The re-emergence of pertussis: implications for diagnosis and surveillance
Vitali SintchenkoA Centre for Infectious Diseases and Microbiology-Public Health, Sydney West Area Health Service, Western Clinical School, University of Sydney.
NSW Public Health Bulletin 19(8) 143-145 https://doi.org/10.1071/NB07005
Published: 20 October 2008
Abstract
Pertussis, or whooping cough, a highly contagious disease caused by Bordetella pertussis, is making a comeback globally and nationally in spite of reasonable vaccination coverage. This paper provides an update on laboratory testing methods that assist the confirmation of clinical disease and investigation of outbreaks. Laboratory confirmation of the diagnosis by polymerase chain reaction or serology should be attempted, especially when atypical pertussis is suspected clinically. Genetic and antigenic variations in virulence factors of strains circulating in the population should also be monitored.
Pertussis, or whooping cough, is a highly contagious disease caused by Bordetella pertussis. This update summarises developments in laboratory testing of pertussis that assist clinicians with the confirmation of the clinical disease and the investigation of outbreaks.
Current laboratory methods
Tests currently used to confirm pertussis infection are shown in the Table 1.
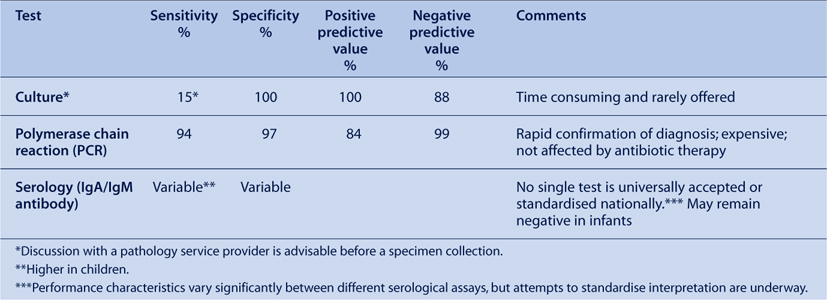
|
Specimen collection
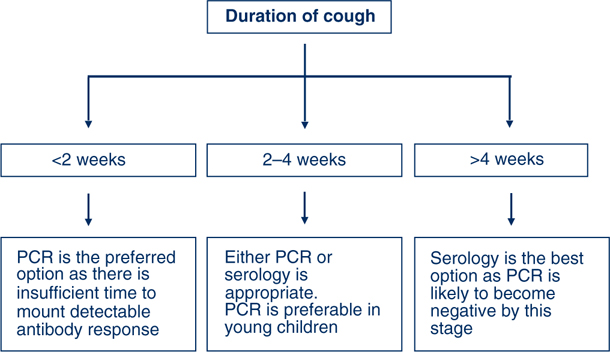
Proper technique and timeliness of specimen collection are important (Figure 1). Nasopharyngeal aspirates are the preferred specimens for polymerase chain reaction (PCR), but are often difficult to collect except from very young children. Aspirates produce a higher recovery of organisms than swabs, and specimens can be split for multiple tests. A swab of the nasopharynx is better than a swab of the anterior nostril for PCR. The polyester swab should be gently inserted into the base of a nostril, advanced as far as possible and rotated in the posterior pharynx for ten seconds before withdrawing. Throat swabs are also acceptable specimens for PCR. Nasopharyngeal aspirates or swabs are the only suitable specimens for culture.
|
Bordetella pertussis is fastidious and quite difficult to grow in the laboratory. It can be recovered from patients only in the first 3 to 4 weeks of illness, and is particularly difficult to isolate from previously immunised persons (Figure 1).1
Polymerase chain reaction
Laboratory confirmation of the diagnosis by PCR or serology should be attempted, especially when atypical pertussis is suspected clinically. The use of PCR has made the rapid diagnosis of pertussis possible and is more sensitive than culture.1,2 The PCR assay is also less affected by antimicrobial therapy. However, as with culture, the sensitivity of PCR decreases with the duration of symptoms. There are occasional false-positive PCR results caused by contamination, which may occur at any stage between sample collection and the laboratory.2
Serology
Natural infection with B. pertussis is followed by an increase in the serum concentration of IgA, IgG and IgM antibodies. In contrast to natural infection, primary immunisation induces mainly IgG and IgM antibodies.1 The greatest specificity for the serological diagnosis of B. pertussis infection is achieved by the measurement of IgG and IgA antibodies against pertussis toxin. Either a significant increase in serum antibody level (preferably) or single high level, in sera obtained at least 2–3 weeks into the illness may be used for diagnosis. It is rarely possible to demonstrate seroconversion because initial symptoms are non-specific and the first (acute) serum is often not collected until 2–3 weeks after the onset of cough. Anti-pertussis toxin IgG levels of >100–125 European or International Units (using standardised methodology) have been shown to be specific for recent exposure to B. pertussis, but this criterion was established in the context of vaccine trials and may be less sensitive and reliable for routine diagnosis.
Many different commercial and in-house serological tests – usually enzyme immunoassays (EIA) – are currently in use; they employ various antigens including pertussis toxin alone or in combination with other, less specific, B. pertussis antigens or a crude preparation of whole bacterial cells. The sensitivity and specificity of EIA-based assays vary considerably, but may be as low as 50–60%. The absence of established cut-off points or diagnostic criteria limit the usefulness of serological confirmation.1,3 Despite these limitations, serological testing (most commonly conducted by a commercial assay, which detects IgA against a whole cell pertussis antigen) has been the basis for notification of the majority of pertussis cases in older children and adults, in Australia.
New methods to confirm pertussis infection
Efforts to control outbreaks of pertussis in a community are costly and require: intensive surveillance; detailed alerts to health-care professionals; enhanced vaccination coverage and public education; and aggressive measures involving treatment, prophylaxis and the isolation of suspected cases.4,5
During the past decade, the demonstration of polymorphism in B. pertussis genes encoding the expression of pertussis toxin and pertactin (another immunogenic B. pertussis virulence factor) led to the suggestion that vaccine-driven evolution has resulted in decreased vaccine efficacy.6,7 Several research groups have also accumulated data suggesting that isolates circulating in a community may be antigenically distinct from vaccine strains and from strains circulating before the introduction of the pertussis vaccination.7,8 Recent evidence from Europe and Australia indicates that we may face the emergence of successful clones of Bordetella harbouring new variants of pertussis toxin.7,9,10
Genetic and antigenic variations in virulence factors of strains circulating in the population can be monitored to detect potential escape from immune protection. However, identification of these variants currently requires time-consuming and expensive sequence analyses. Moreover, as PCR increasingly replaces culture for diagnosis of pertussis, fewer clinical isolates are available for testing. To address this problem, researchers at the Centre for Infectious Diseases and Microbiology – Public Health, in partnership with colleagues from the Universities of Sydney and New South Wales, have been developing new culture-independent methods for molecular subtyping of B. pertussis directly from clinical specimens. This method will allow monitoring future epidemiological changes that predict significant antigenic variation and the potential escape from immune protection.
Acknowledgment
The author thanks Professor Lyn Gilbert for her critical appraisal of the manuscript.
[1] Fry NK, Tzivra O, Li YT, McNiff A, Doshi NP, Maple AC, et al. Laboratory diagnosis of pertussis infections: the role of PCR and serology. J Med Microbiol 2004; 53 519–25.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

[2] Riffelmann M, von Konig CHW, Caro V, Guios N. Nucleic acid amplification tests for diagnosis of Bordetella infections. J Clin Microbiol 2005; 43 4925–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

[3] Cherry JD, Grimprel E, Guiso N, Heininger U, Mertsola J. Defining pertussis epidemiology: Clinical, microbiology and serological perspectives. Pediatr Infect Dis 2005; 25 S25–34.

[4] Tozzi AE, Celentano LP, Atti MLC, Salmaso S. Diagnosis and management of pertussis. Can Med Assoc J 2005; 172 509–15.
| Crossref | GoogleScholarGoogle Scholar |

[5] Wright SW. Pertussis infection in adults. South Med J 1998; 91 702–8.
| PubMed | CAS |

[6] Elomaa A, Advani A, Donnelly D, Antila M, Mertsola J, He Q, et al. Population dynamics of Bordetella pertussis in Finland and Sweden, neighbouring countries with different vaccination histories. Vaccine 2007; 25 918–26.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[7] Van Amersfoorth SCM, Schouls LM, van der Heide HGJ, Advani A, Hallander HO, Bondeson K, et al. Analysis of Bordetella pertussis populations in European countries with different vaccination policies. J Clin Microbiol 2005; 43 2837–43.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

[8] Fingermann M, Fernandez J, Sisti F, Rodriguez ME, Gatti B, Bottero D, et al. Differences in circulating Bordetella pertussis population in Argentina from the strain used in vaccine production. Vaccine 2006; 24 3513–21.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

[9] Poynten M, McIntyre PB, Mooi FR, Heuvelman KJ, Gilbert GL. Temporal trends in circulating Bordetella pertussis strains in Australia. Epidemiol Infect 2004; 132 185–93.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

[10] Byrne S, Slack AT. Analysis of Bordetella pertussis pertactin and pertussis toxin types from Queensland, Australia, 1999–2003. BMC Infect Dis 2006; 6 53.
| Crossref | GoogleScholarGoogle Scholar | PubMed |


