Communicable Diseases Report, NSW, January and February 2007
Communicable Diseases Branch, NSW Department of Health
NSW Public Health Bulletin 18(4) 66-71 https://doi.org/10.1071/NB07032
Published: 8 June 2007
For updated information, including data and facts on specific diseases, visit www.health.nsw.gov.au and click on Infectious Diseases.
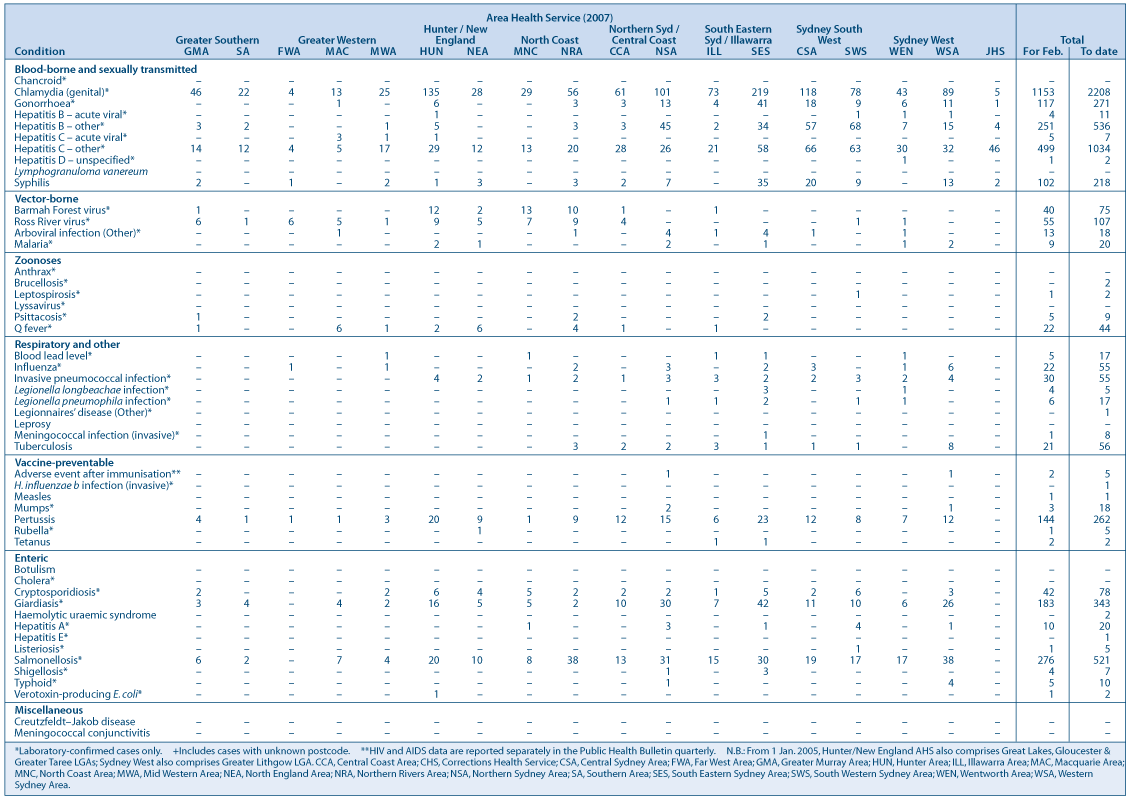
Tables 1 and 2 and Fig. 1 show reports of communicable diseases received through to the end of January and February 2007 in NSW.

|
|
Legionnaires’ disease – Circular Quay
In January 2007, 10 cases of legionellosis due to Legionella pneumophila (serogroup 1) were notified to NSW Health. Six of these cases (five men and one woman, aged between 46 and 64 years) had visited Circular Quay on 31 December 2006, which was within the incubation period. A seventh case, a traveller who had also visited Circular Quay on 31 December, was diagnosed in the United Kingdom and was notified to NSW Health through the European Working Group for Legionella Infections.
NSW Health initiated an investigation into the cause of the outbreak. Active case finding was initiated: public health units solicited reports of additional suspected cases from local emergency departments, respiratory physicians and intensive care units and information about the cluster was faxed to NSW general practitioners and respiratory physicians. Substantial media coverage prompted several patients to present to medical practitioners and the diagnosis was confirmed in one of these.
Environmental health officers from South Eastern Sydney/Illawarra Public Health Unit and the City of Sydney Council initiated an environmental investigation of potential sources of contaminated aerosols in the Circular Quay area, such as cooling towers. Twenty-five cooling towers in the area were evaluated for compliance with Public Health (Microbial Control) Regulation 2000.1 One of these, an inadequately maintained cooling tower in a building at the east end of Circular Quay, returned a high Legionella count (1400 colony forming units per mL). The cooling tower was immediately shut down, cleaned and decontaminated. It is uncertain whether or not this cooling tower was the source of the cases.
This cluster highlights the importance of registration and maintenance of cooling towers, especially in places where many people may be exposed.
Mumps in North Coast Area Health Service
NSW Health was notified of five cases of mumps from among children aged 10–16 years old who attended the ‘South Pacific Pathfinder Camporee’, a church-based event held on the Mid North Coast of NSW in January 2007. Approximately 2900 of 6700 participants in the event were visitors from overseas. All five cases were in children from the Pacific Islands (two cases were from the Solomon Islands, two from Vanuatu and one case was from Fiji).
The cases appear to have been infected in their homelands before travelling to Australia and were otherwise unrelated. Unlike Australia, a mumps-containing vaccine is not included in the standard immunisation schedule of these countries.2
With each clinical diagnosis of mumps, event officials rapidly isolated each case. The North Coast Public Health Unit initiated active case finding for the duration of the camp, and written information about mumps and measles–mumps–rubella (MMR) vaccination was given to all camp participants. MMR immunisation was not feasible because informed consent could not be readily obtained for children from overseas and contraindications to immunisation could not be reliably identified.
The large majority of Australian and New Zealand children at the camp were expected to be immune through previous immunisation with MMR. No information about immunisation status was collected from any of the participants at registration.
Subsequently no secondary cases were reported in NSW.
Norovirus outbreak in a tour group
South Eastern Sydney/Illawarra Public Health Unit investigated an outbreak of gastroenteritis among a group of Japanese school students who visited Sydney from 27 to 30 January 2007. Fifty-eight of the 237 students were reported unwell with vomiting and/or diarrhoea.
By the time the hotel doctor reported the outbreak, most of the students were on their way back to Japan, but several had delayed their return as they were too unwell to travel. Public health unit investigators interviewed the tour organisers and the hotel doctor about illness in the students, the nature of the tour and meals consumed, and arranged for stools from the students to be tested for a range of pathogens.
Of the 237 students, the ill students were among a subset of 116 who visited a farm and other locations (where meals were consumed) over a 2-day period. The high attack rate, nature of the symptoms and close clustering in time of the onset of illness suggested that the outbreak was likely due to norovirus infection acquired at a single event (i.e. a point-source outbreak), most likely during the consumption of a common meal.
Norovirus was detected in stools from one student in Australia, and reported from one ill student tested in Japan. As norovirus has an incubation period of 24 to 48 hours, the investigation focused on the exposures that the ill students had had during that period before onset of illness.
The NSW Food Authority inspected the restaurant and the farm where the students ate and stayed during the incubation period. No ill food handlers were identified and no likely source of contamination was identified at either facility.
Norovirus is a common cause of vomiting and diarrhoea, particularly in the winter months. Norovirus is highly infectious and spreads easily from person to person through contact with faeces or vomitus, or through contact with surfaces that have the virus on them.
While the source of this outbreak remains unconfirmed, a likely explanation appears to be contamination of a shared meal by a food handler or a patron. Sick food handlers should stay away from work for 48 hours after their diarrhoea has stopped, and ready-to-eat food should be presented in a way that protects it from contamination by consumers.
Histamine (scombroid) fish poisoning
In February 2007, NSW Health was notified of two outbreaks of histamine fish poisoning. In the first outbreak, Western Sydney Public Health Unit (Parramatta Office) was notified by an emergency department doctor of three cases who had eaten home-cooked tuna kebabs. The cases had developed facial flushing, a burning sensation of the face and mouth, increased heart rate, headache and rash within 45 min of eating the kebabs. On the same day, Western Sydney Public Health Unit (Penrith Office) was notified of a second outbreak involving two patients, who presented to hospital after eating tuna in a restaurant in the Blue Mountains. All cases recovered.
In a trace-back investigation, the NSW Food Authority found that the tuna in both outbreaks had been caught wild in Indonesia and separately imported into Queensland. Further investigation is underway.
Histamine fish poisoning is among the more common causes of illness linked to fish consumption. It is usually associated with eating fish from the scombroid family, such as tuna and mackerel, but can be caused by other fish.3 Histamine is produced in the fish during bacterial decomposition, following its capture. The formation of histamines can be prevented by gutting the fish, removing the gills and rapid refrigeration throughout the supply chain.4
False positive pertussis serological tests
Linda HuestonA,B,C, Jan LanserA,B, Heather GiddingA,B and Lyn GilbertA,B
ACentre for Infectious Diseases and Microbiology.
BInstitute of Clinical Pathology and Medical Research.
CCorresponding author. Email: lindah@icpmr.wsahs.nsw.gov.au
Following substantial increases in pertussis notifications in Australia based on positive serological tests (but not other diagnostic criteria) in 2005 and 2006, concerns were raised by laboratory-based serologists about the accuracy of serology as a diagnostic criterion for pertussis.
In September 2006, PanBio (whose pertussis whole cell IgA antibody test is used by more than 80% of Australian laboratories), in consultation with the Therapeutic Goods Administration, issued a ‘recall for product correction’ for three batches of its pertussis IgA serology kit. This kit had been used over the previous 12 months in Australia. A new ‘cut-off’ for reporting a positive result, 2.5 times higher than previously recommended, was issued for the kits until a new version could be released.
The Centre for Infectious Diseases and Microbiology (CIDM) recalculated all PanBio pertussis IgA enzyme immunoassay results obtained between 25 January and 9 October 2006 using the new cut-off. Of the 1547 samples tested for pertussis IgA with the original cut-off, 405 had been notified as positive and 171 equivocal. With the new cut-off, only 64 (16% of the original number that were positive) would have been positive and 40 equivocal.
In response to these results, CIDM compared the new and old PanBio kits and several other commercial kits. Panels of positive and negative sera were chosen on the basis of clinical criteria and positive or negative results from a combination of three serological assays used as ‘gold standard’– complement fixation, immunofluorescence and a commercial Western blot.
The evaluation showed that:
-
Approximately 75% of samples that were positive with the old PanBio kit in 2006 were negative when tested with ‘gold standard’ methods. The majority of false-positive results were due to non-specific cross-reactions with the filamentous haemagglutinin as demonstrated by Western blot. Although cross-reactions with filamentous haemagglutinin antigen are known to occur in some other respiratory illnesses, including influenza and mycoplasma infection, all negative sera used in this evaluation were from healthy adults without a cough.
-
Neither of the two commercial kits used by laboratories in NSW in 2006 performed well when compared with the gold standard tests and some of the other commercially available kits.
-
Three commercial kits performed significantly better than all others, based on a combination of specificity (98–99%) and sensitivity (74–78%) and will be further tested in a prospective study.
-
The Western blot kit (MarDx Bordetella pertussis IgA Marblot test) is a useful confirmatory test.
Since the recall of the testing kit in September 2006, the number of pertussis notifications in NSW has fallen from more than 700 cases per month to less than 300 (see: http://www.health.nsw.gov.au/data/diseases/pertussis.html, verified 27 April 2007). Pertussis is classically a clinical diagnosis, based on symptoms of a persistent paroxysmal cough, which in children is typically followed by an inspiratory whoop, and vomiting. Diagnosis can be confirmed by culture, polymerase chain reaction testing or serology, the results of which must be interpreted in light of clinical features of the case.
Note: Use of trade names does not imply endorsement by NSW Health.
[1]
[2]
[3]
[4]


