Breeding parameters of the Sooty Shearwater (Ardenna grisea) on Long Island, New Zealand
Amelia F. Geary A C D , Steve E. Corin B and Nicola J. Nelson AA Allan Wilson Centre for Molecular Ecology and Evolution, School of Biological Sciences, Victoria University of Wellington, PO Box 600, Wellington 6140, New Zealand.
B Synapt Consulting Limited, PO Box 25563, Wellington 6146, New Zealand.
C Present address: Department of Conservation, Private Bag, Port Fitzroy, Great Barrier Island 0963, New Zealand.
D Corresponding author. Email: amelia.geary@gmail.com
Emu 114(1) 74-79 https://doi.org/10.1071/MU13031
Submitted: 24 April 2013 Accepted: 7 June 2013 Published: 27 September 2013
Abstract
The Sooty Shearwater (Ardenna grisea) is one of the better studied petrels of New Zealand. Although one of New Zealand’s most abundant seabirds, smaller populations of Sooty Shearwaters may be less resilient to stochastic events in the long term. We investigated aspects of the breeding biology of Sooty Shearwaters and burrow dynamics on Long Island, in the Marlborough Sounds, New Zealand, from November 2008 to May 2009. Burrows were usually simple and unbranched, with an average length of 83.6 cm (s.e. 4.3 cm). The incubation period was 54.3 days (s.e. 1 day) and the nestling period 104.5 days (s.e. 2 days). Breeding success was 40.1%, within the range expected based on published studies of other populations of Sooty Shearwaters and of congeners. This study paves the way for further investigation into the viability and persistence of a small island population.
Introduction
The Sooty Shearwater (Ardenna grisea), also commonly known in New Zealand as the tītī or muttonbird, is an abundant seabird in New Zealand, with a population of >21 million individuals (Newman et al. 2009a). Of the total New Zealand population, 98% breeds on the satellite islands of Stewart Island and on islands of the Snares Group (Newman et al. 2009a), with the rest in a few mainland sites and on scattered islands along the New Zealand coast as far north as the Three Kings Islands (Warham and Wilson 1982). Breeding populations of Sooty Shearwaters are also found on islands off south-eastern Australia (Lane and White 1983) and Chile (Marin 1984).
Recent evidence suggests that Sooty Shearwater populations have declined worldwide (Scofield and Christie 2002; Moller et al. 2009), with declines reflected in the annual harvest of birds in New Zealand (Lyver et al. 1999), in observed densities in the North Pacific Ocean (Veit et al. 1996, 1997), and in a marked reduction in the density of burrows on some islands (Scott et al. 2008). Like many seabirds today, Sooty Shearwaters are affected by oceanic fisheries (Uhlmann et al. 2005), introduced predators (Jones et al. 2008) and increased frequency of climatic anomalies (Lyver et al. 1999). Small populations of Sooty Shearwaters have disappeared from predator-free islands in the Hauraki Gulf, New Zealand, in the last century (Scott et al. 2008).
The Marlborough Sounds have several islands with breeding populations of Sooty Shearwaters, many of which have never been studied. Biological data from small shearwater populations are needed to understand better the decline of Sooty Shearwaters in New Zealand. In this study, we obtained baseline data on the biology of Sooty Shearwaters on Long Island in the Marlborough Sounds, including burrow architecture, breeding phenology and breeding success and compared our results with published data on other populations of Sooty Shearwater and closely related species. Our overall aim was to understand better the basic life-history characteristics of the Long Island population of Sooty Shearwaters as a proxy to estimate the resilience to harvesting of other small populations of the Marlborough Sounds.
Methods
Study site
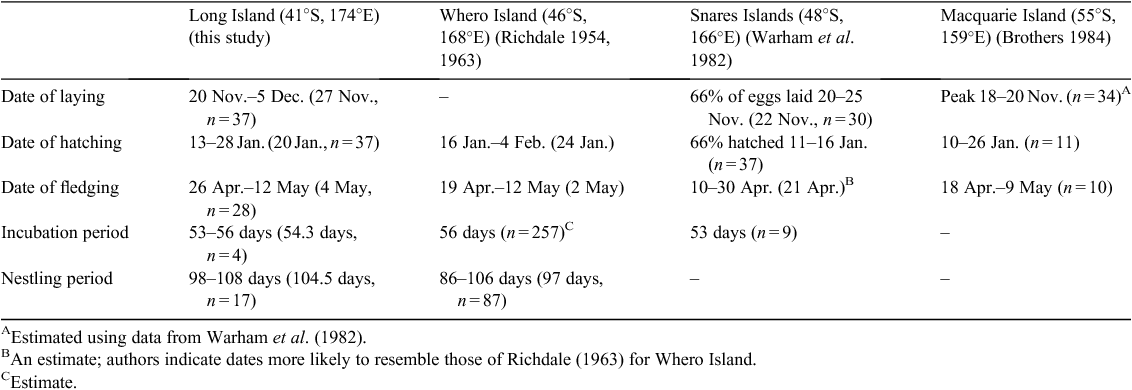
Long Island (41°07′S, 174°17′E) is in the Marlborough Sounds, at the northern end of the South Island of New Zealand. The island is a 142-ha, predator-free scenic reserve with a single colony of Sooty Shearwaters, intermixed with Fluttering Shearwaters (Puffinus gavia), restricted to an area of 1 ha at the northern tip of the island (Fig. 1). Sooty Shearwater nests were systematically identified through progressive inspection of all burrows on the northern part of the island. Burrows were inspected using a burrow-scope (TAUPE with orange LEDs, Sextant Technology Ltd, Wellington, New Zealand) (Dyer and Hill 1991) during the 2008–09 breeding season. Once the presence of a Sooty Shearwater was confirmed, the burrow was monitored daily using the burrow-scope until the chick fledged or nesting failed. Data were collected on 20–22 and 27–28 November 2008, 5–6 December 2008, 12–23 January 2009 and 15 April–14 May 2009.
|
Architecture, habitat and density of burrows
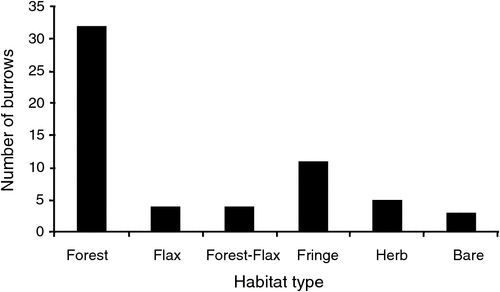
All Sooty Shearwater nests with an incubating adult present were inspected visually and the length of each burrow measured using markers spaced 10 cm apart on the burrow-scope. All plants growing within a radius of 1 m of the entrance to the burrow were recorded and identified to species level. Habitat types were then determined using a combination of species present and composition of the surrounding vegetation. ‘Forest’ was composed of two species of tree, Taupata (Coprosma repens) and Ngaio (Myporum laetum); ‘Flax’ (Phormium cookianum) was vegetation of that species only; ‘Forest–Flax’ is a combination of said habitats; ‘Herb’ describes burrows found entirely under coastal herbs such as Senecio sterquilinus, Apium prostratum, Disphyma australe and Einadia triandra; ‘Fringe’ describes combinations of Forest and Flax habitats with coastal herb vegetation; ‘Bare’ is bare ground with no vegetation growing within 1 m of the burrow entrance. We did not account for subterranean roots.
The density of burrows was determined by counting all Sooty Shearwater burrows within two randomly selected plots (108 and 188 m2) within the overall breeding colony. Sooty Shearwater burrows were identified by the presence of an incubating adult. Burrows of other nesting seabirds were not included in the analyses.
Breeding phenology
Burrows were visually inspected with burrow-scopes to determine the dates of laying and hatching. As Sooty Shearwaters lay only one egg per breeding attempt we recorded the laying and hatching dates as the first day an egg or chick was present when an empty burrow or an egg had been clearly sighted the day before. Similarly, the fledging date was recorded as the day the chick was no longer in the burrow.
Dates of laying or hatching that were missed were estimated from the incubation and nestling periods of nests with complete data. Eggs that did not hatch and for which laying date was not known were excluded from further analyses.
Breeding success
Breeding success was defined as rearing a chick to fledging (i.e. the chick no longer in the burrow). Burrows initially considered active based on only a single record of an adult Sooty Shearwater present early in the breeding season and with no confirmation of laying were removed from analysis as they may have been adults prospecting for a burrow (Bradley et al. 1999) or otherwise not breeding (Serventy 1967).
Breeding success was measured using the ‘Mayfield-40%’ method (Miller and Johnson 1978; Johnson 1979). The daily survival rate of eggs or chicks was calculated using:
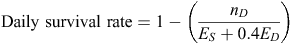
where nD is the number of nests that failed, ES is the combined total exposure of surviving and ED is the exposure of failed nests (‘exposure’ being the number of days the nest was in existence until an endpoint in the calculation, i.e. survival (hatching or fledging) or death). The daily survival rate could then be used to determine the probability of survival for any given period of the nesting cycle. We determined survival for two periods: the incubation period (from the day of laying to the day of hatching) and the nestling (or chick-rearing) period (from the date of hatching to the date of fledging). To estimate survival for each period of x days, the daily survival rate was raised to the power of x. Combining estimated survival for both periods gave the total probability of breeding success.
Standard errors (s.e.) were calculated using the method developed by Johnson (1979), using the equation:
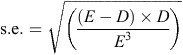
where E is exposure (number of nest days observed) and D is the number of deaths for that period.
Results
Architecture, habitat and density of burrows
The average length of Sooty Shearwater burrows on Long Island was 83.6 cm (s.e. 4.3, range 35–170, n = 51). Of the 51 burrows examined, all had a single nesting chamber; one burrow had two entrances whereas the rest had a single entrance. Density of occupied Sooty Shearwater burrows was estimated to be 0.06 burrows m–2. The tree Taupata was the dominant plant species growing within 1 m of 79.6% of Sooty Shearwater burrows. The frequency distribution of habitat types within a 1-m radius of burrow entrances is shown in Fig. 2.
|
Breeding phenology
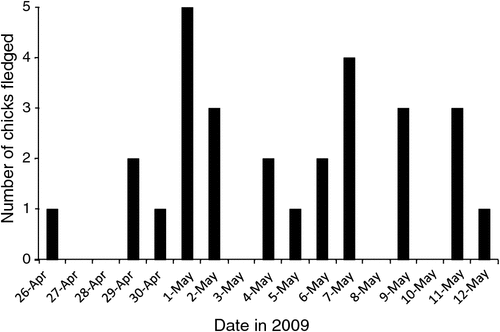
The incubation period in four nests for which the date of laying was directly observed was 54.3 days (s.e. 1 day, range 53–56). Laying in these four nests occurred on the night of 27 November. No eggs were found during the 20–22 November trip. When we returned on 5 December, all eggs had been laid. The date of laying for another 33 nests was estimated from the date of hatching using the mean incubation period. The combined data suggest laying occurred over a period of 16 days between 20 November and 5 December (n = 37). Similarly, the hatching period was estimated from the date of fledging using the average nestling period. The observed nestling period was 104.5 days (s.e. 2 days, range 98–109, n = 17) and the estimated mean date of hatching was 20 January (s.e. 3.5 days, range 13–28 January, n = 37). The mean date of fledging was 4 May (s.e. 4.3 days, range 26 April–12 May, n = 28; Fig. 3).
|
Breeding success
The survival of eggs to hatching was 62.4% (s.e. 0.2), which was 7.0% lower than the survival of chicks to fledging, 67.1% (s.e. 0.1). Overall breeding success was 40.1% (s.e. 0.01).
Causes of loss of eggs and chicks were difficult to determine. Competition for burrows with Little Penguins (Eudyptula minor) was likely to have been a significant cause of failure (A. F. Geary, pers. obs.). Of the chicks that hatched but failed to survive (n = 13), ~60% were provisioned for a minimum of 3 weeks before they died (Geary 2010).
Discussion
Architecture, habitat and density of burrows
The length of burrows on Long Island is very similar to that of burrows on Tītī Island, in Cook Strait, also in the Marlborough Sounds region (Geary 2010), but notably shorter than the average length of Sooty Shearwater burrows reported for three other islands off southern New Zealand (McKechnie et al. 2007) (Table 1). Average length of Sooty Shearwater burrows on Long Island are similar to those of other Ardenna species, such as Wedge-tailed Shearwaters (A. pacificus) on Heron and Erskine Islands, Australia (90 ± 4 cm; Dyer and Hill 1992), Buller’s Shearwaters (A. bulleri) on the Poor Knights Islands, New Zealand (101 ± 20 cm; Harper 1983), and Flesh-footed Shearwaters (A. carneipes), which range from 105 ± 2 cm on Woody Island, Australia (Powell et al. 2007), to 120 ± 10 cm on Lord Howe Island (Priddel et al. 2006). Measuring the depth of soil was beyond the scope of our study but we speculate that the prevalence of burrows near trees may be because the root system of the vegetation helps to stabilise the burrows.

|
Burrows on Long Island did not have the high levels of bifurcation and complexity found on islands off southern New Zealand, where large numbers of Sooty Shearwaters breed. For example, Hamilton (2000) found only 5% of burrow entrances led to a single chamber that was not connected to another. On Long Island, the density of occupied burrows was 0.06 whereas the mean density of burrows was 0.14 burrows m–2 (Geary 2010), which is comparable with the 0.08 burrows m–2 estimated for Tītī Island, although no distinction was made between occupancy by Sooty or Flesh-footed Shearwaters (Baker et al. 2009). The only similarly low density of Sooty Shearwater burrows was 0.09 burrows m–2 reported on Whenua Hou off Stewart Island (Charleton et al. 2009). These densities are much lower than those reported on islands nearer the centre of the breeding range of the species, which vary from 0.98 burrows m–2 (McKechnie et al. 2007) to 0.33 m–2 (Charleton et al. 2009). The lack of burrow complexity on Long Island may be a result of the small population and low density of Sooty Shearwaters in this colony (Geary 2010; A. F. Geary, pers. obs.) and possible reduced competition for space. Long Island also has a long human history. Sooty Shearwaters have historically been harvested from the island (A. F. Geary, unpubl. data) and early European attempts to farm the island resulted in extensive burning of the native vegetation which, in combination with of grazing stock, would almost certainly have had a negative impact on the island’s seabird populations. There are not, however, historical data on seabird populations and densities for Long Island, so it is not possible to determine whether burrow densities and complexity were higher in the past. It is possible that the low density of burrows and their simple structure is related to a population decline either from human influences or a reflection of the on-going decline in Sooty Shearwater numbers in New Zealand (e.g. Scott et al. 2008).
Breeding phenology
Sooty Shearwaters, along with most other migratory petrels, have highly synchronous annual cycles (Warham et al. 1982; Shaffer et al. 2006). The breeding chronology on Long Island is similar to that of other Sooty Shearwater populations occurring at much higher latitudes in New Zealand (Table 2) although laying and hatching on Long Island are possibly slightly later than for other populations, which may be an area for future study. Breeding synchrony has been reported in other migratory Ardenna species (e.g. Harper 1983; Cuthbert 2005; Powell et al. 2007). For example, in a study on Great Dog Island, Australia, up to 90% of Short-tailed Shearwater (A. tenuirostris) eggs were laid within 3 days of the mean laying date, with all eggs laid over a period of 16 days to 9 December (Meathrel et al. 1993).
Breeding success
The breeding success of 40.1% observed in the Long Island population fell within the annual variation reported for Sooty Shearwaters on the Snares Islands (mean breeding success 35%, range 7–67%), Whenua Hou, off Stewart Island (mean 76%, range 37–90%; Newman et al. 2009b) and Tuhawaiki Island, a predator-free islet off the Otago coast (mean 48 ± 2%; Jones et al. 2003). Our data also support the general pattern that most breeding failure occurs during the incubation period (Warham 1990). On Long Island, failure was 7% more likely during the incubation period than during the nestling period in the breeding season studied here.
Sooty Shearwaters can forage >2000 km from their breeding colonies (Shaffer et al. 2009). The ability of Sooty Shearwaters, and other seabirds, to travel vast distances and to forage for long periods (Weimerskirch 1998) may counteract any spatial inconsistencies in food availability in any given year (Shaffer et al. 2009). Of six Sooty Shearwaters tracked for one breeding season from Mana Island, which is near the Marlborough Sounds, only one bird travelled to more-productive oceanic waters to the south-east of the island and the rest remained in less-productive coastal waters (Shaffer et al. 2009). This pattern differed from the two other southern populations studied, which showed the use of multiple habitats, hypothesised to allow them greater flexibility in their resource acquisition (Shaffer et al. 2009). The distance of the Marlborough Sounds Sooty Shearwater colonies from productive Southern Ocean waters may affect breeding success and condition of juvenile Shearwaters compared with those populations breeding at higher latitudes. Studies of the breeding success and foraging range of different colonies, and the availability of food around colonies, would be a useful area of future research.
Conclusion
The breeding biology of the Sooty Shearwater population on Long Island is similar to that of populations at higher latitudes off southern New Zealand and to that of congeners. Although baseline biological data are provided here, longer term studies using standardised methods are needed to determine demographic parameters particular to the Marlborough Sounds to help assess annual variation in breeding success, the resilience of the population to traditional harvest and to guide future management.
Acknowledgements
Many thanks to the New Zealand Department of Conservation staff at Sounds Area Office for logistical support, and Peter Gaze at Nelson Marlborough Conservancy for technical support. Thanks to all the field assistants that helped collect data: Lorraine Cook, Ilse Corkery, Dawson Dunning, Fiona Hodge, Peter Martin, Frank Pega and Briar Smith. We appreciate the hard work of three anonymous reviewers who helped improve this manuscript. Thanks also to Dr Kristina Ramstad for assisting A. F. Geary during research for her M.Sc. Research was conducted under New Zealand Department of Conservation permits NM-25084 and NM-225556-RES and Victoria University of Wellington Animal Ethics Committee permit 2008R15. This study was made possible by a Te Tipu Pūtaiao grant from the New Zealand Foundation for Research Science and Technology (grant VUWX0801).
References
Baker, B., Hedley, G., Waugh, S., and Cunningham, R. (2009). Data collection of demographic, distributional and trophic information on the Flesh-footed Shearwater to allow estimation of effects of fishing on population viability: 2008–09 field season. Latitude 42 Environmental Consultants, Kettering, Tas.Bradley, J. S., Gunn, B. M., Skira, I. J., Meathrel, C. E., and Wooller, R. D. (1999). Age-dependent prospecting and recruitment to a breeding colony of Short-tailed Shearwaters Puffinus tenuirostris. Ibis 141, 277–285.
| Age-dependent prospecting and recruitment to a breeding colony of Short-tailed Shearwaters Puffinus tenuirostris.Crossref | GoogleScholarGoogle Scholar |
Brothers, N. P. (1984). Breeding, distribution and status of burrow-nesting petrels at Macquarie Island. Australian Wildlife Research 11, 113–131.
| Breeding, distribution and status of burrow-nesting petrels at Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
Charleton, K., Bragg, C., Knight, B., Fletcher, D., Moller, H., Newman, J., and Scott, D. (2009). Spatial variation in burrow entrance density of the Sooty Shearwater (Puffinus griseus). Notornis 56, 1–10.
Cuthbert, R. J. (2005). Breeding biology, chick growth and provisioning of Great Shearwaters (Puffinus gravis) at Gough Island, South Atlantic Ocean. Emu 105, 305–310.
| Breeding biology, chick growth and provisioning of Great Shearwaters (Puffinus gravis) at Gough Island, South Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Dyer, P. K., and Hill, G. J. E. (1991). A solution to the problem of determining the occupancy status of Wedge-tailed Shearwater Puffinus pacificus burrows. Emu 91, 20–25.
| A solution to the problem of determining the occupancy status of Wedge-tailed Shearwater Puffinus pacificus burrows.Crossref | GoogleScholarGoogle Scholar |
Dyer, P. K., and Hill, G. J. E. (1992). Active breeding burrows of the Wedge-tailed Shearwater in the Capricorn Group, Great Barrier Reef. Emu 92, 147–151.
| Active breeding burrows of the Wedge-tailed Shearwater in the Capricorn Group, Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Geary, A. F. (2010). Harvest and conservation of Sooty Shearwaters (Puffinus griseus) in the Marlborough Sounds, New Zealand. M.Sc. Thesis, Victoria University of Wellington, Wellington.
Hamilton, S. (2000). How precise and accurate are data obtained using an infra-red scope on burrow-nesting Sooty Shearwaters Puffinus griseus? Marine Ornithology 28, 1–6.
Harper, P. C. (1983). Biology of the Buller’s Shearwater (Puffinus bulleri) at the Poor Knights Islands, New Zealand. Notornis 30, 299–318.
Johnson, D. H. (1979). Estimating nest success, the Mayfield method and an alternative. Auk 96, 651–661.
Jones, C., Bettany, S., Moller, H., Fletcher, D., Lyver, P., and de Cruz, J. (2003). Burrow occupancy and productivity at coastal Sooty Shearwater (Puffinus griseus) breeding colonies, South Island, New Zealand: can mark-recapture be used to estimate burrowscope accuracy? Wildlife Research 30, 377–388.
| Burrow occupancy and productivity at coastal Sooty Shearwater (Puffinus griseus) breeding colonies, South Island, New Zealand: can mark-recapture be used to estimate burrowscope accuracy?Crossref | GoogleScholarGoogle Scholar |
Jones, H. P., Tershy, B. R., Zavaleta, E. S., Croll, D. A., Keitt, B. S., Finkelstein, M. E., and Howald, G. R. (2008). Severity of the effects of invasive rats on seabirds: a global review. Conservation Biology 22, 16–26.
| Severity of the effects of invasive rats on seabirds: a global review.Crossref | GoogleScholarGoogle Scholar | 18254849PubMed |
Lane, S. G., and White, G. (1983). Nesting of the Sooty Shearwater in Australia. Emu 83, 117–118.
| Nesting of the Sooty Shearwater in Australia.Crossref | GoogleScholarGoogle Scholar |
Lyver, P. O., Moller, H., and Thompson, C. (1999). Changes in Sooty Shearwater Puffinus griseus chick production and harvest precede ENSO events. Marine Ecology Progress Series 188, 237–248.
| Changes in Sooty Shearwater Puffinus griseus chick production and harvest precede ENSO events.Crossref | GoogleScholarGoogle Scholar |
Marin, A. M. (1984). Breeding record for the Sooty Shearwater (Puffinus griseus) from Chiloe Island, Chile. Auk 101, 192.
McKechnie, S., Fletcher, D., Moller, H., Scott, D. S., Newman, J., and Bragg, C. (2007). Estimating and correcting for bias in population assessments of Sooty Shearwaters. Journal of Wildlife Management 71, 1325–1335.
| Estimating and correcting for bias in population assessments of Sooty Shearwaters.Crossref | GoogleScholarGoogle Scholar |
Meathrel, C. E., Skira, I. J., Bradley, J. S., and Wooller, R. D. (1993). The influence of egg-size, mass and composition upon hatching success in the Short-tailed Shearwater Puffinus tenuirostris (Aves:Procellariiformes). Journal of Zoology 230, 679–686.
| The influence of egg-size, mass and composition upon hatching success in the Short-tailed Shearwater Puffinus tenuirostris (Aves:Procellariiformes).Crossref | GoogleScholarGoogle Scholar |
Miller, H. W., and Johnson, D. H. (1978). Interpreting the results of nesting studies. Journal of Wildlife Management 42, 471–476.
| Interpreting the results of nesting studies.Crossref | GoogleScholarGoogle Scholar |
Moller, H., Fletcher, D., Johnson, P. N., Bell, B. D., Flack, D., Bragg, C., Scott, D., Newman, J., McKechnie, S., and Lyver, P. O. B. (2009). Changes in Sooty Shearwater (Puffinus griseus) abundance and harvesting on the Rakiura Tītī Islands. New Zealand Journal of Zoology 36, 325–341.
| Changes in Sooty Shearwater (Puffinus griseus) abundance and harvesting on the Rakiura Tītī Islands.Crossref | GoogleScholarGoogle Scholar |
Newman, J., Scott, D., Bragg, C., McKechnie, S., Moller, H., and Fletcher, D. (2009a). Estimating regional population size and annual harvest intensity of the Sooty Shearwater in New Zealand. New Zealand Journal of Zoology 36, 307–323.
| Estimating regional population size and annual harvest intensity of the Sooty Shearwater in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Newman, J., Fletcher, D., Moller, H., Bragg, C., Scott, D., and McKechnie, S. (2009b). Estimates of productivity and detection probabilities of breeding attempts in the Sooty Shearwater (Puffinus griseus), a burrow-nesting petrel. Wildlife Research 36, 159–168.
| Estimates of productivity and detection probabilities of breeding attempts in the Sooty Shearwater (Puffinus griseus), a burrow-nesting petrel.Crossref | GoogleScholarGoogle Scholar |
Powell, C. D. L., Wooller, R. D., and Bradley, J. S. (2007). Breeding biology of the Flesh-footed Shearwater (Puffinus carneipes) on Woody Island, Western Australia. Emu 107, 275–283.
| Breeding biology of the Flesh-footed Shearwater (Puffinus carneipes) on Woody Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Priddel, D., Carlile, N., Fullagar, P., Hutton, I., and O’Neill, L. (2006). Decline in the distribution and abundance of Flesh-footed Shearwaters (Puffinus carneipes) on Lord Howe Island, Australia. Biological Conservation 128, 412–424.
| Decline in the distribution and abundance of Flesh-footed Shearwaters (Puffinus carneipes) on Lord Howe Island, Australia.Crossref | GoogleScholarGoogle Scholar |
Richdale, L. E. (1954). Duration of parental attentiveness in the Sooty Shearwater. Ibis 96, 586–600.
| Duration of parental attentiveness in the Sooty Shearwater.Crossref | GoogleScholarGoogle Scholar |
Richdale, L. E. (1963). Biology of the Sooty Shearwater Puffinus griseus. Proceedings of the Zoological Society of London 141, 1–117.
| Biology of the Sooty Shearwater Puffinus griseus.Crossref | GoogleScholarGoogle Scholar |
Scofield, R. P., and Christie, D. (2002). Beach patrol records indicate a substantial decline in Sooty Shearwater (Puffinus griseus) numbers. Notornis 49, 158–165.
Scott, D., Scofield, P., Hunter, C., and Fletcher, D. (2008). Decline of Sooty Shearwaters, Puffinus griseus, on the Snares, New Zealand. Papers and Proceedings of the Royal Society of Tasmania 142, 185–196.
Serventy, D. L. (1967). Aspects of the population ecology of the Short-tailed Shearwater Puffinus tenuirostris. Proceedings of the International Ornithological Congress 14, 338–343.
Shaffer, S. A., Tremblay, Y., Weimerskirch, H., Scott, D., Thompson, D. R., Sagar, P. M., Moller, H., Taylor, G. A., Foley, D. G., Block, B. A., and Costa, D. P. (2006). Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proceedings of the National Academy of Sciences of the United States of America 103, 12 799–12 802.
| Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptVyms78%3D&md5=5b05060018f768e630b23de1cf5abb34CAS |
Shaffer, S. A., Weimerskirch, H., Scott, D., Pinaud, D., Thompson, D. R., Sagar, P. M., Moller, H., Taylor, G. A., Foley, D. G., Tremblay, Y., and Costa, D. P. (2009). Spatiotemporal habitat use by breeding Sooty Shearwaters Puffinus griseus. Marine Ecology Progress Series 391, 209–220.
| Spatiotemporal habitat use by breeding Sooty Shearwaters Puffinus griseus.Crossref | GoogleScholarGoogle Scholar |
Uhlmann, S., Fletcher, D., and Moller, H. (2005). Estimating incidental takes of shearwaters in driftnet fisheries: lessons for the conservation of seabirds. Biological Conservation 123, 151–163.
| Estimating incidental takes of shearwaters in driftnet fisheries: lessons for the conservation of seabirds.Crossref | GoogleScholarGoogle Scholar |
Veit, R. R., Pyle, P., and McGowan, J. A. (1996). Ocean warming and long-term change in pelagic bird abundance within the California current system. Marine Ecology Progress Series 139, 11–18.
| Ocean warming and long-term change in pelagic bird abundance within the California current system.Crossref | GoogleScholarGoogle Scholar |
Veit, R. R., McGowan, J. A., Ainley, D. G., Wahls, T. R., and Pyle, P. (1997). Apex marine predator declines ninety percent in association with changing oceanic climate. Global Change Biology 3, 23–28.
| Apex marine predator declines ninety percent in association with changing oceanic climate.Crossref | GoogleScholarGoogle Scholar |
Warham, J. (1990). ‘The Petrels: Their Ecology and Breeding Systems.’ (Academic Press: London.)
Warham, J., and Wilson, G. J. (1982). The size of the Sooty Shearwater Puffinus griseus population at the Snares Islands, New Zealand. Notornis 29, 23–30.
Warham, J., Wilson, G. J., and Keeley, B. R. (1982). The annual cycle of the Sooty Shearwater Puffinus griseus at the Snares Islands, New Zealand. Notornis 29, 269–292.
Weimerskirch, H. (1998). How can a pelagic seabird provision its chick when relying on a distant food resource? Cyclic attendance at the colony, foraging decision and body condition in Sooty Shearwaters. Journal of Animal Ecology 67, 99–109.
| How can a pelagic seabird provision its chick when relying on a distant food resource? Cyclic attendance at the colony, foraging decision and body condition in Sooty Shearwaters.Crossref | GoogleScholarGoogle Scholar |