2014 Accepted: 2 July 2014 Published: 24 July 2014
Journal Compilation © Australasian College for Infection Prevention and Control 2015
Abstract
The prevention and control of multi-resistant organisms (MRO) in residential aged care facilities (RACF) has significant implications for health services. The use of broad-spectrum antibiotics is associated with the development of MRO and therefore antimicrobial stewardship is a key initiative in decreasing development and spread of these organisms. This pilot study aims to assess the role of the infection control clinical nurse consultant in the antimicrobial stewardship team in two RACFs.
Implications
-
A simple educational intervention, utilising an infection prevention clinical nurse consultant to drive the program, led to a significant reduction in antibiotic use in a residential aged care facility.
-
Programs such as this could also conceivably be driven by other nursing staff or pharmacists with an interest in antimicrobial stewardship.
Introduction
The prevention and control of antimicrobial-resistant pathogens in residential aged care facilities (RACFs) has significant implications for health services.1 Challenges include the following: limited infection-control resources; lack of robust prevalence data; and complexities in service provision and governance.2 Antimicrobial stewardship in this setting is difficult because of multiple service providers, lack of understanding of organism susceptibility and the desire to treat residents before they become seriously unwell.3
Frequent antibiotic use and multiple hospital visits results in residents within RACF having an increased risk of acquiring multi-drug resistant organisms (MRO).4,5 The emergence of MRO is a major public health concern. Recent studies have shown that outcomes of MRO infections are worse than those due to susceptible organisms and residents from RACF may be a source of transmission in the acute-care setting.4,5
Antimicrobial stewardship is a key initiative in decreasing development and spread of MRO. Antibiotics are among the most frequently prescribed medications in RACF, with studies showing that 50–80% of residents will receive at least one course a year,6,7 but more importantly up to 50% of antibiotics prescribed may be inappropriate.8
This pilot study aimed to assess the role of the infection control clinical nurse consultant (CNC) in the antimicrobial stewardship team in RACF.
Methods
Two RACF facilities (130 available beds) associated with our health service were involved in this pilot study. Hostel A (100 beds) and Hostel B (30 beds) are both high care facilities. The RACF are situated in urban settings. Each facility has two general practitioners (GPs) that attend regularly but are often also visited by locum GPs if the facility GP is unavailable. Each RACF has an associated community pharmacy for medication supply and delivery, and the choice of private pathology services is at the discretion of the attending GP. Pharmacists are not involved in the daily monitoring of antimicrobial use at these facilities.
The infection control team performs surveillance activities for common infection types at each of these facilities (respiratory tract infections, skin and soft tissue infections, urinary tract infections, gastrointestinal tract infections). Such surveillance is audited against the McGeer criteria.9 The McGeer criteria were updated in 2012 and the new criteria were employed in the participating facilities in January 2013.10
The study included three phases: a pre-intervention data collection phase, an intervention phase and then a post-intervention data collection phase. The initial pre-intervention period of 3 months (September to November 2012) involved baseline-data collection. Data was collected by the CNC and included: type and dose of antibiotics commenced; reason why an antibiotic was commenced; whether specimens were taken before commencement and results of these; whether results were seen by a GP; total duration of antibiotics and patient outcome. Topical antimicrobials were not addressed in this pilot study.
Following the 3-month baseline-data collection period, an intervention period followed. During this time the CNC took on a role whereby they were the intermediary between the prescribing GP and an off-site infectious diseases (ID) physician who could provide expert advice on antimicrobial prescribing. Prior to the study commencement there was no ID advice that was easily available for attending GPs. The CNC was involved in education of nursing staff and GPs, data collection, monitoring of pathology results (via review of incoming paper results) and discussions between GPs and the ID physician. Education took the form of small lectures, posters on the wards (detailing in particular the importance of using antibiotics wisely), letters to GPs detailing findings of the baseline audit and reminders on appropriate prescribing. Additionally, twice-weekly rounds occurred whereby results from pathology and current patient status was evaluated and discussed with the GP when available.
Specific education campaigns targeted the management of urinary sepsis in residents. This focussed on not treating asymptomatic bacteriuria, and decreasing the duration of prescribed antibiotics for urinary sepsis. Additionally, education of the threat of MROs in the RACF setting occurred. Follow-up data collection for the intervention period occurred during the 3-month period from May to July 2013.
Occupied bed days (OBD) were obtained from facility administrative databases. Antibiotic usage (in the form of days of antibiotics administered) was calculated daily from resident drug charts. The Chi-squared or Fishers exact test was performed for comparison of categorical data with P ≤ 0.05 indicating significance.
During the course of this study there were no changes to the GP servicing or pharmacy provision.
Results
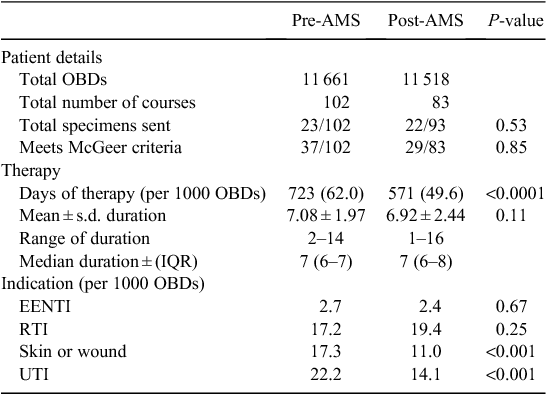
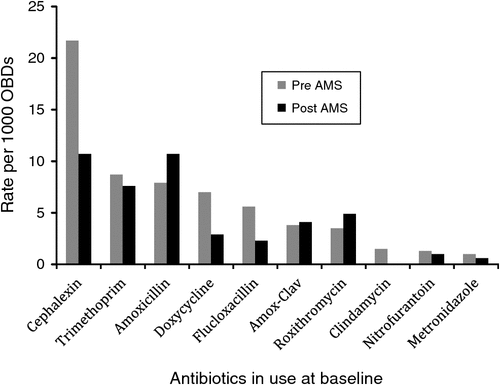
Occupied bed days for the baseline and post-intervention period were 11 661 and 11 518 respectively. There were 102 courses of antimicrobials prescribed at baseline and 83 during the post-intervention period, with a significant reduction in total days of antimicrobials prescribed (P < 0.0001) (Table 1). There were statistically significant reductions in the use of cephalexin (P < 0.001), doxycycline (P < 0.001), flucloxacillin (P < 0.001), clindamycin (P < 0.001), and metronidazole (P = 0.03) (Fig. 1). The major decreases were seen for the indications of urinary tract sepsis and skin and soft tissue infections (P < 0.001 for both indications).
|
|
Rates of infection types from the surveillance data remained stable over the two data collection periods, although respiratory tract infection rates increased in Hostel A (data not shown).
Discussion
In this pilot study, we found that a simple educational intervention, utilising an infection prevention CNC to drive the program, led to a significant reduction in antibiotic use in a RACF. The CNC was an intermediary between an ID physician, the GPs and nurses in the RACF and the microbiology results. This simple intervention was effective in educating, reminding and driving antibiotic use in two RACF attached to our health service. Although an infection prevention CNC was used in this pilot study, programs such as this could also conceivably be driven by other nursing staff or pharmacists with an interest in antimicrobial stewardship.
Significant changes in prescribing were evident especially in the cases of urinary tract sepsis and skin and soft tissue infections. These areas have been highlighted previously as being targets for antimicrobial stewardship activities in RACF.3 This was reflected in a decrease in commonly used antibiotics to treat these conditions (cephalexin, doxycycline, flucloxacillin and clindamycin). Although there were slight increases in the use of amoxicillin, amoxicillin-clavulanic acid and roxythromycin, these potentially could have been explained by an increase in the incidence of respiratory tract infections in the post-intervention period.
Limitations of this study include the fact that the numbers were small in this pilot study and that only two RACF were included such that our findings may not be applicable to other RACF settings. Nevertheless in this age of increasing need to undertake antimicrobial stewardship activities in resource-limited RACF, further study is justified on the role of the infection prevention CNC, and indeed other nurses, in this area.
Funding
This study was undertaken with support from a Covidien Infection Control Scholarship.
Conflicts of interest
The authors report no conflicts of interest.
References
[1] Ben-Ami R, Schwaber MJ, Navon-Venezia S, Schwartz D, Giladi M, Chmelnitsky I, et al Influx of extended-spectrum β-lactamase-producing Enterobacteriaceae into the hospital. Clin Infect Dis 2006; 42 925–34.| Influx of extended-spectrum β-lactamase-producing Enterobacteriaceae into the hospital.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjtlelsro%3D&md5=804356ea83ac18fdddceef4cfb6b8639CAS | 16511754PubMed |
[2] Moro M, Jans B, Cookson B, Fabry J. The burden of healthcare-associated infections in European long-term care facilities. Infect Control Hosp Epidemiol 2010; 31 S59–S62.
| 20929373PubMed |
[3] Lim CJ, Kong DCM, Stuart RL. Reducing inappropriate antibiotic prescribing in the residential care setting: current perspectives. Clin Interv Aging 2014; 9 165–77.
| 24477218PubMed |
[4] O’Fallon E, Pop-Vicas A, D’Agata E. The emerging threat of multidrug-resistant Gram-negative organisms in long-term care facilities. J Gerontol A Biol Sci Med Sci 2009; 64A 138–41.
| The emerging threat of multidrug-resistant Gram-negative organisms in long-term care facilities.Crossref | GoogleScholarGoogle Scholar |
[5] Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D’Agata EMC. Multidrug-resistant Gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc 2008; 56 1276–80.
| Multidrug-resistant Gram-negative bacteria in a long-term care facility: prevalence and risk factors.Crossref | GoogleScholarGoogle Scholar | 18557965PubMed |
[6] Katz PR, Beam TR, Brand F, Boyce K. Antibiotic use in the nursing home. Physician practice patterns. Arch Intern Med 1990; 150 1465–8.
| Antibiotic use in the nursing home. Physician practice patterns.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3czhsVKjtw%3D%3D&md5=2831eb44dc9cb3973c106492a587d14dCAS | 2369244PubMed |
[7] Loeb M, Simor AE, Landry L, McArthur M, Duffy J, Kwan D, et al Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med 2001; 16 376–83.
| Antibiotic use in Ontario facilities that provide chronic care.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzntlOrtg%3D%3D&md5=2604412472ecb50df3870c493e550c15CAS | 11422634PubMed |
[8] Stuart RL, Wilson J, Bellaard-Smith E, Brown R, Wright L, Vandergraaf S, et al Antibiotic use and misuse in residential aged care facilities. Intern Med J 2012; 42 1145–9.
| Antibiotic use and misuse in residential aged care facilities.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38rhvFaguw%3D%3D&md5=b16e55ffd08fafea2dd3a532377a62bcCAS | 22472087PubMed |
[9] McGeer A, Campbell B, Emori TG, Hierholzer W, Jackson M, Nicolle L, et al Definitions of infection for surveillance in long-term care facilities. Am J Infect Control 1991; 19 1–7.
| Definitions of infection for surveillance in long-term care facilities.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M3hsVSmuw%3D%3D&md5=910c5f4dba2bc63511ea2a3ad6e75855CAS | 1902352PubMed |
[10] Stone ND, Ashraf MS, Calder J, Crnich C, Crossley K, Drinka P, et al Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012; 33 965–77.
| Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria.Crossref | GoogleScholarGoogle Scholar | 22961014PubMed |

