NSW Annual Immunisation Coverage Report, 2009
Brynley Hull A D , Aditi Dey A , Deepika Mahajan A , Sue Campbell-Lloyd B , Robert I. Menzies A and Peter B. McIntyre A CA National Centre for Immunisation Research and Surveillance,The Children’s Hospital at Westmead
B AIDS and Infectious Diseases Branch,NSW Department of Health
C Sydney Medical School, The University of Sydney
D Corresponding author. Email: brynleyh@chw.edu.au
NSW Public Health Bulletin 21(10) 210-223 https://doi.org/10.1071/NB10045
Published: 18 November 2010
Abstract
Aims: This is the first in a series of annual immunisation coverage reports that document trends in NSW for a range of standard measures derived from Australian Childhood Immunisation Register data, including overall coverage at standard age milestones and for individual vaccines. This report includes data up to and including 2009. Methods: Data from the Australian Childhood Immunisation Register, the NSW Health Survey and the NSW School Immunisation Program were used to calculate various measures of population coverage relating to childhood vaccines, adult influenza and pneumococcal vaccines and adolescent vaccination, respectively. Results: Immunise Australia Program targets have been reached for children at 12 and 24 months of age but not for children at 5 years of age. Delayed receipt of vaccines is an issue for vaccines recommended for Aboriginal children. Pneumococcal vaccination in the elderly has been steadily rising, although it has remained lower than the influenza coverage estimates. For adolescents, there is better coverage for the first and second doses of human papillomavirus vaccine and the dose of dTpa than for varicella. Conclusion: This comprehensive analysis provides important baseline data for NSW against which future reports can be compared to monitor progress in improving immunisation coverage. Immunisation at the earliest appropriate age should be a public health goal for countries such as Australia where high levels of vaccine coverage at milestone ages have been achieved.
This is the first New South Wales (NSW) Annual Immunisation Coverage Report. A series of annual reports will provide NSW Health with information on important trends and issues in immunisation coverage in NSW. It provides a detailed summary for 2009 that includes: vaccination coverage at the standard milestone ages for vaccines not included in standard assessments; vaccination coverage for adolescents, the elderly and Aboriginal children; data for small geographic areas on coverage; and information on the prevalence of conscientious objectors to immunisation.
This report uses the longstanding international practice of reporting coverage at key milestone ages to measure coverage against national targets and to track trends over time. It is adapted from annual national immunisation reports published since 2008.1
High levels of reporting to the Australian Childhood Immunisation Register are maintained by a system of incentive payments for immunisation providers and carers. These have been discussed in detail elsewhere.2 However, changes to immunisation policy, the incentive payment system and changes to the ‘fully immunised’ coverage algorithms may have an impact on reported vaccination coverage; some recent changes are highlighted in Box 1 and also referred to in this report.
The Australian Childhood Immunisation Register was established on 1 January 1996 by incorporating demographic data from Medicare on all enrolled children aged less than 7 years.3 Medicare constitutes a nearly complete population register, as approximately 99% of children are registered by 12 months of age. The operations of the Australian Childhood Immunisation Register have been discussed in detail elsewhere.2
Table 1 presents the NSW Immunisation Program for children in 2009. No new vaccines were introduced to the NSW Immunisation Program during 2009.

|

|
Methods
Measuring immunisation coverage using the Australian Childhood Immunisation Register
The cohort method has been used for calculating coverage at the population level (national and state/territory)4 since the inception of the Australian Childhood Immunisation Register. Cohort immunisation status is assessed at 12 months of age (for vaccines due at 6 months), 24 months of age (for vaccines due at 12 and 18 months), and 5 years of age (for vaccines due at 4 years). A minimum 3-month lag period is allowed for the late notification of immunisations to the Australian Childhood Immunisation Register.4 If a child's records indicate receipt of the last dose of a vaccine that requires more than one dose to complete the series, it is assumed that earlier vaccinations in the sequence have been given. This assumption has been shown to be valid.5,6
The proportion of children designated as ‘fully immunised’ was calculated using the number of Medicare-registered children completely immunised with the vaccines of interest by the designated age as the numerator and the total number of Medicare-registered children in the age cohort as the denominator. ‘Fully immunised’ at 12 months of age was defined as a child having a record on the Australian Childhood Immunisation Register of three doses of a diphtheria (D), tetanus (T) and pertussis-containing (P) vaccine, three doses of polio vaccine, three doses of Haemophilus influenzae type b (Hib) vaccine and three doses of hepatitis B vaccine. ‘Fully immunised’ at 24 months of age was defined as three or four doses of a DTP-containing vaccine, three doses of polio vaccine, four doses of Hib vaccine, three doses of hepatitis B vaccine, and one dose of a measles, mumps and rubella-containing (MMR) vaccine. ‘Fully immunised’ at 5 years of age was defined as four or five doses of a DTP-containing vaccine, four doses of polio vaccine, and two doses of an MMR-containing vaccine.
Immunisation coverage estimates were also calculated for individual National Immunisation Program vaccines, including the National Immunisation Program vaccines not included in calculations for incentive payments and ‘fully immunised’ status. They were: the third dose of 7vPCV and second dose of rotavirus vaccine by 12 months of age; and the first dose of varicella vaccine and first dose of meningococcal C vaccine by 24 months of age.
Timeliness
Age-appropriate immunisation was defined as receipt of a scheduled vaccine dose within 30 days of the recommended age. We categorised delayed vaccination as 1–6 months and greater than 6 months. All children included in the analysis were old enough to potentially experience delays in immunisation greater than 6 months for immunisation due by 24 months of age or earlier. Timeliness of different vaccines and doses was also compared by plotting the cumulative percentage receiving each vaccine dose by age, with the proportion ever immunised set as 100%.
Aboriginal status
Aboriginal status on the Australian Childhood Immunisation Register is recorded as ‘Aboriginal’, ‘non-Aboriginal’ or ‘unknown’, as reported by the child’s carer to Medicare or by the immunisation provider to the Australian Childhood Immunisation Register. For this report we considered two categories of children: ‘Aboriginal’ and ‘non-Aboriginal’; children with unknown Aboriginal status were presumed to be ‘non-Aboriginal’. Coverage estimate time trends are presented from 2004 only, due to poor rates of reporting of Aboriginal status prior to that time.8
Small area coverage
Coverage was calculated for Australian Bureau of Statistics (ABS)-defined Statistical Subdivisions.9 We chose ABS-defined Statistical Subdivisions as areas to be mapped because they provide more detail than area health services (AHSs) but are not too small to render maps unreadable (child population sizes for Statistical Subdivisions in NSW range from 89 to 7000 children). Maps were created using version 10 of the MapInfo mapping software (version 10, MapInfo Corporation, New York, USA) and the ABS Census Boundary Information. As postcode is the only geographical indicator on the Australian Childhood Immunisation Register, the ABS Postal Area to Statistical Local Area Concordance 2006 was used to match the Australian Childhood Immunisation Register residential postcodes of the children to Statistical Subdivsions.10
Conscientious objection/No vaccine recorded
A child must be registered with Medicare before its parent(s) can lodge a conscientious objection to immunisation. Conscientious objectors are eligible for immunisation incentive payments, however parents may also object to immunisation but refuse to lodge any official objection. We used the percentage of children with no vaccines recorded on the Australian Childhood Immunisation Register as a proxy measure of the number of these children. Proportions of conscientious objectors and children with no vaccines recorded by AHSs were calculated from the cohort of children registered with Medicare and born between 1 January 2003 and 31 December 2008. At the time of data extraction they were 12–72 months of age. We chose this cohort when calculating proportions so that children under the age of 12 months were not included, to allow sufficient time for registration of objection and to exclude infants late for vaccination.
Coverage in the elderly and adolescents
Influenza and pneumococcal vaccination coverage estimates in the elderly were from the NSW Health Survey. This is a rolling random digit-dialled telephone survey, with vaccination status determined from patient recall at the time of the interview. Methods and results are presented in more detail elsewhere.7 Coverage for vaccines given to adolescents were collected from the NSW School Immunisation Program. Vaccination status is recorded by school immunisation teams and counts collated by AHSs and NSW Health. The denominator is the school population. Methods and more results are presented in the accompanying paper in this issue.11
Results
Overall coverage estimates
In NSW, coverage for all individual vaccines and ‘fully immunised’ for the 12-month and 24-month age groups is greater than the Immunise Australia Program’s target of 90% in all AHSs (except for rotavirus at 12 months of age and varicella at 24 months of age) (Tables 2 and 3). Recorded coverage for the 5-year age group is below the target, at around 82% for all vaccines and even lower in particular AHSs (Table 4). Figure 1 shows time trends in ‘fully immunised’ coverage at three milestone ages. The proportion ‘fully immunised’ at 1 and 2 years of age increased steadily over the period. Coverage estimates at 6 years of age (for vaccines due at 4 years) increased steadily from early 2002 to late 2007, including a noticeable increase in June 2006 corresponding with the introduction of combination vaccines. However, from the beginning of 2008 the assessment age was changed from 6 years to 5 years, resulting in substantially lower coverage for this age group by December 2009.

|

|
Coverage estimates for individual vaccines
Coverage for the Hib and hepatitis B vaccines at 12 months of age are greater than DTPa and polio coverage prior to the change in algorithm to measure coverage that occurred in the later half of 2009. The change tightened the rules regarding Hib and hepatitis B vaccines for 12-month olds to lead to more accurate measures of Hib and hepatitis B vaccine coverage in NSW. Coverage has since lowered, becoming similar to the coverage estimates of DTPa and polio in the last two cohorts of 2009 (Figure 2). Almost all AHSs had 12-month coverage at more than 90% for all vaccines except rotavirus (Table 2).
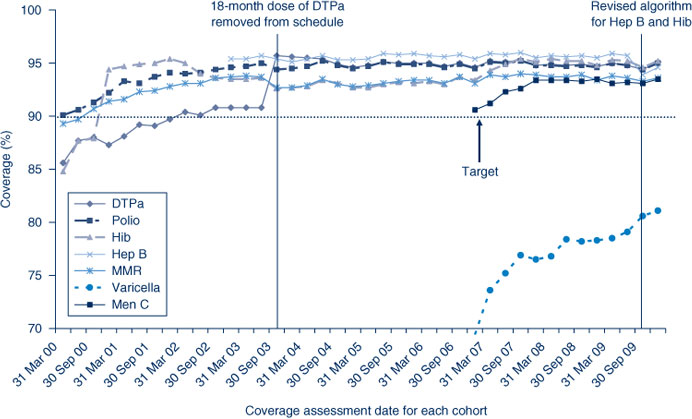
The trends in childhood vaccination coverage in Australia for individual vaccines at 24 months of age are shown in Figure 3. Coverage for the 24-month age group increased substantially and suddenly in September 2003 following the removal of the 18-month dose of DTPa from the immunisation schedule. For most of the study period, hepatitis B coverage was higher than for all other vaccines, at just under 95%, due to the different coverage algorithm described above. Coverage was lowest for MMR and Hib, the only vaccines that have a 12-month dose used in coverage calculations. Almost all AHSs had 24-month coverage estimates at more than 90% for all vaccines except varicella (Table 3).
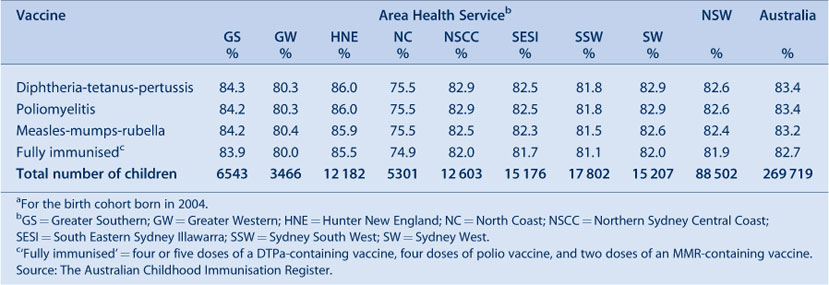
The coverage trends in NSW for individual vaccines at 6 years of age (5 years of age from December 2007) are shown in Figure 4. Coverage for all three vaccines was almost identical and remained steady until mid-2006 when a sharp increase of almost 5% was recorded, likely due to the introduction of combination vaccines. From December 2007, due to the change in assessment age discussed previously, coverage was markedly lower for all vaccines due by 5 years of age. The overall ‘fully immunised’ estimate for 5-year coverage was approximately 82% in NSW, slightly lower than the national 5-year coverage rate of 82.7%. No AHSs in NSW had 5-year coverage estimates at more than 90%, the lowest being the North Coast AHS with around 75% (Table 4).
|
Coverage estimates for Aboriginal children
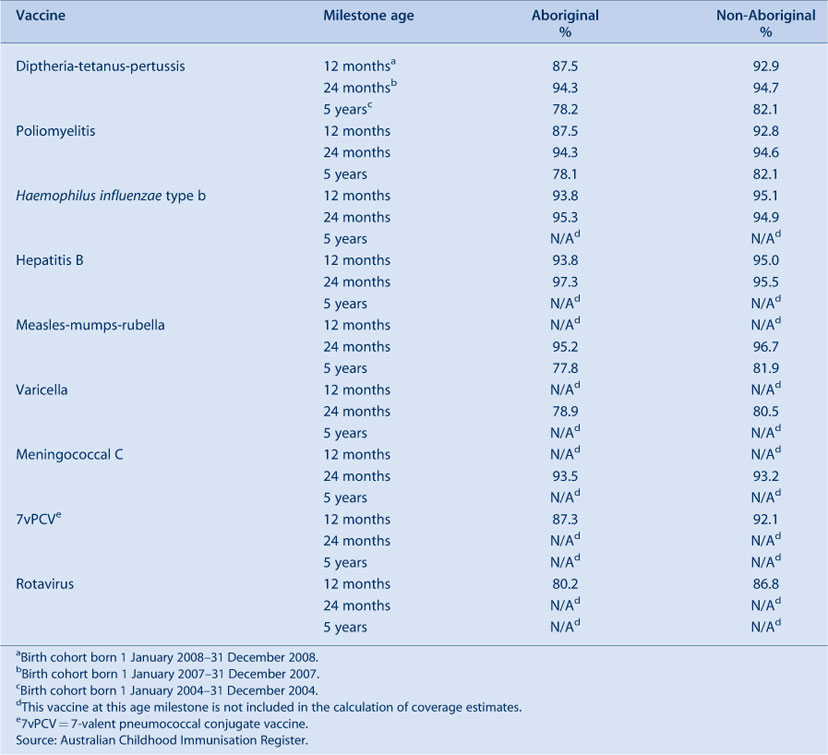
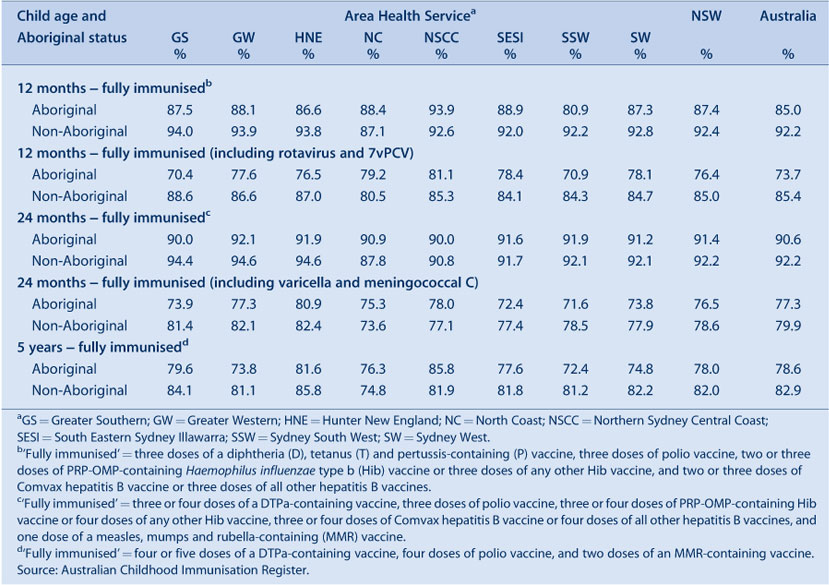
Vaccination coverage estimates in 2009 for the three milestone ages for individual vaccines by Aboriginal status are shown in Table 5. These show that coverage was lower for Aboriginal children than non-Aboriginal children at the 12-month and 5-year age milestones, with the difference being greater at 12 months of age.
|
The proportion of Aboriginal children fully immunised at 12 months of age was lower compared with coverage for non-Aboriginal children (Table 6). Coverage was lower among Aboriginal children in most AHSs, except in the Northern Sydney Central Coast and North Coast AHSs where coverage in Aboriginal children was 1.3% higher than in non-Aboriginal children. The extent of the difference varied among AHSs, reaching more than 11% in some. However, by 24 months of age, coverage disparities between Aboriginal and non-Aboriginal children had almost disappeared in most AHSs (Table 6). All AHSs had more than 90% coverage for Aboriginal children with the highest Aboriginal 24-month coverage observed in the Greater Western AHS.
|
At 5 years of age, the proportion recorded as being ‘fully immunised’ was lower than that of earlier age milestones. There was 4% lower coverage in Aboriginal children compared to non-Aboriginal children while, for individual AHSs, coverage in Aboriginal children was lower in most AHSs than in non-Aboriginal children and higher in one AHS.
Coverage estimates for National Immunisation Program vaccines not routinely reported elsewhere
7vPCV and rotavirus
Coverage for 7vPCV vaccine has remained at high levels since first calculated for NSW in early 2006 (Figure 2). Coverage is similar in all AHSs at greater than 91%, except the North Coast (Table 2).
Rotavirus vaccine was added to the National Immunisation Program in July 2007; coverage for three doses at 12 months of age was calculated only from the July 2008 quarter onwards. Coverage increased in NSW from July 2008 to December 2009 (Figure 2). Rotavirus coverage was lower and had greater variation between AHSs compared with other vaccines given at 2, 4 and 6 months of age, which is expected from the vaccine most recently introduced to the National Immunisation Program. Reported coverage for two doses of rotavirus at 12 months of age varied amongst AHSs (Table 2).
Meningococcal C and varicella
Meningococcal C vaccine was added to the National Immunisation Program in January 2003. Coverage for this vaccine has remained at high levels since first calculated for NSW in early 2006 (Figure 3). Coverage is similar in all AHSs at greater than 92%, except the North Coast (Table 3).
Figure 3 shows coverage for varicella vaccine has consistently been lower than that for meningococcal C vaccine. Coverage is similar in all AHSs except the North Coast (Table 3).
Coverage estimates for vaccines for the elderly(pneumococcal and influenza)
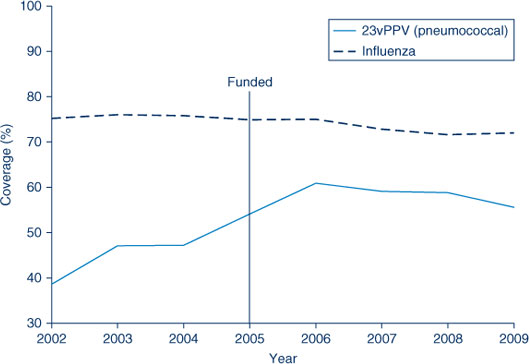
The proportion of people aged 65 years and over vaccinated for influenza in the past 12 months remained relatively stable at over 70% during the period 2002–2009. However, the coverage rate of the elderly receiving a dose of pneumococcal vaccination during the previous 5 years (23vPPV) has been steadily rising: from 39% in 2002 to 55% in 2009. Despite this rise it remains lower than the influenza coverage estimates. The highest coverage rate for pneumococcal vaccination in the elderly was observed in 2006, the year after its inclusion in the National Immunisation Program (Figure 5 and Table 7).
|

|
In 2009, influenza (vaccinated in the past 12 months) and pneumococcal (vaccinated in the past 5 years) vaccine coverage in the elderly was highest in the Hunter New England AHS and lowest in the Sydney West AHS (Table 7).
Coverage estimates for adolescents
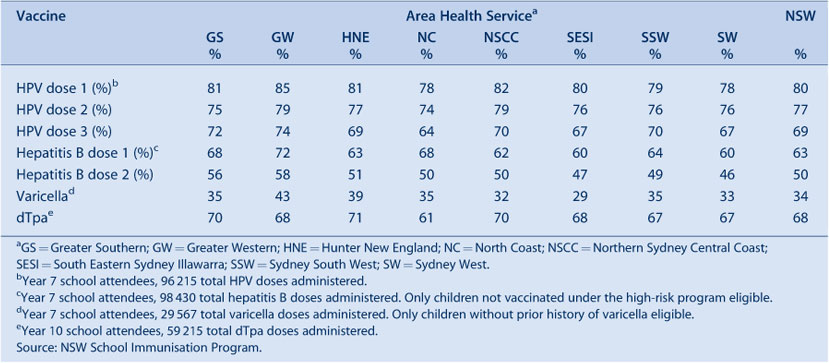
NSW Adolescent Vaccination Program coverage data for high school students for 2009 are shown in Table 8. Coverage varies by vaccine, dose and AHS with better coverage for the first and second doses of human papillomavirus vaccine and the dose of dTpa. Varicella coverage was lower, with no AHS achieving coverage greater than 43%. This lower coverage for varicella is likely due to some children gaining natural immunity as a result of having a prior varicella infection and hence not requiring vaccination.
|
Timeliness of immunisation
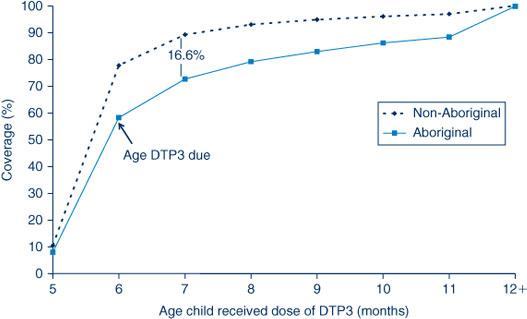
For the third dose of DTPa, there was significantly greater delay in immunisation for Aboriginal children than non-Aboriginal children, with a 16.6% differential at 7 months of age (Figure 6). A similar difference was found for timeliness of the second dose of MMR, but with a smaller differential of 5% (Figure 7). However, timeliness of this dose was poor for both population groups with only 29–33% of children receiving the vaccine on time.
|
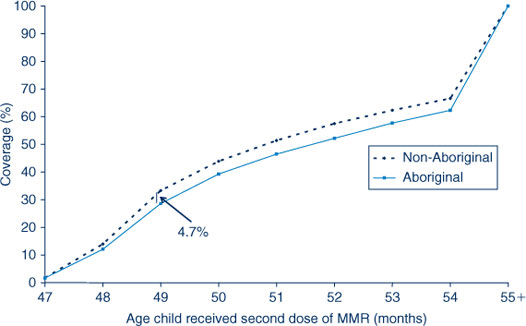
|
A similar pattern is illustrated in Tables 9 and 10 where delay is categorised into 1–6 months and greater than 6 months. The Northern Sydney Central Coast and Sydney West AHSs had the lowest degree of vaccination delay for both Aboriginal and non-Aboriginal children and for both delay categories. The AHSs experiencing the greatest delay were Greater Southern and Greater Western, especially for Aboriginal children. The degree of vaccination delay for the second dose of MMR vaccine was very high for all AHSs and for both Aboriginal and non-Aboriginal children (Table 10).

|
Conscientious objectors and no vaccines recorded
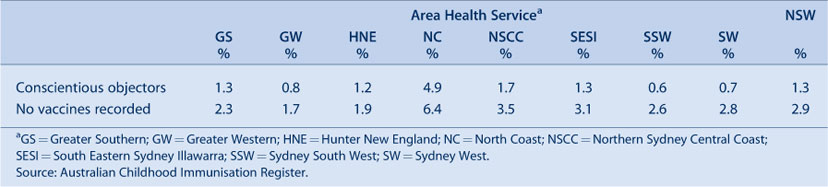
The percentage of children with no vaccines recorded in NSW is greater than those recorded as conscientious objectors (Table 11). Both indicators varied by AHS with a high percentage of objectors and children with no vaccines recorded in the North Coast AHS and the lowest in the Greater Western AHS.
Small area coverage
‘Fully immunised’ coverage in NSW by Statistical Subdivision for the 5-year milestone age group in 2009 varies substantially, with many Statistical Subdivisions having recorded coverage below 80%, putting them at higher risk of outbreaks of contagious diseases such as measles and pertussis12 (Figure 8). In fact, there are very few small areas (Statistical Subdivisions) in NSW with ‘fully immunised’ coverage for vaccines due at 4 years of age above national target levels.

|
First dose of DTPa at 6 weeks of age
In response to the current pertussis epidemic and to provide early protection for young infants, it was recommended in March 2009 that NSW immunisation providers give the first dose of DTPa vaccine (Infanrix-hexa) at 6 weeks of age instead of 8 weeks of age. Figure 9 shows the age at which children in NSW were given the first dose of DTPa by month of vaccination during 2009. Prior to the recommendation, very few children received the vaccine dose at 6 weeks of age; the percentage rose during 2009 with more than 50% of children receiving the dose prior to 8 weeks of age in the last quarter.
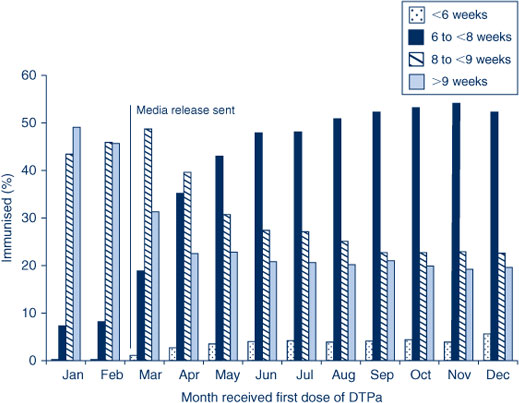
|
Discussion
These data reveal that Immunise Australia Program coverage targets have been reached for children at both 12 and 24 months of age in NSW and for all AHSs except the North Coast. However, this is not the case for children aged 5 years, with relatively poor coverage of 82% for NSW and for all AHSs. This is also the case in other jurisdictions.1
Coverage at 24 months of age exceeds that at 12 months of age in NSW and all AHSs. This is likely related to the greater period of time between due date and assessment time (12 versus 6 months respectively), and potentially an impact of the maternity incentive payment which is assessed at 18–24 months. It should be noted that several vaccines are not included in this assessment, including some with relatively high coverage (pneumoccocal conjugate, meningococcal C), and some with lower coverage (rotavirus, varicella). The change in December 2007 in assessment age from 6 to 5 years for vaccines due at 4 years resulted in lower coverage estimates for vaccines due at this age and has revealed that many children are not fully protected in a timely way from the diseases these vaccines guard against. This was of particular concern during the pertussis epidemic of 2008 and 2009, when children aged 5–9 years were seriously affected.13
A number of vaccines included in the National Immunisation Program are excluded when calculating ‘fully immunised’ status and eligibility for incentive payments. While this annual report provides coverage data on these vaccines, data for the more longstanding and established vaccines are also available in data provided to GP Divisions and immunisation providers. Coverage estimates for 7vPCV and meningococcal C vaccines are comparable with estimates for vaccines that are included in ‘fully immunised’ calculations, but estimates for varicella and rotavirus are lower. As these abovementioned vaccines have been routinely incorporated into the childhood immunisation schedule for some time, their inclusion in official coverage assessments for ‘fully immunised’ and wider dissemination should be considered to facilitate monitoring of program delivery.
Although most children eventually complete the scheduled series by the 24-month milestone, many still do not do so in a timely manner. This is more pronounced for Aboriginal children, as has been noted previously.1,14 This is of particular concern for diseases where multiple vaccine doses are required for protection, where the disease risk among young infants is significant (e.g. pertussis, pneumococcal disease), and for Aboriginal children for whom early vaccination is critical for any impact on the incidence of otitis media and pneumonia.
Coverage for the elderly has been consistently high for the influenza vaccine but less so for the pneumococcal vaccine, perhaps due to greater awareness of annual influenza vaccination programs.
There are some limitations to the data used to compile this report. Common to almost all analyses of Australian Childhood Immunisation Register data is the problem of underreporting of immunisation encounters by providers. We do know from a previous study that underreporting of immunisation encounters leads to underestimation of coverage rates.15 However, the level of underreporting over the past 4–5 years has decreased somewhat as a result of a number of initiatives,15 and we have no evidence to suggest that dates of administration of immunisations provided to the Australian Childhood Immunisation Register by providers are incorrect. Vaccine coverage data for the elderly is limited as the NSW Health Survey relies on self-reported vaccination status.7 Finally, vaccination coverage for adolescents only includes school attendees and school-administered doses.
Conclusion
Data provided by the Australian Childhood Immunisation Register in this report reflect the successful delivery of the National Immunisation Program in NSW, while identifying some areas for improvement. The Australian Childhood Immunisation Register, the NSW Health Survey and monitoring through the NSW School Vaccination Program continue to be very useful tools for administering the National Immunisation Program and monitoring its implementation in NSW.
[1] Hull B, Deeks S, Menzies R, McIntyre P. Immunisation coverage annual report, 2007. Commun Dis Intell 2009; 33 170–87.
| PubMed | (Cited 7 June 2010.)
[8] Rank C, Menzies RI. How reliable are Australian Childhood Immunisation Register coverage estimates for Aboriginal children? An assessment of data quality and coverage. Commun Dis Intell 2007; 31 283–7.
| PubMed | (Cited 6 December 2008.)
[11] Ward KF, Menzies RI, Quinn HE, Campbell-Lloyd S. School-based vaccination in NSW. N S W Public Health Bull 2010; 21(9–10): 237–42.
| Crossref | GoogleScholarGoogle Scholar |

[12] Hanna JN, Symons DJ, Lyon MJ. A measles outbreak in the Whitsundays, Queensland: the shape of things to come? Commun Dis Intell 2002; 26 589–92.
| PubMed |

[13] Department of Health and Ageing National Notifiable Diseases Surveillance System – Quarterly report. Commun Dis Intell 2009; 33 63–9.

[14] Hull BP, McIntyre PB. Timeliness of childhood immunisation in Australia. Vaccine 2006; 24 4403–8.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[15] Hull BP, Lawrence GL, MacIntyre CR, McIntyre PB. Immunisation coverage in Australia corrected for under-reporting to the Australian Childhood Immunisation Register. Aust N Z J Public Health 2003; 27(5): 533–8.
| Crossref | GoogleScholarGoogle Scholar | PubMed |