How many of Australia’s ground-nesting birds are likely to be at risk from the invasive Cane Toad (Rhinella marina)?
Christa Beckmann A B and Richard Shine AA School of Biological Sciences A08, University of Sydney, Sydney, NSW 2006, Australia.
B Corresponding author. Present address: Centre for Integrative Ecology, School of Life & Environmental Sciences, Deakin University, Waurn Ponds, Pigdons Road, Geelong, Vic. 3217, Australia. Email: c.beckmann@deakin.edu.au
Emu 112(2) 83-89 https://doi.org/10.1071/MU11028
Submitted: 7 April 2011 Accepted: 25 July 2011 Published: 23 April 2012
Abstract
Cane Toads (Rhinella marina; hereafter ‘toads’) are large, toxic American anurans that were introduced to Australia in 1935. Research on their ecological impact has focussed on the lethal ingestion of toxic toads by native frog-eating predators. Less attention has been paid to the potential impacts of Cane Toads as predators, although these large anurans sometimes eat vertebrates, such as nestling birds and bird eggs. We review published and unpublished data on interactions between Cane Toads and Australian ground-nesting birds, and collate distributional and breeding information to identify the avian taxa potentially at risk of having eggs or chicks eaten by Cane Toads. Cane Toads are currently sympatric with 80 ground-nesting bird species in Australia, and five additional species of bird occur within the predicted future range of the toad. Although many species of bird are potentially at risk, available data suggest there is minimal impact of Cane Toads on ground-nesting species. Future research could usefully address both direct and indirect impacts of the invasion by Cane Toads, ideally with detailed field observations of these impacts on nesting success and of changes in bird breeding success as a function of invasion by toads.
Additional keywords: alien species, Bufo marinus, chick, ecological impact, egg, invasive species.
‘The eggs and nestlings of all our ground-nesting birds will be snapped up by these night-hunting marauders.’ [Froggatt 1936; p. 163]
Introduction
Invasive species are widely viewed as a threat to global biodiversity (Vitousek et al. 1997; Mack et al. 2000), and in 2008 the International Union for the Conservation of Nature ranked invasive predators as the third most significant threat to bird populations (Birdlife International 2008). Invasive predators such as rats, cats and snakes have had devastating effects on some bird populations (Fritts and Rodda 1998; Nogales et al. 2004; Jones et al. 2008). Such impacts have attracted considerable research, but much remains to be learnt about the ways in which the arrival of an invasive species influences native taxa.
One widely studied invasive species is the Cane Toad (Rhinella marina or Bufo marinus under previous nomenclatural schemes; Pramuk 2006) (hereafter ‘toad’). Intentionally introduced to Australia in 1935 for the control of agricultural pests, the toad has spread widely and its distribution now covers >1 × 106 km2 of tropical and subtropical Australia (Lever 2001). Research on the ecological impacts of toads has focussed on their toxicity when they are eaten by native predators. Many native predators have experienced population declines owing to lethal ingestion of toxic toads. Such predators include native frogs (Crossland et al. 2008), marsupial quolls (Burnett 1997), crocodiles (Letnic et al. 2008), elapid snakes (Phillips et al. 2003; Phillips and Shine 2004), varanid lizards (Doody et al. 2006; Smith and Phillips 2006; Griffiths and McKay 2007) and scincid lizards (Price-Rees et al. 2010). In contrast, predatory birds do not seem to be at substantial risk from poisoning following ingestion of toads: few bird species show ill-effects after eating toads, possibly owing to phylogenetic affinities with other toad-eating species or the ability to learn to avoid the toxin, or some combination of both (Beckmann and Shine 2009, 2010, 2011; Beckmann et al. 2011).
The toxicity of toads is not their only possible impact on native wildlife; they might also affect Australian taxa by eating them. Cane Toads primarily prey on insects, and can reduce insect abundances and affect species composition (Catling et al. 1999; Greenlees et al. 2006). These large anurans, which can weigh up to 2.8 kg, also take vertebrates as prey, albeit as a minor portion of their total diet (reviewed in Lever 2001). Recorded vertebrate prey include mammals, such as the Common Planigale (Planigale maculata) (Redhead 1983) and Common Field Rat (Rattus rattus minanensis) (Rabor 1952), and reptiles such as neonate Brown Tree Snakes (Boiga irregularis) (Caudell et al. 2000), blind snakes (L. Pizzatto, unpubl. data), Coral Snakes (Micrurus circinalis) (Quesnel 1986), burrowing snakes (Typhlops sp.) (Rabor 1952), pygopodids (T. Madsen, pers. comm.) and geckos (Pemberton 1934). Other records of vertebrate taxa eaten by Cane Toads include Australian frogs (Freeland and Kerin 1988) and birds (see detailed references below). In the only detailed published analysis of predation of birds by toads, Boland (2004a) studied the impact of toads on the burrow-nests of Rainbow Bee-eaters (Merops ornatus). He reported that toads were the most common nest predator, with nest predation and usurpation by toads causing one-third of all nesting attempts to fail, and mean fledgling numbers per nest to fall by 34%. Likewise, at one site in the Northern Territory, Australia, we found 67% of nesting attempts by Rainbow Bee-eaters close to water failed owing to toads (C. Beckmann, unpubl. data). This substantial impact suggests that other ground- and burrow-nesting birds might be similarly at risk (Froggatt 1936; Boland 2004a).
The first step in evaluating potential impacts of Cane Toads on ground-nesting birds is to identify the species at risk. Thus, we reviewed available literature to: (a) determine the number of species of ground-nesting birds that overlap in range with the current and future predicted ranges of the Cane Toad in Australia; and (b) predict which of these bird species might be at risk of being eaten by toads. We also reviewed the (sparse) literature on dietary habits of Cane Toads for records of actual or attempted predation by Cane Toads on birds.
Methods
To identify which species of native ground-nesting birds might be at risk of having their eggs or chicks eaten by Cane Toads, or having their nests usurped, we compiled a list of the species of ground-nesting birds whose ranges overlap with either the current or the predicted future range of the Cane Toad in mainland Australia (distributions of Cane Toads from Kearney et al. 2008; see Fig. 1). Our analysis excluded Christmas, Lord Howe, Norfolk, Macquarie, Heard and Cocos-Keeling Islands and the islands of Torres Strait. We used range maps published in Pizzey and Knight (2007) and the Australian species list (Christidis and Boles 2008). The proportion of the range of each species of bird that overlaps with the range of Cane Toads was classified into one of five categories: 0% currently but overlap with future predicted range of the toad, 1–25% of current range, 26–50%, 51–75%, 76–100%. As the range maps in Pizzey and Knight (2007) do not specify breeding range, for some non-sedentary species we may be overestimating the proportion of the population that is at risk.
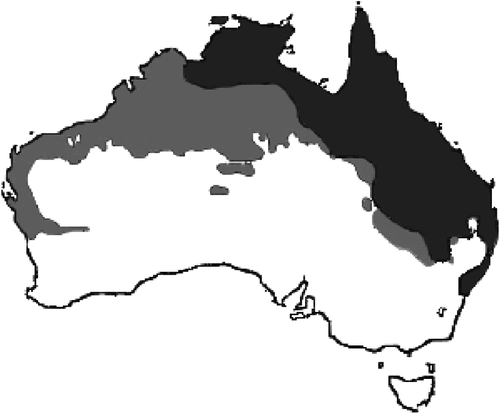
|
Toads are gape-limited predators, and previous work found that toads were unable to eat hard objects with a diameter of ≥38 mm (Beckmann 2011). We thus limited our search to species with an egg diameter (measured at the widest point along the longitudinal axis) of ≤35 mm. However, in doing this we may be overestimating the vulnerability of bird species with the largest eggs, because only the largest toads would be capable of eating them. Equally, however, as nestling birds are pliable (unlike eggs), toads might be able to eat nestlings from eggs >35 mm in diameter even if they could not eat the eggs from which the chick emerged, which would lead us to underestimate the vulnerability of birds with larger eggs. To determine which species are ground nesters and the size of their eggs, we used species accounts in the Handbook of Australian, New Zealand and Antarctic Birds (Marchant and Higgins 1990, 1993; Higgins and Davies 1996; Higgins 1999; Higgins et al. 2001, 2006; Higgins and Peter 2002). All species known to nest on the ground (including both obligate and some occasional ground nesters as well as burrow-nesting species) and with eggs small enough for toads to ingest were considered to be at potential risk of having nest contents eaten by toads.
We next examined published and grey literature on the diet of Cane Toads for reports of toads eating birds. We entered the search terms ‘cane toad and bird’ and ‘cane toad diet’ into the following search engines: Searchable Ornithological Research Archives, Ornithological Worldwide Literature, Web of Knowledge, Biosis Previews and Google Scholar. Journals that are not available through search engines (Corella, Herpetofauna, Reptilia and the Australian Reptile Club Journal) were manually searched.
Results
The birds with ranges that overlap the current distribution of Cane Toads in Australia include 80 species (from 32 families) of obligate or occasional ground nesters with egg diameters ≤35 mm (Appendix 1). An additional five species (from four families) have ranges that overlap the predicted future range of Cane Toads (Appendix 1). Of the 80 species of birds whose ranges overlap with the current range of Cane Toads: 17 (21.3%) have 1–25% of their range overlapping with that of Toads, 35 (43.8%) have 26–50% overlap, 8 (10.0%) have 51–75% overlap and 20 (25.0%) have 76–100% overlap (Appendix 1). When the projected future range of toads is included, the percentage overlap in range with toads will increase by one category for 27 species (34.6%) and percentage overlap will increase by two categories for four species (5.1%). Of the five additional species within the predicted future range of Cane Toads, all have 0–25% overlap with toads (Appendix 1).
There are few published reports of Cane Toads eating eggs or nestlings of ground- and burrow-nesting birds, or usurping nests of such species. Most of these reports are anecdotal and either describe interactions between a single bird and a single toad or simply describe the stomach contents of toads. There were no reports of adult birds being taken by Cane Toads. We found only 12 reports of toads eating birds or eggs, or usurping nests; of these, only nine birds were identified to species. Three of these encounters were in nests: of Brown Quail (Coturnix ypsilophora), Rainbow Bee-eater, and Striated Pardalote (Pardalotus striatus) (Table 1). Two of the reports were from outside Australia, and reported Cane Toads taking a fledgling Shining Cowbird (Molothrus bonariensis) in Trinidad and Tobago and a fledgling Common Myna (Acridotheres tristis) in Fiji (see Table 1). Cane Toads have also been observed eating chicks of domestic poultry (see Table 1). Whereas some encounters were in nests, others involved chicks on the ground, possibly outside a nest, or were simply reports of the stomach contents of toads (Table 1). At least three of the records do not involve a species of ground-nesting bird. Toads ate birds from a diversity of phylogenetic lineages, ranging from passerines to waterbirds, and ate both chicks and eggs. In summary, Cane Toads appear to eat birds only rarely, and some of the nestlings that were eaten belong to bird species that are not ground-nesters.
Discussion
Our literature survey suggests that although Cane Toads eat birds, they do so only infrequently. However, predation rates may be greater in some circumstances, such as among colonially nesting birds (where a small number of toads could have a substantial impact), birds whose nests also provide a refuge for toads (such as burrow-nesting species), and birds that nest in areas with high density of toads. A combination of these factors is likely to be required for toads to be a serious threat to local populations of ground-nesting birds. The overall impact of the invasion by Cane Toads on Australian ground-nesting birds may be minimal, despite occasional deaths of individual birds or destruction of a few nests.
The low occurrence of birds in the diet of Cane Toads is important because our survey shows that many species (80) of Australia’s mainland ground-nesting bird species are already sympatric with Cane Toads, and the distribution of a further five overlaps with the future predicted range of toads. Bird species with a greater percentage of range overlap with toads may be at greater risk than those with a smaller overlap, as they will have a smaller percentage of their range which could act as a ‘refuge’ from toads for the species. Of these 85 species, only six species are listed as threatened by the Australian government. The Partridge Pigeon (Geophaps smithii smithii, and G. s. blaauwi), Squatter Pigeon (Geophaps scripta scripta), Black-breasted Button-quail (Turnix melanogaster), and Plains-wanderer (Pedionomus torquatus) are listed as vulnerable, whereas the Night Parrot (Pezoporus occidentalis) and Buff-breasted Button-quail (Turnix olivii) are considered endangered. Most species of ground-nesting birds that will coexist with Cane Toads already do so, and the additional threat of predation by Cane Toads only applies to a few threatened species.
Toads have been recorded to eat the young of at least five species of bird (see Table 1). Young of birds that are not ground-nesting species are also potentially at risk, with the introduced Common Myna (Acridotheres tristis) and House Sparrow (Passer domesticus), both cavity nesters, eaten by toads (Table 1). Toads have been reported to pull live chicks out from beneath a brooding domestic Chicken (Gallus gallus) (Anon. 1939). Boland (2004a, 2004b) found that Cane Toads disrupted one-third of nesting attempts by Rainbow Bee-eaters in southeast Queensland, and were responsible for most of the nest failures due to predation. The birds did not respond to Cane Toads as they did to other native predators, that is with alarm calling or mobbing (Boland 2004a). In his study, the presence of toads was associated with a decline in production of fledglings from 1.2 to 0.8 fledglings per nest (Boland 2004a). The toads were confirmed to have eaten nestlings, and Boland (2004a) suspected they also ate eggs (toads were found in empty nests that had held eggs on the previous check). Cane Toads can locate stationary prey (i.e. carrion, faeces and pet food) by olfaction (Rossi 1981, 1983) and Boland (2004a) found that toads used olfactory cues to locate Bee-eater nests and therefore were active, rather than opportunistic, nest predators.
Our literature survey also reveals a large gap in knowledge on interactions between ground-nesting birds and Cane Toads. For example we have little information about behavioural responses to toads by birds (e.g. will parents defend their nests against toads?), nor on the ecological determinants of encounter rates between Cane Toads and ground-nesting birds. The likelihood of toads encountering bird nests or fledglings will depend on factors such as: (a) population densities of both birds and toads; (b) hunger levels and feeding rates of toads; (c) timing of the nesting season of a bird relative to seasonal activity schedules of toads; and (d) body-size distributions of toads (proportion of individual toads large enough to eat eggs or nestlings).
In the short term, the arrival of invasive Cane Toads may cause an increase, rather than a decrease, in nest survival in many bird species (not just ground nesters), because populations of other nest-predators (such as quolls and varanid lizards) crash owing to lethal toxic ingestion of toads (Doody et al. 2006; Smith and Phillips 2006; Griffiths and McKay 2007; Shine 2010). Thus, any additional mortality due to Cane Toads may be more than offset by reduced predation from other species of native predators. Over time, however, numbers of native predators tend to rebound from invasion by toads (Shine 2010), such that predation by toads may become additive and populations of ground-nesting birds may be adversely affected. A well-designed field study could address these questions, by evaluating reproductive success and identifying predators of ground nesters both before and after the arrival of toads. More generally, we need additional field-based research to elucidate more fully the potential impacts of invasive Cane Toads as predators on Australia’s avian fauna.
Acknowledgements
For funding, we thank the Australian Research Council and Birds Australia. We thank the many authors who have published accounts of the breeding habits of Australia’s birds.
References
Anon. (1939). The bushlover: toad takes chicken. The Courier-Mail, 9 September 1933, p. 18.Beckmann, C. (2011). Impacts of the invasive cane toad on Australia’s native birds. Ph.D. Thesis, University of Sydney.
Beckmann, C., and Pizzatto, L. (2011). Invasive cane toad consumes nestling bird in Australia. Herpetological Review 42, 592.
Beckmann, C., and Shine, R. (2009). Impact of invasive cane toads on Australian birds. Conservation Biology 23, 1544–1549.
| Impact of invasive cane toads on Australian birds.Crossref | GoogleScholarGoogle Scholar |
Beckmann, C., and Shine, R. (2010). The power of myth: the (non) impact of invasive cane toads (Bufo marinus) on domestic chickens (Gallus gallus). Animal Production Science 50, 847–851.
| The power of myth: the (non) impact of invasive cane toads (Bufo marinus) on domestic chickens (Gallus gallus).Crossref | GoogleScholarGoogle Scholar |
Beckmann, C., and Shine, R. (2011). Toad’s tongue for breakfast: exploitation of a novel prey type, the invasive cane toad, by scavenging raptors in tropical Australia. Biological Invasions 13, 1447–1455.
| Toad’s tongue for breakfast: exploitation of a novel prey type, the invasive cane toad, by scavenging raptors in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Beckmann, C., Crossland, M. R., and Shine, R. (2011). Responses of Australian wading birds to a novel toxic prey type, the invasive cane toad (Bufo marinus). Biological Invasions 13, 2925–2934.
| Responses of Australian wading birds to a novel toxic prey type, the invasive cane toad (Bufo marinus).Crossref | GoogleScholarGoogle Scholar |
Birdlife International (2008). ‘State of the World’s Birds: Indicators for Our Changing World.’ (BirdLife International: Cambridge, UK.)
Boland, C. R. J. (2004a). Introduced cane toads Bufo marinus are active nest predators and competitors of Rainbow Bee-eaters Merops ornatus: observational and experimental evidence. Biological Conservation 120, 53–62.
| Introduced cane toads Bufo marinus are active nest predators and competitors of Rainbow Bee-eaters Merops ornatus: observational and experimental evidence.Crossref | GoogleScholarGoogle Scholar |
Boland, C. R. J. (2004b). Breeding biology of Rainbow Bee-eaters (Merops ornatus): a migratory, colonial, cooperative bird. The Auk 121, 811–823.
| Breeding biology of Rainbow Bee-eaters (Merops ornatus): a migratory, colonial, cooperative bird.Crossref | GoogleScholarGoogle Scholar |
Burnett, S. (1997). Colonizing cane toads cause population declines in native predators: reliable anecdotal information and management implications. Pacific Conservation Biology 3, 65–72.
Catling, P. C., Hertog, A., Burt, R. J., Wombey, J. C., and Forrester, R. I. (1999). The short-term effect of cane toads (Bufo marinus) on native fauna in the Gulf Country of the Northern Territory. Wildlife Research 26, 161–185.
| The short-term effect of cane toads (Bufo marinus) on native fauna in the Gulf Country of the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Caudell, J. N., Janes, B., and Lawie, P. (2000). Boiga irregularis (brown tree snake). Predation. Herpetological Review 31, 245.
Christidis, L., and Boles, W. E. (2008). ‘Systematics and Taxonomy of Australian Birds.’ (CSIRO Publishing: Melbourne.)
Crossland, M. J., Brown, G. P., Anstis, M., Shilton, C. M., and Shine, R. (2008). Mass mortality of native anuran tadpoles in tropical Australia due to the invasive toad (Bufo marinus). Biological Conservation 141, 2387–2394.
| Mass mortality of native anuran tadpoles in tropical Australia due to the invasive toad (Bufo marinus).Crossref | GoogleScholarGoogle Scholar |
Doody, J. S., Green, B., Sims, R., Rhind, D., West, P., and Steer, D. (2006). Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta). Wildlife Research 33, 349–354.
| Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta).Crossref | GoogleScholarGoogle Scholar |
Freeland, W. J., and Kerin, S. H. (1988). Within habitat relationships between invading Bufo marinus and Australian species of frog during the tropical dry season. Australian Wildlife Research 15, 293–305.
| Within habitat relationships between invading Bufo marinus and Australian species of frog during the tropical dry season.Crossref | GoogleScholarGoogle Scholar |
Fritts, T. H., and Rodda, G. H. (1998). The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annual Review of Ecology and Systematics 29, 113–140.
| The role of introduced species in the degradation of island ecosystems: a case history of Guam.Crossref | GoogleScholarGoogle Scholar |
Froggatt, W. W. (1936). The introduction of the great Mexican toad Bufo marinus into Australia. Australian Naturalist 9, 163–164.
Greenlees, M. J., Brown, G. P., Webb, J. K., Phillips, B. L., and Shine, R. (2006). Effects of an invasive anuran [the cane toad (Bufo marinus)] on the invertebrate fauna of a tropical Australian floodplain. Animal Conservation 9, 431–438.
| Effects of an invasive anuran [the cane toad (Bufo marinus)] on the invertebrate fauna of a tropical Australian floodplain.Crossref | GoogleScholarGoogle Scholar |
Griffiths, A. D., and McKay, J. L. (2007). Cane toads reduce the abundance and site occupancy of Merten’s water monitor (Varanus mertensi). Wildlife Research 34, 609–615.
| Cane toads reduce the abundance and site occupancy of Merten’s water monitor (Varanus mertensi).Crossref | GoogleScholarGoogle Scholar |
Higgins, P. J. (Ed.) (1999). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 4: Parrots to Dollarbird.’ (Oxford University Press: Melbourne.)
Higgins, P. J., and Davies, S. J. F. (Eds) (1996). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 3: Snipe to Pigeons.’ (Oxford University Press: Melbourne.)
Higgins, P. J., and Peter, J. M. (Eds) (2002). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 6: Pardalotes to Shrike-thrushes.’ (Oxford University Press: Melbourne.)
Higgins, P. J., Peter, J. M., and Steele, W. K. (Eds) (2001). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 5: Tryant-flycatchers to Chats.’ (Oxford University Press: Melbourne.)
Higgins, P. J., Peter, J. M., and Cowling, S. J. (Eds) (2006). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 7: Boatbill to Starlings.’ (Oxford University Press: Melbourne.)
Jones, H. P., Tershy, B. R., Zavaleta, E. S., Croll, D. A., Keitt, B. S., Finkelstein, M. E., and Howald, G. R. (2008). Severity of the effects of invasive rats on seabirds: a global review. Conservation Biology 22, 16–26.
| Severity of the effects of invasive rats on seabirds: a global review.Crossref | GoogleScholarGoogle Scholar |
Kearney, M., Phillips, B. L., Tracy, C. R., Christian, K. A., Betts, G., and Porter, W. P. (2008). Modeling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434.
| Modeling species distributions without using species distributions: the cane toad in Australia under current and future climates.Crossref | GoogleScholarGoogle Scholar |
Krakauer, T. (1968). The ecology of the neotropical toad, Bufo marinus, in South Florida. Herpetologica 24, 214–221.
Letnic, M., Webb, J. K., and Shine, R. (2008). Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biological Conservation 141, 1773–1782.
| Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Lever, C. (2001). ‘The Cane Toad. The History and Ecology of a Successful Colonist.’ (Westbury: Otley, UK.)
Mack, R. N., Simberloff, D., Lonsdale, W. M., Evans, H., Clout, M., and Bazzaz, F. (2000). Biotic invasions: causes, epidemiology, global consequences and control. Issues in Ecology 5, 1–20.
Marchant, S., and Higgins, P. J. (Eds) (1990). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 1: Ratites to Ducks.’ (Oxford University Press: Melbourne.)
Marchant, S., and Higgins, P. J. (Eds) (1993). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 2: Raptors to Lapwings.’ (Oxford University Press: Melbourne.)
Nogales, M., Martin, A., Tershy, B. R., Donlan, C. J., Veitch, D., Puerta, N., Wood, B., and Alonso, J. (2004). A review of feral cat eradication on islands. Conservation Biology 18, 310–319.
| A review of feral cat eradication on islands.Crossref | GoogleScholarGoogle Scholar |
Pemberton, C. E. (1934). Local investigations on the introduced tropical American toad, Bufo marinus. Hawaii Planters’ Rec. 38, 186–192.
Phillips, B. L., and Shine, R. (2004). Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proceedings of the National Academy of Sciences of the United States of America 101, 17150–17155.
| Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFagu7bK&md5=f27b902254d0f92d3e91e6860fdf72f8CAS |
Phillips, B. L., Brown, G. P., and Shine, R. (2003). Assessing the potential impact of cane toads on Australian snakes. Conservation Biology 17, 1738–1747.
| Assessing the potential impact of cane toads on Australian snakes.Crossref | GoogleScholarGoogle Scholar |
Pippett, J. R. (1975). The marine toad, Bufo marinus, in Papua New Guinea. Papua and New Guinea Agricultural Journal 26, 23–30.
Pizzey, G., and Knight, F. (2007). ‘Graham Pizzey and Frank Knight: The Field Guide to the Birds of Australia.’ 8th edn. (Ed. P. Menkhorst). (HarperCollins Publishers: Sydney.)
Pramuk, J. B. (2006). Phylogeny of South American Bufo (Anura : Bufonidae) inferred from combined evidence. Zoological Journal of the Linnean Society London 146, 407–452.
| Phylogeny of South American Bufo (Anura : Bufonidae) inferred from combined evidence.Crossref | GoogleScholarGoogle Scholar |
Price-Rees, S. J., Brown, G. P., and Shine, R. (2010). Predation on toxic cane toads (Bufo marinus) may imperil bluetongue lizards (Tiliqua scincoides intermedia, Scincidae) in tropical. Australian Wildlife Research 37, 166–173.
| Predation on toxic cane toads (Bufo marinus) may imperil bluetongue lizards (Tiliqua scincoides intermedia, Scincidae) in tropical.Crossref | GoogleScholarGoogle Scholar |
Quesnel, V. V. (1986). An unusual prey for the marine toad, Bufo marinus 1985–1986. Living World 1986, 25.
Rabor, D. S. (1952). Preliminary notes on the giant toad Bufo marinus (Linne) in the Philippine Islands. Copeia 1952, 281–282.
| Preliminary notes on the giant toad Bufo marinus (Linne) in the Philippine Islands.Crossref | GoogleScholarGoogle Scholar |
Redhead, T. D. (1983). Common planigale. In ‘The Australian Museum Complete Book of Australian Mammals’. (Ed. R. Strahan.) p. 75. (Angus and Robertson: Sydney.)
Rossi, J. V. (1981). Bufo marinus in Florida: some natural history and its impact on native vertebrates. M.Sc. Thesis, Department of Botany, University of South Florida, Tampa.
Rossi, J. V. (1983). The use of olfactory cues by Bufo marinus. Journal of Herpetology 17, 72–73.
| The use of olfactory cues by Bufo marinus.Crossref | GoogleScholarGoogle Scholar |
Shine, R. (2010). The ecological impact of invasive cane toads (Bufo marinus) in Australia. The Quarterly Review of Biology 85, 253–291.
| The ecological impact of invasive cane toads (Bufo marinus) in Australia.Crossref | GoogleScholarGoogle Scholar |
Simmonds, H. W. (1957). The giant toad Bufo marinus in Fiji. Fiji Agricultural Journal 28, 77–78.
Smith, J. G., and Phillips, B. L. (2006). Toxic tucker: the potential impact of cane toads on Australian reptiles. Pacific Conservation Biology 12, 40–49.
Vitousek, P. M., Mooney, H. A., Lubchenco, J., and Melillo, J. M. (1997). Human domination of earth’s ecosystems. Science 277, 494–499.
| Human domination of earth’s ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkvVektLs%3D&md5=69e3c1b803e2f7e0b7c649abee57bf97CAS |
Weber, N. A. (1938). The food of the Giant Toad, Bufo marinus (L.), in Trinidad and British Guiana with special reference to the ants. Annals of the Entomological Society of America 31, 499–503.
|


