Geographical variation in the morphology of the Wedge-tailed Shearwater (Puffinus pacificus)
Leigh S. BullSchool of Biological Sciences, Victoria University of Wellington, PO Box 600, Wellington, New Zealand. Present address: Universite Paris-Sud XI, Laboratoire Ecologie Systematique et Evolution, Batiment 362, F-91405 Orsay cedex, France. Email: leigh.bull@ese.u-psud.fr
Emu 106(3) 233-243 https://doi.org/10.1071/MU05050
Submitted: 26 September 2005 Accepted: 15 June 2006 Published: 18 August 2006
Abstract
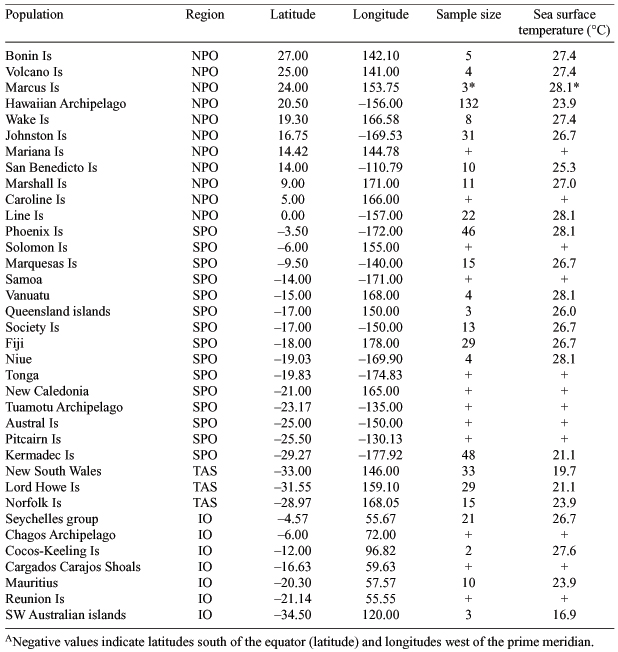
Geographical variation in the morphology of the Wedge-tailed Shearwater (Puffinus pacificus) was assessed using multivariate analyses, including MANOVA and canonical discriminant analysis. This study tested the roles of climatic (sea surface temperature) and geographical (latitude, longitude and inter-population distance) factors as potential agents of natural selection on the body dimensions of this geographically variable species. Patterns of morphological variation, represented by phenotypic distances, were found to be unrelated to the geographical proximity of colonies. The Wedge-tailed Shearwater exhibited the trend in body size predicted by Bergmann’s Rule, increasing significantly with increasing latitude and decreasing temperature. Allen’s Rule proposes that an animal’s extremities should be relatively shorter in colder climates, but the data did not support this hypothesis with regards to variation in bill-shape of the Wedge-tailed Shearwater. Owing to the interrelated nature of ecological parameters, such as food, climate, distribution, life history and breeding biology, it is unlikely that a single factor is responsible for the observed morphological variation. However, food, through its influence on competition, dispersal, growth, fecundity and survival, may play an important role in the patterns of morphological variation found in this study. Therefore, the heterogeneity of the foraging habitat over the range of the species may be responsible for the observed variation, owing to differences in the distance required to travel to the feeding grounds and the availability and quality of food.
Acknowledgments
I thank the collection managers and curators of the bird collections in: the National Museum of New Zealand Te Papa Tongarewa (Wellington, NZ); the American Museum of Natural History (New York, USA); the National Museum of Natural History (Smithsonian Institute, Washington DC, USA); the Natural History Museum (Tring, UK); and the Australian Museum (Sydney, Australia) for access to specimens and facilities. This study was funded by the following sources: Collection study grant, the American Museum of Natural History; Hutton Fund, the Royal Society of New Zealand; Victoria University of Wellington Science Faculty grants; Helen Stewart Royle Scholarship; and the Victoria University of Wellington Postgraduate Scholarship. Thanks go to Bruce Norris and Svend Andersen for developing the computer program to calculate geographical distances, and to Ben Bell and Shirley Pledger for their advice and feedback throughout this study. Two anonymous referees provided helpful comments, which improved the paper. Many thanks go to Shirley Pledger for calculation of the Mantel’s test.
Adkison, M. (1995). Population differentiation in pacific salmon: local adaptation, genetic drift, or the environment? Canadian Journal of Fisheries and Aquatic Sciences 52, 2762–2777.
Austin, J. J. , White, R. W. G. , and Ovenden, J. R. (1994). Population-genetic structure of a philopatric, colonially nesting seabird, the Short-tailed Shearwater (Puffinus tenuirostris). Auk 111, 70–79.
Bergmann, C. (1847). Üeber die Verhältnisse der Wärmeökonomie de Thiere zu ihrer Grösse. Göttinger Studien 3, 595–708.
Boyce, M. S. (1978). Climatic variability and body size variation in Muskrats (Ondatra zibethicus) of North America. Oecologia 36, 1–20.
| Crossref | GoogleScholarGoogle Scholar |
Bull, L. S. , Haywood, J. , and Pledger, S. (2004). Components of phenotypic variation in the morphometrics of Shearwater (Puffinus) species. Ibis 146, 38–45.
| Crossref | GoogleScholarGoogle Scholar |
Croxall, J. P. (1995). Sexual size dimorphism in seabirds. Oikos 73, 399–403.
Davis, J. (1954). Seasonal changes in bill length of certain passerine birds. Condor 56, 142–149.
Fairbairn, J. , and Shine, R. (1993). Patterns of sexual size dimorphism in seabirds of the southern hemisphere. Oikos 68, 139–145.
Freeman, S. , and Jackson, W. M. (1990). Univariate metrics are not adequate to measure avian body size. Auk 107, 69–74.
Geist, V. (1987). Bergmann’s rule is invalid. Canadian Journal of Zoology 65, 1035–1038.
Grant, P. R. (1968). Bill size, body size, and the ecological adaptations of bird species to competitive situations on islands. Systematic Zoology 17, 319–333.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Macdonald, D. W. , Courtenay, O. , Forbes, S. , and Mathews, F. (1999). The Red Fox (Vulpes vulpes) in Saudi Arabia: loose-knit groupings in the absence of territoriality. Journal of Zoology 249, 383–391.
| Crossref | GoogleScholarGoogle Scholar |
McNab, B. K. (1971). On the ecological significance of Bergmann’s rule. Ecology 52, 845–854.
| Crossref | GoogleScholarGoogle Scholar |
Newton, I. , and Dale, L. (1996). Relationships between migration and latitude among west European birds. Journal of Animal Ecology 65, 137–146.
| Crossref | GoogleScholarGoogle Scholar |
Skira, I. J. (1991). The Short-tailed Shearwater: a review of its biology. Corella 15, 45–52.
Spear, L. B. , and Ainley, D. G. (1997). Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis 139, 221–233.
van Heezik, Y. M. (1990). Seasonal, geographical and age-related variations in the diet of the Yellow-eyed Penguin (Megadyptes antipodes). New Zealand Journal of Zoology 17, 201–212.
Waugh, S. M. , Prince, P. A. , and Weimerskirch, H. (1999). Geographical variation in morphometry of Black-browed and Grey-headed Albatrosses from four sites. Polar Biology 22, 189–194.
| Crossref | GoogleScholarGoogle Scholar |
Wigginton, J. D. , and Dobson, F. S. (1999). Environmental influences on geographic variation in body size of Western Bobcats. Canadian Journal of Zoology 77, 802–813.
| Crossref | GoogleScholarGoogle Scholar |

Wikelski, M. , and Trillmich, F. (1997). Body size and sexual size dimorphism in Marine Iguanas fluctuate as a result of opposing natural and sexual selection: an island comparison. Evolution 51, 922–936.
| Crossref | GoogleScholarGoogle Scholar |

Wright, S. (1943). Isolation by distance. Genetics 28, 114–138.

Zink, R. M. , and Remsen, J. V., Jr (1986). Evolutionary processes and patterns of geographic variation in birds. Current Ornithology 4, 1–69.

Zusi, R. L. (1982). Infraspecific geographic variation and the subspecies concept. Auk 99, 606–608.

|

