Museum collections in ornithology: today’s record of avian biodiversity for tomorrow’s world
Leo JosephAustralian National Wildlife Collection, CSIRO Ecosystem Sciences, PO Box 284, Canberra, ACT 2601, Australia. Email: leo.joseph@csiro.au
Emu 111(3) i-xii https://doi.org/10.1071/MUv111n3_ED
Published: 17 August 2011
Museum collections have always played a fundamental role in the study of avian biology. They underpin all classifications of birds and understanding of changes in bird populations on recent (Olsen et al. 1993) or historical time-scales (Donnellan et al. 2009). The widely scattered collections of birds pertinent to Emu’s southern hemisphere scope and readership are no exception (Mearns and Mearns 1998; Craig and Nuttall 2010). Here, my aim is to highlight the scientific importance of current bird collections, highlight issues that relate to their expansion and anticipate their ever-increasing diversity of scope and applications. This complements Gill’s (2006) inventory and analysis of 10 Australian and four New Zealand bird collections, which together comprise some 500 000 specimens. I address the vulnerability of collections at a time of variable and increasingly uncertain institutional support and touch on the scientific need for voucher specimens (especially in molecular genetics studies; Bates et al. 2004; Peterson et al. 2007) and the need for carefully and ethically conducted scientific collecting of birds (Remsen 1995). If has not already been dispelled, I also hope to dispel the whimsical image shown in Fig. 1.

|
Collections of natural history specimens once were the domain of the well to-do, the aristocracy and curious natural historians. With the advent of the theory of evolution by natural selection in July 1858, collections immediately became repositories of the evidence for and results of evolution. Along with contemporary databases of gene sequences, collections still serve this purpose. Also in the mid-19th century, the great colonial powers were scientifically exploring the world, especially the southern hemisphere. Discoveries in natural history were documented in the form of specimens, which poured into the now venerable museums of Europe and North America. By 1900, countries like Australia, New Zealand, Argentina, Brazil and present-day South Africa had established their own natural history museums in regional capitals. Critically, these were often established under and protected by a parliamentary act. Sensibly enough for the times, these museums each developed collections pertinent to the states or provinces of which they were capitals, though not exclusively so. The legacy is a multitude of collections that have always been a first port of call when preparing checklists, atlases and handbook-style reference works. They can now be linked electronically in ways never imagined by their founders.
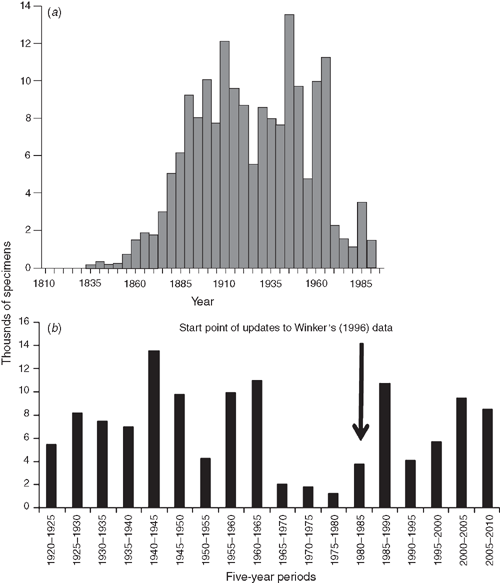
By the latter half of the 20th century, bird collecting had declined, often being seen outside museum circles as increasingly anachronistic, even unnecessary. Winker (1996) used data from one major North American collection to show the decline in specimens collected between 1950 and 1985–90. He called this part of the crumbling infrastructure of biodiversity. However, since the mid-1980s, that same collection and others have seen something of an upsurge in collecting (Fig. 2; Winker 2004), due in part to the advent of molecular techniques, renewed interest in the evolution of avian anatomy, and exploration of still little known areas. In Australia, for example, CSIRO’s Australian National Wildlife Collection (ANWC, Canberra) has added 22 400 bird specimens since 1985 and the Western Australian Museum have actively collected in the Pilbara and Kimberley regions of that state. We may ask whether more or fewer specimens should have been added in that time? How many birds died through anthropogenic causes in the same time? Are we now facing another episode of decline in collecting?
|
Several publications since Mearns and Mearns (1998) and Gill (2006) describe bird collections pertinent to Emu’s readership. The always impressive but never comprehensive collections from particular countries and regions are helpfully listed in a range of sources (e.g. Escalante-Pliego and Vuilleumier 1989 for the Neotropics). The uses of particular kinds of specimens are often evaluated (e.g. papers on sound, bones, eggs, genetic resources, electronic data, isotopes and contaminants accompanying Winker (2005)). The world’s cryofrozen avian tissues were reviewed by Stoeckle and Winker (2009) who only mentioned 1400 Australian specimens in the Australian Museum (>30 000 are held in the ANWC and the Australian Biological Tissue Collection, South Australian Museum). The general value and perception of collections is reasonably often articulated (Winker 1996), and increasingly so in high-level government reports (IWGSC 2009; Commonwealth of Australia 2009) and popular science literature (Suarez and Tsutsui 2004; Winker 2004; Cherry 2009; Lister and Climate Change Research Group 2011).
Some research is still totally dependent on museum specimens
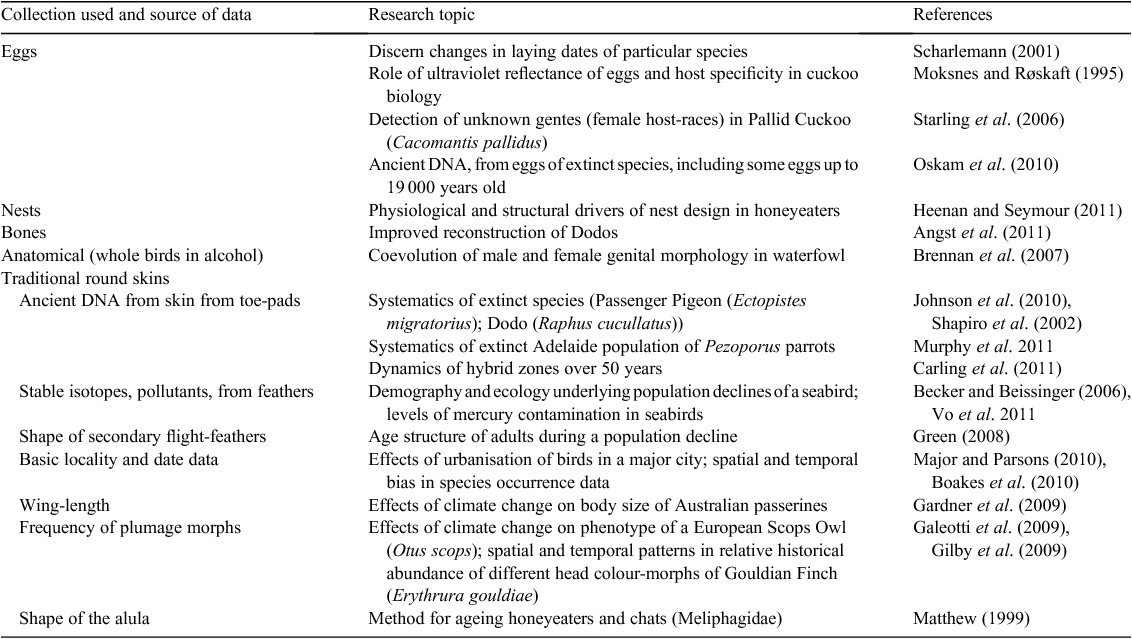
Specimens in collections continue to underpin primary research papers reporting diverse results that simply could not be achieved without ever-changing uses of specimens: from phylogenetic analyses on deep time-scales of evolutionary history through to present-day landscape connectivity. Table 1 lists noteworthy examples of recent research totally dependent on museum specimens. Even in a relatively well-known avifauna such as that of Australia, critically important research projects are still either dependent on existing specimens or effectively inhibited because critical specimens are not in collections. The following examples span a telling range of topics.
|
Is there an undescribed population of grasswrens Amytornis spp. in the southern Kimberleys of Western Australia? Had specimens been collected in or soon after 1991 when an individual of the alleged population was photographed, the matter would be settled (see Anonymous 1996). Searches by my group in 2010 followed much burning of the relevant area and were unsuccessful. So the mystery remains in 2011.
Conservation managers, taxonomists and birdwatchers alike frequently ask whether there are there two or more species of Cicadabird (Coracina tenuirostris) in Australia. The 19 cryofrozen tissues available in two collections (all vouchered) inadequately span the geographical range of the bird and would not be enough to disentangle movements of seasonally overlapping populations. They are unlikely to provide the basis for robust analyses of the issue to pass muster for publication in a peer-reviewed journal.
Similarly, how many species of shrike-tits (Falcunculus spp.) exist? There are no specimens of the western and northern populations of the Crested Shrike-tit (Falcunculus frontatus) appropriate for easy inclusion in a modern DNA-based comparison with the better-collected eastern populations. Apart from only being able to obtain limited DNA sequences from skins (see below) and whether the question of specific versus subspecific status of the shrike-tits is even meaningful given the geographical isolation of the various taxa (Joseph and Omland 2009), it is striking that those asking these questions expect an answer without new data from new specimens that few want to see collected. Well-labelled blood samples would help with the shrike-tits because the relevant populations are all geographically isolated from each other. In contrast, the Cicadabird problem needs vouchered specimens to sort out seasonal movements. Ornithologists of all stripes could join forces to start solving these problems.
Researchers trying to understand the conservation status of any species with few specimens (fewer than ~30) and mostly old specimens in collections (e.g. apparent decline of the Carpentarian Grasswren (Amytornis dorotheae)) cannot easily address population genetics with existing collections. There are simply insufficient specimens collected recently with tissue samples; using DNA from skins is technically difficult and likely to be inadequate.
To conserve and manage effectively the rarer and more poorly known waterbirds, such as Frecked Duck (Stictonetta naevosa) and Banded Stilt (Cladorhynchus leucocephalus), population dynamics need to be understood. Researchers need to be able to quickly receive the permits necessary to benefit from unusual weather events leading to breeding when the birds can be relatively easily sampled in large numbers.
Should a species be judged to be currently in decline, now is precisely the time at which one or two specimens would be a record for study of cause and nature of the declines and whether other species might be so affected. Green (2008) beautifully illustrated this. Using specimens of Corncrakes (Crex crex) collected during a population decline, he showed that lowered recruitment of young adults was a key contributing factor to the decline, which was ultimately caused by mechanisation of hay harvesting.
Illustrators of field guides are dependent on collections and are familiar with their incomplete nature in terms of having adequate material of all plumages, especially of distinctive juvenal and immature plumages. Specimens taken during the wet season across monsoonal northern Australia are a gap. I have heard these problems for all of my ornithological life but they remain valid. In January 2011, an illustrator for a forthcoming field guide to Australian birds consulted the ANWC for specimens of juvenal Myiagra flycatchers. He found very few specimens for his needs and thus those of field observers. This helps explain why collections are never complete. It reiterates the need for specimens from across all age-classes and the geographical range of a species. Also, the question of whether geographical patterns of variation in genetic and external characters are the same brings novel insights to population biology and history, and examples abound (Joseph and Wilke 2004, 2006; Kearns et al. 2010).
New research questions require new specimens. In recent years, my group has collected the following in response to specific requests from individual researchers: cloacal swabs of waterfowl for study of avian influenza; ectoparasitic lice and nematodes from feathers and internal cavities for taxonomic and evolutionary studies (Johnson et al. 2007); reproductive tracts of female waterfowl for studies of evolutionary biology of anseriform birds (see Brennan et al. 2007, 2010); enlarged testes of reproductively active males for a study of sperm morphology; stomach and crop contents, although their value is often compromised by grinding of the gizzard; and whole eyes for study of opsin gene evolution.
What’s in a bird specimen?
Bird collections fairly bristle with data for research. Consider that older museum specimens of birds usually had just a date of collection, a place name for a locality, maybe some basic natural history and biological notes on habitat, associations with other animals, and the collector’s name. A modern avian specimen, however, whether a skin, spread wing, skeleton or specimen in alcohol, should have all of those ancillary data as well as georeferenced locality, weight, amount and location on the skin of fat deposits, even if none are present, developmental state and size of reproductive organs and, for females, whether the oviduct is straight or convoluted (related to laying), degree of ossification of the skull (indicative of age) and presence or absence and size of the Bursa of Fabricius – an outgrowth of the lower alimentary tract associated with the development of the immune system but which is usually only present in the first 6 months of life. Routinely, we still note the fresh colours of exposed skin, which fades after death. This subjective practice was no doubt formerly more pioneering and cutting-edge than it is now. Its main point is still to pinpoint gross differences among age-classes (e.g. brown v. blue and white irides of immature and adult corvids), populations (colour of the skin around the eye in Galahs (Eolophus roseicapillus)), or closely related species (Melithreptus honeyeaters). Objective colour measurements, through reflectance spectrophotometry or other means, is rarely appropriate or indeed the point. Taking accompanying frozen tissue samples for genetic analyses is now de rigeur. Spread wings of all age-classes and both sexes of each species should be added to collections for the study of moult and for illustrators. The above is a minimum. Keeping everything that one could from every single bird accessioned into a collection unfortunately is not realistic. Nests and eggs are not easy to curate but when appropriate to gather them they should also be accompanied by date and a georeferenced locality. Especially for specimens collected on field trips, as distinct from salvaged material prepared in the laboratory, all or most accompanying data should be captured electronically in the field as well as on paper to facilitate inclusion in databases. I stress here that collections have a temporal element, as records of species in time not just geographical space.
Critically, there are probably fewer than 15 people in Australia who really know how to prepare and curate modern bird specimens and fewer still who could prepare birds, mammals, reptiles and amphibians. Unlike universities overseas, those in Australia have no strong tradition of teaching vertebrate ‘-ology’ courses, which is where collection management staff are often nurtured and trained if not in museums themselves.
Electronic linking of collections: a new historical phase
Table 2 includes recent electronic initiatives to link collections and make them searchable from desktops. The Online Zoological Collections of Australian Museums (URLs are found in Table 2) will eventually link all Australian museum collections and serve the Atlas of Living Australia. The Atlas will be a source of data on faunal and floral collections as well as other sources, such as Birds Australia’s Atlas of Australian Birds data. North American-based ORNIS is an initiative relevant to the readership of Emu as it currently includes 42 North American collections some of which have extensive southern hemisphere holdings. The Global Biodiversity Information Facility is an international initiative, begun and funded by government, focussed on making biodiversity data available to all, for scientific research, conservation and sustainable development. A key problem here is obtaining the funds to enter some large collections into databases. Electronic linking of collections emphasises their current nature as elements of a meta-collection. This contrasts with the historical basis of their establishment, in isolation from each other.

|
Where are the collections one needs?
For traditional voucher specimens (skins, skeletons, eggs), and their locality and date data, the major collections of southern hemisphere birds are indeed generally held in larger natural history museums of relevant countries (see inventories in Mearns and Mearns 1998; and Gill 2006; Table 2). Larger European and North American museums hold important 19th and early 20th century collections of southern hemisphere birds, especially type specimens to which scientific names are anchored. Data accompanying the earlier specimens may be scant but the historical record that their specimens hold is invaluable. Table 2 lists some of the more important ones.
The most significant library of Australian bird sounds is that of the ANWC in Canberra. Generous donations of digitised collections from professionally minded amateurs as well as earlier analogue recordings from CSIRO researchers are its key materials. Sound files are increasingly being uploaded to freely accessible websites but the sounds and data are not curated and archived to the standard that the major collections strive to achieve (see Table 2 for the website of a well-curated sound file).
‘Cinderella collections’ are those squirreled away and often languishing in universities or government departments and not protected by an act of parliament. Usually assembled for teaching purposes or by university academics for research, they often house extremely valuable material, especially of fossils and invertebrates, and occasionally skins of recently extinct birds. Ideally, they should be safely housed in an appropriate museum collection. Their long-term security poses severe challenges. They are not simple to move. They usually come with no funds to help in their housing or for addition to databases. They are a very current concern (Anonymous 2011). Perhaps most significant in ornithology today are privately held egg collections that have been amassed, shall we say, under the legal radar. At the ANWC, Ian Mason, working with governmental authorities, has succeeded in having many of these collections in Australia legalised and safely housed. It is vital that such valuable specimens are relocated (Russell et al. 2010).
Museum collections in today’s world
Why are such obviously useful and beneficial enterprises as collections so often written about so defensively as in the title, Why Museums Matter, of Collar et al.’s (2003) edited compilation? The answer is in the all too often declining state of funding for the natural history museums that usually house collections. Make no mistake – funding is tough and worsening for most museums. So, too, there is pressure on museums to somehow be more relevant to a society ever more concerned with fast-paced entertainment. Collections workers may struggle with how to be more relevant beyond housing, displaying, researching and making available such fundamental biodiversity data. We need to shake off cultural inhibitions, such as reluctance to speak to the media; we need to get our stories to the electronic and print media. Electronic initiatives mentioned earlier will do a better job of communicating our collection riches and potential uses. I have been told of three major natural history museums in Australia where budgets no longer have any significant operating allocations – funds to actually do any work funded by the museum itself. At the time of writing, six major museums in Australia have no dedicated doctorate-level (Ph.D.) research ornithologist, which is what the traditional position of curator has mostly become in museums. One’s instinct may be to decry this as an adverse development but in 2011 is it necessarily so? Should some such museum research curators be more integrative across vertebrates, not just birds, and work more with postgraduate students on a range of vertebrates? Species discovery is most active in Australia in ichthyology, herpetology and mammalogy, so perhaps they need different kinds of museum researchers than ornithology needs. Might ornithological positions in future be more usefully oriented to interfaces between systematics (from ordinal to species levels) and population genetics? Perhaps so, but these positions need to maintain their links to the roots of museum ornithology as well as to the growth and maintenance of collections; further, species-level taxonomy should not suffer.
Similarly, collection managers, who curate specimens and data often work across several ‘-ologies’, not just ornithology. As a result, bird collections in Australia and New Zealand are mostly being curated by collection managers who are variously stretched to look after their institutions’ collections of all vertebrates, depending on the museum. This does not seem an appropriate way to treat such valuable resources.
The strains felt by this limited number of collection managers as they perform a sterling job of servicing the research community can be seen in another ANWC example. Between 2006 and 2010 inclusive, the average number of specimen loans per year (i.e. regardless of what kind of specimen, and almost all involve multiple specimens) has been 72 – more than one a week, all year. Specimens had to be retrieved from the collection, paperwork done, specimens carefully packed and sent, and the loan itself entered to a database. Eventually, loans are returned and the whole process is repeated in reverse. This is typical of the day-to-day service work of most big collections.
Similarly, there is strong and increasing demand from students and researchers for frozen tissues, toe-pads or skin samples and even samples drilled from eggshells and bones for DNA-based work and other kinds of research. The labour to service these requests – which we should not forget is why the collections exist – is substantial. For example, in 2010 alone, the ANWC supplied 536 individual tissue samples to 18 Australian and nine overseas researchers. Each sample first had to be tracked through a database to its spot in the freezer. A subsample of each had to be taken and placed in ethanol, prepared for transport, the relevant paperwork and database entries had to be done, all necessary export and import permits obtained, and the material finally dispatched. The parent samples all had to be returned to the freezer. Recall also the effort and cost of collecting and curating these samples in the first place. To locate tissues of Australian material one should search the list of Australian and North American collections in Table 2. They have relevant holdings either because of their basic mission or because they have had staff working on Australian birds. It is worth reiterating here that preserved tissues and DNA sequences derived from them mean more when accompanied by a voucher specimen. Similarly, a DNA sequence adds value to a voucher specimen.
Notwithstanding negative facets of collection funding or the undeniable fact that many see collections as dusty anachronisms (dust is the last thing we want in collections), there is broad community and institutional support for collections in Australia. For example, I conducted seven major bird collecting expeditions and participated in or directed three others in Australia between 2001 and 2010, each requiring government support through collecting permits. At least five US-based museums have collected in Australia in the last 15 years. To be sure, scientific collecting of birds is unpalatable to many but the granting of permits by committees that try to represent community interests as well as the number and diversity of users of collections are all testimony to the broad, community-level support that collections still have. The Atlas of Living Australia (Table 2) also represents substantial government support for getting data from collections to the community.
Collections are not just about taxonomy
What of the demographic, geographical, temporal and ecological dimensions of collections? This also concerns the oft-asked question ‘Why do collections have so many specimens of one species’? First every specimen is different, and even genetically identical twins differ owing to non-genetic effects. Beyond that, the point is that collections document diversity at different hierarchical levels of individual, age, sex, population and geography. There is variation at the level of DNA as well as the external appearance of the bird. Genes are expressed (i.e. turned on and active), in different parts of an organism. All cells have all genes but not all are active in every cell or in different parts of a species’ range, or at different times of the year or of an individual’s life. To study all of these levels of variation for even the most common birds from across their geographical ranges is surprisingly difficult because collections have rarely been built so systematically.
Ponder what would be involved to document all of these levels of variation for even a common, widespread bird such as the Galah. Only since 2005 have we sampled tissues of Galahs from huge parts of their range, such as the Great Victoria Desert, the Kimberleys, western Cape York Peninsula, and large swathes of the Northern Territory. Toon et al. (2007) reported molecular phylogeography of the Australian Magpie (Gymnorhina tibicen) but had no access to any samples from most of inland Australia west of the Great Divide (see their Fig. 2). Despite adding a few from these gaps since then, there is little vouchered tissue from most of northern and inland Australia. More daunting to contemplate is such research in species, groups and remote areas that have not even been sampled for tissues. Three distinctive Australian birds – the Nullarbor Quail-thrush (Cinclosoma cinnamomeum alisteri), Western Quail-thrush (C. castaneothorax marginatum) and Naretha Blue Bonnet (Northiella haematogaster narethae) – were each sampled for tissues for the first time as late as 2004–08. Further, there are no tissue samples for a few Australian birds, apart from some specimens in alcohol, such as the Chestnut-breasted Whiteface (Aphelocephala pectoralis) and Banded Fruit-Dove (Ptilinopus cinctus) (as per Christidis and Boles 2008).
The relevance of collections to avian ecology is being reinvigorated and, again, the absolute dependence on museum collections for some of this work is reiterated. Examples pertinent to understanding climate change and population declines were cited above. Another active area is that of understanding reflectance within the ultraviolet wavelengths in avian biology. Eaton and Lanyon (2003) surveyed collections for 312 bird species from 142 families and documented the ubiquity among birds of plumage that reflects ultraviolet light. This is a foundation for study of its significance or otherwise in the biology of individual species, and certainly of its evolution. Similarly, Starling et al. (2006) applied reflectance spectrophotometry to eggs in collections and showed that the colour of the eggs of the Pallid Cuckoo (Cacomantis pallidus) does indeed closely mimic the eggs of the host in which they are laid. Thus, Pallid Cuckoos have cryptic gentes, or female-based host races, diagnosable on host-specific egg-types that had not been detected by humans in the spectra that are visible to us.
New methodologies drive new collections
From c. 1980, the technique of allozyme electrophoresis allowed indirect access to the genome of any individual of any natural population of any species and began the molecular revolution in population genetics and systematics. It was quickly followed by direct access to the genome, at first through mitochondrial DNA and then, fuelled by the advent of the polymerase chain reaction (PCR), to nuclear DNA. Rapid sequencing of complete genomes is the most obvious sign of the present and astounding pace of change. If allozymes were akin to knocking on the genome’s front door, today’s technologies allow us to go inside the genome’s house, rummage around in its rooms and open boxes hidden on the highest shelves.
Until c. 1980, collections regrettably did not cryofreeze internal organs from most avian specimens collected, most of which were preserved only as skins. Sadly, it just was not a part of the culture of collecting. Allozyme electrophoresis drove re-collection of many species and within regions to provide the raw materials – tissue samples with accompanying voucher specimens – with which researchers could address old, intractable questions as well as new ones, all needing data based on DNA sequences. In theory at least, museum collections acquired a new lease of life and this was realised in countries with the appropriate resources (e.g. Fig. 2). It fired a wave of collecting into new areas too, such as the explosion of work in the Neotropics by various North American and, eventually, South American museums and in Australia by a small group of Australian and North American researchers. This also helped stimulate collecting of avian skeletons and alcohol-preserved whole bodies for anatomical study.
The current revolution in genomics could drive another wave of collecting. Through RNA, not DNA, we can determine which genes are actively expressed in which organs and tissues of an individual as well as where in the geographical range of populations those genes are being expressed. This opens potentially new ways to study ecologically important genes and natural selection in changing environments. RNA is notoriously difficult to study, however, because unlike DNA it rapidly degrades post mortem. Simple freezing of tissues is not adequate. The tissues need to be immediately preserved in a solution such as RNAlater (Barrett et al. 1999). To date, there have been few collections of avian organs and tissues in this way (Wolf et al. 2010) and, apart from some of our own experimentation at the ANWC, I know of no systematic attempt to add these specimens to museum collections.
DNA can be obtained from dried museum skins, blood and feathers. This means that extinct and highly endangered species or populations can be included in genetic work (Table 1). Shephard et al. (2005) illustrate the value of careful molecular work with feathers from a species in which taking voucher specimens would have been inappropriate, ludicrous and, moreover, simply not permitted, the White-bellied Sea-Eagle (Haliaeetus leucogaster).
The use of blood samples is more complicated, and the risks of using blood as a source of mtDNA have often been explained (see Bates et al. 2004). Avian red blood-cells are nucleated and so multiple nuclear DNA copies, or paralogs, can exist of target mtDNA, which is rare in blood anyway. Genetic comparisons among individuals are valid when made between orthologous (like with like) pieces of DNA not paralogous ones (like with unlike). Bates et al. (2004) show how erroneous biogeographical conclusions could have appeared had the presence of paralogs not been detected. An even clearer example is a report of DNA allegedly from the time of dinosaurs but which turned out to be paralogs of the gene in question that were essentially contaminants from humans (see Woodward et al. 1994; and Hedges and Schweitzer 1995)! The use of blood or feathers should never obviate the need to anchor a study with some specimens lodged in a museum collection. I suggest that the need for this is inversely proportional to how well known a population already is.
Students and non-systematists interested in including DNA in their work often believe that DNA can be obtained from skins easily enough. Unfortunately DNA does degrade and as museum skins age, shorter and shorter sequences are obtainable. Skins are thus far from optimal sources of DNA.A If there is substantial divergence in a piece of DNA that can be recovered consistently from all the available skins, then skin-based DNA work may suit the kind of taxonomic question posed above for Cicadabirds and Crested Shrike-tits. The study of Pezoporus parrots cited in Table 1 benefited from a major phylogenetic break existing between eastern and western Australian populations but still involved just one individual of an extinct population. Also, skins cannot easily provide long pieces of DNA one needs for extensive sampling at the level of nucleotides to answer questions of population size, genetic connectivity and dispersal. Regardless of how many individuals are sampled, this limits the statistical and biological power in analyses and results. Further, it is still not widely grasped that the protocols for extracting DNA from skins are far more complicated and fraught with risk of obtaining erroneous data relative to those involving fresh tissue samples (Austin et al. 1997; Willerslev and Cooper 2005). In short, this way of thinking should be focussed on extinct and highly endangered species or populations or those critically necessary to include but difficult to sample afresh.
Museum collections face challenges in whether they should be archiving DNA extracts from non-vouchered blood and feathers, which can be excellent sources of DNA (e.g. Hogan et al. 2008). The alternative is discarding the DNA when students and researchers move on. Then the work itself is not repeatable. Notwithstanding the importance of vouchering tissue samples, museums should confront the facts that valuable DNA is being extracted from unvouchered blood and feathers and that it needs to be safeguarded, somewhere. To be sure there will be some low frequency of misidentified specimens but that is why repeatability is important. Archiving unvouchered material is a further cultural challenge for many museum workers, but perhaps museum collections, not university laboratories, are the most logical place to archive these DNA extracts. Do we have the staff and resources?
Stable isotopes are among the most exciting new uses for collections, especially as they relate to how specimens can document changes in physical environments over time. Elements such as carbon and hydrogen exist in various isotopic forms. Some are unstable and radioactive (think of carbon dating with the radioactive carbon isotope 14C), but many isotopes are stable. There is often more than one stable isotope of a given element. The ratio at which these isotopes exist at a point in the environment can be mapped as an ‘isoscape’, analogous to a map of rainfall isohyets or temperature isotherms. Feathers retain signatures of stable isotope ratios from the environments in which the feathers grew, thus providing a basis for studying temporal changes in isotopic signatures. Potentially, then, if a continent’s isoscape has been mapped, stable isotopes provide a means of knowing the movements of an individual bird without having to capture, mark, release and recapture. Insights into breeding biology have been gained by so linking how and when an individual bird uses different quality habitats in its breeding and non-breeding grounds (Marra et al. 1998). The potential for using museum specimens, especially old ones, in this work is vast: although they have little more than their date and locality, their age imparts value. Becker and Beissinger (2006) compared isotopic signatures from 50-year-old museum specimens of Marbled Murrelets (Brachyramphos marmoratus) with present-day birds. They showed that the birds now feed at a lower trophic level than in the past. Overfishing has depleted their earlier, more nutritious food source, which was at a higher trophic level, and so forced the birds to adopt a less-nutritious diet. A major implication of these examples is that collections need to grow to maintain the temporal element, and not just the spatial and taxonomic elements, of their value.
Permits and ethical considerations
Scientific collecting of birds must be rigorously controlled by a permit system. Increasingly, in Australia at least, an ethics committee decides what can be submitted to a second committee that separately processes applications for scientific collecting. Some ethics committees involve lay members of the community who represent broad community interests, and for whom laboriously detailed explanations and justifications of the scientific work are required. The time taken here is fair but costly for the community. A risk is that opportunities to grow the temporal value of collections as well as that of slowly building the number of modern specimens available for common species might be compromised. Increasingly in Australia, one may require permission from indigenous landowners to enter and collect on land under their control. This can be very time-consuming and culturally challenging for all concerned but is, of course, well worth doing. Opportunities to work with indigenous groups might even arise. For example, many an Australian Bustard (Ardeotis australis) ends up in the cooking pot but might we all work together to preserve tissues and other biological data from these birds?
In awarding permits, consistent decisions must be based on the benefit of having the specimens versus the cost of not collecting. Decisions on allowing permits should be rapid where necessary (e.g. when waterbirds congregate after unpredictable extreme weather events), fair and helpful.
All species suffer rates of mortality from natural sources that are far higher than occasional scientific collecting without hastening any decline. Consider the effects of episodes of mass mortality, natural or otherwise (e.g. up to 4000 birds of several species near Esperance in December 2006 and January 2007; DEC 2007), as well as predation, starvation, disease, brood parasites, and loss of habitat. Humans too cause deaths of millions of birds, thus far outweighing the numbers taken for collections (see Banks 1979).
Collections into the future
The challenges of ensuring collections remain safe and available for research for hundreds of years can be political as well as practical, and ought not to be underestimated, especially in the tropics. Collections in Australia and New Zealand have in 2011 been tested severely by major floods and earthquakes and generally survived well. The Museu Goeldi (Belém, Brazil) has successfully dealt with challenges of equatorial heat and humidity. Cheke (2003) described challenges faced by a museum in Mauritius, noting that knowing what to do and how to do it can be outweighed by the socio-political challenges. Few collections are housed in ideal conditions: purpose-built modern buildings with truly appropriate cabinetry, in climate-controlled, secure vaults physically isolated from work areas. The USA’s National Science Foundation awards collection improvement grants and their long-term benefit in securing a diversity of natural history collections is profound. Can we wish for something similar in other countries, including Australia? Most Australian bird collections are now in locked vaults physically isolated from work areas. The latter criterion was rarely if ever accounted for in most natural history museums but is increasing in frequency as collections and their staff are rehoused in purpose-built facilities. Climate control and archival quality cabinetry, trays and paper for storage are also vital for the long-term survival of collections and are still fairly rare among collections.
Conclusions
The roll call of topics that can only be addressed with museum collections is growing, as is community demand for knowledge that absolutely requires collections (Table 1). Climate change and its effect on natural populations are among the most obvious examples. Just as the collections of yesteryear help us today to understand environmental change, so too tomorrow’s researchers will need collections from today. Yet bird collections to enable that are growing patchily at best. The need to document and understand biodiversity is a catchcry, yet museum collections are variously having either wonderful ups or some very worrying downs. Collections do not exist in vacuums – they exist in parental institutions where funding and other support are erratic and patchy at best and declining or non-existent at worst. Collections face enormous political, financial, physical and technological challenges in being safely housed into the future. Collections are as much about the people who work in them, the people who utilise their resources and their role in securing their future as they are about the actual specimens and data in the collections and the buildings in which they are housed. Administrators of collection institutions and those working in collections need to remember that they will be judged by how well they secure the future of collections and the passionate people who want to work in them. If we can build the means for funding positions in museum biology, then the passionate and talented people to work in them will come.
Acknowledgements
I thank the Editor, Kate Buchanan, for her persistence in inviting me to write this review and for her comments on drafts. Margaret Cawsey, Michael Cherry, Philippa Horton, John La Salle, Joe Miller, Penny Olsen, Maya Penck, Steve Pruett-Jones and an anonymous reviewer all thoughtfully commented on drafts, and I hope a more balanced result has ensued. Walter Boles, Adrian Craig, James Dean, Ron Johnstone, Wayne Longmore, Golo Maurer, Claire Stevenson, Paul Sweet and Gary Voelker all helped with information. I gratefully acknowledge all permit-granting authorities who have permitted me, and all before and after me, to collect birds.
References
Angst, D., Buffetaut, E., and Abourachid, A. (2011). The end of the fat Dodo? A new mass estimate for Raphus cucullatus. Naturwissenschaften 98, 233–236.| The end of the fat Dodo? A new mass estimate for Raphus cucullatus.Crossref | GoogleScholarGoogle Scholar |
Anonymous, (1996). What’s this grasswren? Wingspan 6, 25.
Anonymous, (2011). Preserve the past. Nature 470, 5–6.
| Preserve the past.Crossref | GoogleScholarGoogle Scholar |
Austin, J., Smith, A. B., and Thomas, R. H. (1997). Palaeontology in a molecular world: the search for authentic ancient DNA. Trends in Ecology & Evolution 12, 303–306.
| Palaeontology in a molecular world: the search for authentic ancient DNA.Crossref | GoogleScholarGoogle Scholar |
Banks, R. C. (1979). Human related mortality of birds in the United States. Special Scientific Report – Wildlife No. 215, United States Department of the Interior Fish and Wildlife Service, Washington, DC.
Barrett, M. T., Glogovac, J., Porter, P., Reid, B. J., and Rabinovitch, P. S. (1999). High yields of RNA and DNA suitable for array analysis from cell sorter purified epithelial cell and tissue populations. Nature Genetics 23, 32–33.
| High yields of RNA and DNA suitable for array analysis from cell sorter purified epithelial cell and tissue populations.Crossref | GoogleScholarGoogle Scholar |
Bates, J. M., Bowie, R. C. K., Willard, D. E., Voelker, G., and Kahindo, C. (2004). A need for continued collecting of avian voucher specimens in Africa: why blood is not enough. Ostrich 75, 187–191.
| A need for continued collecting of avian voucher specimens in Africa: why blood is not enough.Crossref | GoogleScholarGoogle Scholar |
Becker, B., and Beissinger, S. (2006). Centennial decline in the trophic level of an endangered seabird after fisheries decline. Conservation Biology 20, 470–479.
| Centennial decline in the trophic level of an endangered seabird after fisheries decline.Crossref | GoogleScholarGoogle Scholar |
Boakes, E. H., McGowan, P. J. K., Fuller, R. A., Chang-qing, D., Clark, N. E., O’Connor, K., and Mace, G. M. (2010). Distorted views of biodiversity: spatial and temporal bias in species occurrence data. PLoS Biology 8, e1000385.
| Distorted views of biodiversity: spatial and temporal bias in species occurrence data.Crossref | GoogleScholarGoogle Scholar |
Brennan, P. L. R., Prum, R. O., McCracken, K. G., Sorenson, M. D., Wilson, R. E., and Birkhead, T. R. (2007). Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2, e418.
| Coevolution of male and female genital morphology in waterfowl.Crossref | GoogleScholarGoogle Scholar |
Brennan, P., Clark, C., and Prum, R. (2010). Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia. Proceedings of the Royal Society of London. Series B. Biological Sciences 277, 1309–1314.
| Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia.Crossref | GoogleScholarGoogle Scholar |
Carling, M., Serene, L. G., and Lovette, I. J. (2011). Using historical DNA to characterize hybridization between Baltimore Orioles (Icterus galbula) and Bullock’s Orioles (I. bullockii). Auk 128, 61–68.
| Using historical DNA to characterize hybridization between Baltimore Orioles (Icterus galbula) and Bullock’s Orioles (I. bullockii).Crossref | GoogleScholarGoogle Scholar |
Cheke, A. S. (2003). Treasure Island – the rise and decline of a small tropical museum, the Mauritius Institute. Bulletin of the British Ornithologists’ Club 123A, 197–206.
Cherry, M. (2009). What can museum and herbarium collections tell us about climate change? South African Journal of Science 105, 87–88.
Christidis, L., and Boles, W. E. (2008). ‘Systematics and Taxonomy of Australian Birds.’ (CSIRO Publishing: Melbourne.)
Collar, N., Fisher, C., and Feare, C. (Eds) (2003). Why museums matter: avian archives in an age of extinction. Bulletin of the British Ornithologists’ Club 123A, 1–360.
Commonwealth of Australia (2009). Powering ideas: an innovation agenda for the 21st century. Commonwealth of Australia, Canberra. Available at http://www.innovation.gov.au/innovation/policy/pages/PoweringIdeas.aspx [Verified 10 July 2011].
Craig, A. J. F. K., and Nuttall, R. J. (2010). The current status of ornithological collections in southern African museums. Journal of Afrotropical Zoology 6, 111–115.
DEC (2007). Update # 2: Esperance bird deaths. Department of Environment and Conservation (WA). Available at http://portal.environment.wa.gov.au/pls/portal/docs/PAGE/DOE_ADMIN/MEDIA_REPOSITORY/TAB108326/TAB6428377/BIRD_UPDATE23032007.PDF [Verified 10 July 2011].
Donnellan, S., Armstrong, J., Pickett, M., Milne, T., Baulderstone, J., Hollfelder, T., and Bertozzi, T. (2009). Systematic and conservation implications of mitochondrial DNA diversity in emu-wrens, Stipiturus (Aves : Maluridae). Emu 109, 143–152.
| Systematic and conservation implications of mitochondrial DNA diversity in emu-wrens, Stipiturus (Aves : Maluridae).Crossref | GoogleScholarGoogle Scholar |
Eaton, M. D., and Lanyon, S. M. (2003). The ubiquity of avian ultraviolet plumage reflectance. Proceedings of the Royal Society of London. Series B. Biological Sciences 270, 1721–1726.
| The ubiquity of avian ultraviolet plumage reflectance.Crossref | GoogleScholarGoogle Scholar |
Escalante-Pliego, P., and Vuilleumier, F. (1989). ‘Directorio de Colecciones Ornitológicas en los Países de la América Neotropical.’ (American Museum of Natural History: New York.)
Galeotti, P., Rubolini, D., Sacchi, R., and Fasola, M. (2009). Global changes and animal phenotypic responses: melanin-based plumage redness of Scops Owls increased with temperature and rainfall during the last century. Biology Letters 5, 532–534.
| Global changes and animal phenotypic responses: melanin-based plumage redness of Scops Owls increased with temperature and rainfall during the last century.Crossref | GoogleScholarGoogle Scholar |
Gardner, J., Heinsohn, R., and Joseph, L. (2009). Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines. Proceedings of the Royal Society of London. Series B. Biological Sciences 276, 3845–3852.
| Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines.Crossref | GoogleScholarGoogle Scholar |
Gilby, A. J., Pryke, S. R., and Griffith, S. C. (2009). The historical frequency of head-colour morphs in the Gouldian Finch (Erythrura gouldiae). Emu 109, 222–229.
| The historical frequency of head-colour morphs in the Gouldian Finch (Erythrura gouldiae).Crossref | GoogleScholarGoogle Scholar |
Gill, B. J. (2006). Birds in Australian and New Zealand museums – a major resource for ornithology. New Zealand Journal of Zoology 33, 299–315.
| Birds in Australian and New Zealand museums – a major resource for ornithology.Crossref | GoogleScholarGoogle Scholar |
Green, R. E. (2008). Demographic mechanism of a historical bird population collapse reconstructed using museum specimens. Proceedings of the Royal Society of London. Series B. Biological Sciences 275, 2381–2387.
| Demographic mechanism of a historical bird population collapse reconstructed using museum specimens.Crossref | GoogleScholarGoogle Scholar |
Hedges, S. B., and Schweitzer, M. (1995). Detecting dinosaur DNA. Science 268, 1191–1192.
| Detecting dinosaur DNA.Crossref | GoogleScholarGoogle Scholar |
Heenan, C. B., and Seymour, R. S. (2011). Structural support, not insulation, is the primary driver for avian cup-shaped nest design. Proceedings of the Royal Society of London. Series B. Biological Sciences , .
| Structural support, not insulation, is the primary driver for avian cup-shaped nest design.Crossref | GoogleScholarGoogle Scholar |
Hogan, F., Cooke, R., Burridge, C., and Norman, J. (2008). Optimizing the use of shed feathers for genetic analysis. Molecular Ecology Resources 8, 561–567.
| Optimizing the use of shed feathers for genetic analysis.Crossref | GoogleScholarGoogle Scholar |
IWGSC (2009). Scientific collections: mission-critical infrastructure for Federal science agencies. Interagency Working Group on Scientific Collections, National Science and Technology Council, Committee on Science, Washington, DC.
Johnson, K., Reed, D., Parker, S. L. H., Kim, D., and Clayton, D. (2007). Phylogenetic analysis of nuclear and mitochondrial genes supports species groups for Columbicola (Insecta : Phthiraptera). Molecular Phylogenetics and Evolution 45, 506–518.
| Phylogenetic analysis of nuclear and mitochondrial genes supports species groups for Columbicola (Insecta : Phthiraptera).Crossref | GoogleScholarGoogle Scholar |
Johnson, K., Clayton, D., Dumbacher, J., and Fleischer, R. (2010). The flight of the Passenger Pigeon: phylogenetics and biogeographic history of an extinct species. Molecular Phylogenetics and Evolution 57, 455–458.
| The flight of the Passenger Pigeon: phylogenetics and biogeographic history of an extinct species.Crossref | GoogleScholarGoogle Scholar |
Joseph, L., and Omland, K. (2009). Phylogeography: its development and impact in Australo-Papuan ornithology with special reference to paraphyly in Australian birds. Emu 109, 1–23.
| Phylogeography: its development and impact in Australo-Papuan ornithology with special reference to paraphyly in Australian birds.Crossref | GoogleScholarGoogle Scholar |
Joseph, L., and Wilke, T. (2004). When DNA throws a spanner in the taxonomic works: testing for monophyly in the Dusky-capped Flycatcher Myiarchus tuberculifer and its South American subspecies M. t. atriceps Cabanis, 1883. Emu 104, 197–204.
| When DNA throws a spanner in the taxonomic works: testing for monophyly in the Dusky-capped Flycatcher Myiarchus tuberculifer and its South American subspecies M. t. atriceps Cabanis, 1883.Crossref | GoogleScholarGoogle Scholar |
Joseph, L., and Wilke, T. (2006). Molecular resolution of population history, systematics and historical biogeography of the Australian ringneck parrots Barnardius: are we there yet? Emu 106, 49–62.
| Molecular resolution of population history, systematics and historical biogeography of the Australian ringneck parrots Barnardius: are we there yet?Crossref | GoogleScholarGoogle Scholar |
Kearns, A., Joseph, L., and Cook, L. (2010). The impact of Pleistocene climatic and landscape changes on Australian birds: a test using the Pied Butcherbird (Cracticus nigrogularis). Emu 110, 285–295.
| The impact of Pleistocene climatic and landscape changes on Australian birds: a test using the Pied Butcherbird (Cracticus nigrogularis).Crossref | GoogleScholarGoogle Scholar |
Lister, A. M., Climate Change Research Group (2011). Natural history collections as sources of long-term datasets. Trends in Ecology & Evolution 26, 153–154.
| Natural history collections as sources of long-term datasets.Crossref | GoogleScholarGoogle Scholar |
Major, R. E., and Parsons, H. (2010). What do museum specimens tell us about the impact of urbanisation? A comparison of the recent and historical bird communities of Sydney. Emu 110, 92–103.
| What do museum specimens tell us about the impact of urbanisation? A comparison of the recent and historical bird communities of Sydney.Crossref | GoogleScholarGoogle Scholar |
Marra, P. P., Hobson, K. A., and Holmes, R. T. (1998). Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886.
Matthew, J. S. (1999). A new method for ageing some species of Meliphagidae. Corella 23, 69–71.
Mearns, B., and Mearns, R. (1998). ‘The Bird Collectors.’ (Academic Press: London.)
Moksnes, A., and Røskaft, E. (1995). Egg-morphs and host preference in the Common Cuckoo (Cuculus canorus): an analysis of Cuckoo and host eggs from European museum collections. Journal of Zoology 236, 625–648.
| Egg-morphs and host preference in the Common Cuckoo (Cuculus canorus): an analysis of Cuckoo and host eggs from European museum collections.Crossref | GoogleScholarGoogle Scholar |
Murphy, S., Joseph, L., Burbidge, A., and Austin, J. (2011). A cryptic and critically endangered species revealed by mitochondrial DNA analyses – the Western Ground Parrot. Conservation Genetics 12, 595–600.
| A cryptic and critically endangered species revealed by mitochondrial DNA analyses – the Western Ground Parrot.Crossref | GoogleScholarGoogle Scholar |
Olsen, P., Fuller, P., and Marples, T. (1993). Pesticide-related eggshell thinning in Australian raptors. Emu 93, 1–11.
| Pesticide-related eggshell thinning in Australian raptors.Crossref | GoogleScholarGoogle Scholar |
Oskam, C., Haile, J., McLay, E., Rigby, P., Allentoft, M., Olsen, M., Bengtsson, C., Miller, G., Schwenninger, J.-L., Jacomb, C., Walter, R., Baynes, A., Dortch, J., Parker-Pearson, M., Thomas, M., Gilbert, P., Holdaway, R. N., Willerslev, E., and Bunce, M. (2010). Fossil avian eggshell preserves ancient DNA. Proceedings of the Royal Society of London. Series B. Biological Sciences 277, 1991–2000.
| Fossil avian eggshell preserves ancient DNA.Crossref | GoogleScholarGoogle Scholar |
Parkes, K. C. (1963). The contribution of museum collections to knowledge of the living bird. Living Bird 2, 121–130.
Peterson, A. T., Moyle, R. G., Nyari, A., Robbins, M. B., Brumfield, R. T., and Remsen, J. V. (2007). The need for proper vouchering in phylogenetic studies of birds. Molecular Phylogenetics and Evolution 45, 1042–1044.
| The need for proper vouchering in phylogenetic studies of birds.Crossref | GoogleScholarGoogle Scholar |
Remsen, J. V. (1995). The importance of continued collecting of bird specimens to ornithology and bird conservation. Bird Conservation International 5, 145–180.
| The importance of continued collecting of bird specimens to ornithology and bird conservation.Crossref | GoogleScholarGoogle Scholar |
Roselaar, C. S. (2003). An inventory of major European bird collections. Bulletin of the British Ornithologists’ Club 123A, 253–337.
Russell, D. G. D., White, J., Maurer, G., and Cassey, P. (2010). Data-poor egg collections: cracking an important research resource. Journal of Afrotropical Zoology 6, 77–82.
Scharlemann, J. P. (2001). Museum egg collections as stores of long-term phenological data. International Journal of Biometeorology 45, 208–211.
| Museum egg collections as stores of long-term phenological data.Crossref | GoogleScholarGoogle Scholar |
Shapiro, B., Sibthorpe, D., Rambaut, A., Austin, J., Wragg, G. M., Bininda-Emonds, O. R. P., Lee, P. L. M., and Cooper, A. (2002). Flight of the Dodo. Science 295, 1683.
| Flight of the Dodo.Crossref | GoogleScholarGoogle Scholar |
Shephard, J., Hughes, J., Catterall, C., and Olsen, P. (2005). Conservation status of the White-bellied Sea-Eagle Haliaeetus leucogaster in Australia determined using mtDNA control region sequence data. Conservation Genetics 6, 413–429.
| Conservation status of the White-bellied Sea-Eagle Haliaeetus leucogaster in Australia determined using mtDNA control region sequence data.Crossref | GoogleScholarGoogle Scholar |
Starling, M., Heinsohn, R., Cockburn, R., and Langmore, N. (2006). Cryptic gentes revealed in Pallid Cuckoos Cuculus pallidus using reflectance spectrophotometry. Proceedings of the Royal Society of London. Series B. Biological Sciences 273, 1929–1934.
| Cryptic gentes revealed in Pallid Cuckoos Cuculus pallidus using reflectance spectrophotometry.Crossref | GoogleScholarGoogle Scholar |
Stoeckle, M., and Winker, K. (2009). A global snapshot of avian tissue collections: state of the enterprise. Auk 126, 684–687.
| A global snapshot of avian tissue collections: state of the enterprise.Crossref | GoogleScholarGoogle Scholar |
Suarez, A. V., and Tsutsui, N. D. (2004). The value of museum collections for research and society. Bioscience 54, 66–74.
| The value of museum collections for research and society.Crossref | GoogleScholarGoogle Scholar |
Toon, A., Mather, P. B., Baker, A. M., Durrant, K. L., and Hughes, J. M. (2007). Pleistocene refugia in an arid landscape: analysis of a widely distributed Australian passerine. Molecular Ecology 16, 2525–2541.
| Pleistocene refugia in an arid landscape: analysis of a widely distributed Australian passerine.Crossref | GoogleScholarGoogle Scholar |
Vo, A.-T., Bank, M. S., Shine, J. P., and Edwards, S. V. (2011). Temporal increase in organic mercury in an endangered pelagic seabird assessed by century-old museum specimens. Proceedings of the National Academy of Sciences of the United States of America 108, 7466–7471.
| Temporal increase in organic mercury in an endangered pelagic seabird assessed by century-old museum specimens.Crossref | GoogleScholarGoogle Scholar |
Willerslev, E., and Cooper, A. (2005). Ancient DNA. Proceedings of the Royal Society of London. Series B. Biological Sciences 272, 3–16.
| Ancient DNA.Crossref | GoogleScholarGoogle Scholar |
Winker, K. (1996). The crumbling infrastructure of biodiversity: the avian example. Conservation Biology 10, 703–707.
| The crumbling infrastructure of biodiversity: the avian example.Crossref | GoogleScholarGoogle Scholar |
Winker, K. (2004). Natural history museums in a postbiodiversity era. Bioscience 54, 455–459.
| Natural history museums in a postbiodiversity era.Crossref | GoogleScholarGoogle Scholar |
Winker, K. (2005). Bird collections: development and use of a scientific resource. Auk 122, 966–971.
| Bird collections: development and use of a scientific resource.Crossref | GoogleScholarGoogle Scholar |
Wolf, J., Bayer, T., Haubold, B., Schilhabel, M., Rosenstiel, P., and Tautz, D. (2010). Nucleotide divergence vs. gene expression differentiation: comparative transcriptome sequencing in natural isolates from the carrion crow and its hybrid zone with the Hooded Crow. Molecular Ecology 19, 162–175.
| Nucleotide divergence vs. gene expression differentiation: comparative transcriptome sequencing in natural isolates from the carrion crow and its hybrid zone with the Hooded Crow.Crossref | GoogleScholarGoogle Scholar |
Woodward, S., Weyand, N., and Bunnell, M. (1994). DNA sequence from Cretaceous Period bone fragments. Science 266, 1229–1232.
| DNA sequence from Cretaceous Period bone fragments.Crossref | GoogleScholarGoogle Scholar |
A Note added in proof: Rowe et al. (in press, Molecular Ecology Resources, https://doi.org/10.1111/j.1755-0998.2011.03052.x) describe applications of high-throughput next-generation sequencing technologies to obtain genome-scale sequence data from traditionally preserved museum skins of mammals. One looks forward to the application of this technology to museum skins of birds.

