Camera traps provide insight into factors influencing trap success of the swamp wallaby, Wallabia bicolor
Ami Bennett A B and Graeme Coulson AA Department of Zoology, The University of Melbourne, Parkville, Vic. 3010, Australia.
B Corresponding author. Email: bea@unimelb.edu.au
Australian Mammalogy 36(1) 15-20 https://doi.org/10.1071/AM13006
Submitted: 7 March 2013 Accepted: 1 July 2013 Published: 13 September 2013
Abstract
Trapping programs for mammals often have low capture success, which is known to be influenced by a range of environmental factors, in addition to aspects of the traps themselves. However, the behavioural responses to traps by the target species are largely unknown. We simultaneously set camera traps and soft-walled double-layered traps for swamp wallabies, Wallabia bicolor, and used images from the camera traps to investigate responses by the target species. Wallabies mostly visited traps after sunset, with the number of visits declining steadily through the night. Visits to traps were more frequent during crescent and new moon phases and when the moon was set. In the majority (59%) of these visits, wallabies did not enter the traps. In some cases wallabies consumed only the bait outside the trap, or the trap door had been closed, usually by other swamp wallabies or bobucks, Trichosurus cunninghami, but in many cases (28% of visits) we could not discern why wallabies failed to enter. When wallabies did enter traps, just 14% of visits resulted in successful capture, with non-captures mainly occurring because wallabies reached in to obtain bait without triggering the trap.
Additional keywords: activity pattern, capture, macropod, moonlight, moon phase, non-target.
Introduction
Trapping programs for mammals typically report low (<10%) capture success (e.g. Woinarski et al. 2001; Vernes and Pope 2006; Morrant et al. 2010; Albanese et al. 2011). Environmental factors may influence trap success. For example, Caley (1994) reported that captures of feral pigs, Sus scrofa, were affected by season and forest type in tropical habitats of the Northern Territory, and van Hensbergen and Martin (1993) showed that trap success of small mammals in South Africa was influenced by rainfall, wind speed, temperature, relative humidity and moonlight. Aspects of the trapping technique itself are also influential. Intrinsic factors such as the type of trap, arrangement of traps, trap position within a trapping array, free-feeding before trapping, type of bait and practical experience of setting traps can all impact on trap success (Greenberg et al. 1994; Ruette et al. 2003; Cunningham et al. 2005). While these environmental and intrinsic factors are known to influence trap success, there is little information on the ways in which target species interact with traps (but see Jury et al. 2001).
Although most macropod species cannot be trapped using conventional trapping techniques (Coulson 1996), some will enter traps. The red-bellied pademelon, Thylogale billiardierii, and Bennett’s wallaby, Macropus rufogriseus, have often been trapped in Tasmania (Le Mar and McArthur 2005; Wiggins et al. 2010), and rock-wallabies, Petrogale spp., have been trapped in many areas on the Australian mainland (Bluff et al. 2011; Willers et al. 2011). Another macropod species that can be trapped is the swamp wallaby, Wallabia bicolor. We conducted a trapping program for this target species, and simultaneously set cameras to monitor visits to the traps. We investigated (1) the influence of a range of external variables on the rate of visits by the target species, and (2) the behavioural responses of the wallabies to aspects of the traps themselves.
Methods
Study site and species
We conducted this study in the Upper Yarra catchment, one of the three main water catchments located in the Yarra Ranges National Park, situated ~100 km north-east of Melbourne. The forest at this location was burnt by wildfire in 1983 (Ough 2001), and has more recently been subjected to heavy browsing pressure by a high-density population of sambar, Cervus unicolor (Bennett 2008). The study site is bordered by the Upper Yarra Reservoir, located in a valley surrounded by a range of forest types including the Ecological Vegetation Classes (EVCs) Wet Forest, Damp Forest, Riparian Forest, Heathy Dry Forest and Shrubby Foothill Forest (Department of Sustainability and Environment 2008). The aspect in general is north- and west-facing, and the topography is steep in places and dissected by ephemeral streams. The elevation rises from the reservoir at ~380 m to 640 m above sea level on the adjacent ridge. Annual rainfall averages over 1000 mm (Bureau of Meterology 2013), with rainfall and temperature over the study period being similar to the long-term means.
The swamp wallaby is a generally solitary, medium-sized macropod found in a variety of habitat types along the east coast of Australia (Merchant 2008). Swamp wallabies are predominantly active during nocturnal hours, and rest during the day in dense cover (Swan et al. 2008). While commonly described as generalist browsers (Hollis et al. 1986), swamp wallabies feed selectively, preferring shrubs and forbs over other plant types (Davis et al. 2008; Di Stefano and Newell 2008). The main predators of the swamp wallaby are wild dogs, Canis familiaris (Triggs et al. 1984; Glen et al. 2006), and dingoes, Canis lupus dingo (Robertshaw and Harden 1986). While several studies have found swamp wallaby in scats of the red fox, Vulpes vulpes, Lunney et al. (1990) considered that consumption of carrion was the most likely source. Wild dog and fox trapping was regularly conducted at the study site, but had ceased for approximately a year before this study (last trapping program conducted 5 March to 26 April 2010). Following the course of this study, six tagged wallabies were killed by wild dogs between October 2011 and November 2012.
Trapping program
We trapped swamp wallabies to allow the fitting of radio-collars to investigate the habitat use of this species for another study. We used soft-walled double-layered traps (70 × 70 × 100 cm) specifically designed for swamp wallabies (Di Stefano et al. 2005). For ease of transportation, we modified the rigid frame used by Di Stefano et al. (2005) into a folding design. To reduce the potential for injury to trapped animals, we added dense water-repellent ‘pool noodle’ foam to pad the underside of the top cross-bar. To prevent traps rolling, potentially causing injury or allowing wallabies to escape, we secured the frame to the ground with tent pegs. We dug a shallow pit under the trap, which extended almost the full width and a third to half the length of the floor, to allow the trigger mechanism to function, and used a low-fragrance spray lubricant (Inox-mx3) on the trap hinges to ensure the door could swing freely (see Di Stefano et al. 2005 for further details on the trap mechanism). For practical purposes of trap transportation, baiting and checking, we placed all traps within 40 m of access tracks, which form a network across the catchment. Pollock and Montague (1991) recommended traps be distributed a minimum of 15 m apart to reduce distress and disturbance to wallabies caught in adjacent traps. Similarly, J. Di Stefano (University of Melbourne 2011, pers. comm.) recommended the placement of multiple traps within a small (e.g. 50 × 50 m) area so that if one trap were closed, another would be available in close proximity, and to increase the efficiency of checking traps. We therefore distributed traps in groups of two to four, 15–30 m apart, with each group >500 m apart in a variety of forest types (Ecological Vegetation Classes) including Riparian Forest, Damp Forest and Shrubby Foothill Forest. We deployed six folding traps on 9 April but, owing to the apparent low density of the target species, deployed a further eight standard, rigid traps on 1 June and another five on 24 July for a total of 19 traps. To further increase the speed of trap checks and minimise disturbance at traps when checks were conducted, we attached reflective tape to the door and door handle of all traps (S. Garnick, University of Melbourne 2011, pers. comm.) so that it was apparent by torch light from a distance at night whether the door was closed.
We commenced weekly free-feeding with roughly chopped carrots in and around each trap six weeks before trapping. We locked the trap door open to habituate wallabies and encourage them to enter the traps. Initially, we baited traps with only carrots, but after a month tried a mix of carrot, pear and apple, with the occasional addition of peanut butter, as used by Di Stefano and Newell (2008), because the carrots did not appear to be attractive to swamp wallabies. However, these additional baits generated a frenzy of bobuck, Trichosurus cunninghami, activity but apparently little swamp wallaby activity, so we increased the quantity of carrots at each trap, and did not offer other baits. Instead, we trialled bait boxes, robust plastic containers with holes drilled in the lid, containing apple and pear. We placed these boxes in the traps in addition to the carrots in the hope of providing an attractive scent at the trap, but again this did not appear to increase swamp wallaby activity so we removed the bait boxes after a month, and used only carrots thereafter. Following the free-feeding phase and bait trials, we placed most carrots inside each trap, as well as a few outside the trap (to entice swamp wallabies to approach), every day while trapping and at least every three days between trapping bouts. We trapped over 45 nights between 4 May and 8 September 2011, for a total of 620 trap-nights. Initially, we set traps 2–3 h before dusk and checked them once a day in the morning at first light, and then locked the door open. However, the few swamp wallabies that had been tagged in the first two months became regular visitors at dusk, so from June we additionally checked traps 2–3 h after sunset to ensure that these animals did not unnecessarily remain in traps overnight, and to maximise the number of available traps to untagged wallabies.
We set infrared-triggered wildlife cameras (Reconyx HC600 Hyperfire) at 10 traps on 7 July 2011 to better understand the apparent low visitation and capture rate. We installed a further three cameras on 15 July and 11 August for a total of 16 cameras operating until 8 September 2011, when trapping was completed. The number of operational cameras fluctuated according to the number of traps installed at the time and with occasional camera malfunction. We located cameras 1–3 m in front, or to the side, of traps depending on the availability of a suitable mounting point, and aimed the cameras at the door of traps. We set cameras without time delays (24 h) so that, once triggered, three consecutive images were recorded 1 s apart, and were generally sufficient to identify tagged swamp wallabies. Each image file recorded the date, time and temperature. The date and time stamp allowed us to classify visits to traps as daylight, twilight or night, and whether there was moonlight present. Visits falling between times of moonrise and moonset were classified as moonlight present, and all other visits classified as moonlight absent. We did not record cloud cover, so variation of light levels within these two categories were not considered in analysis. The date stamp also allowed us to determine the moon phase at the time of visits, which we classified as either gibbous (first quarter), new, crescent (third quarter) or full, encompassing three days before and after the phase. Infrared images are monochrome, so we used reflective tape in unique patterns on the collar and barrel of the swamp wallaby radio-collars to identify individuals. We recorded the duration (minutes within camera view) of each swamp wallaby visit to traps, whether they entered the trap, and finally whether they were trapped. To allow for the variation in the number of operational cameras, we used the visit rate of swamp wallabies (total visits/cameras) for analyses from 7 July until 8 September 2011. Due to the small sample size, we did not differentiate visits by tagged wallabies from untagged wallabies for analyses. We used camera data to examine the relationship of the swamp wallaby visit rate to time of day, time after sunset, moon phase, moonlight and temperature, which previous studies have suggested may influence swamp wallaby activity (Osawa 1989; Di Stefano 2007; Swan et al. 2008), and to additionally examine the events that led to the successful trapping of a swamp wallaby. Following examination of residual plots, we normalised the data using a square-root transformation, and analysed using analysis of variance in Genstat 14, with Fisher’s least significant difference post hoc tests for paired comparisons of means.
Results
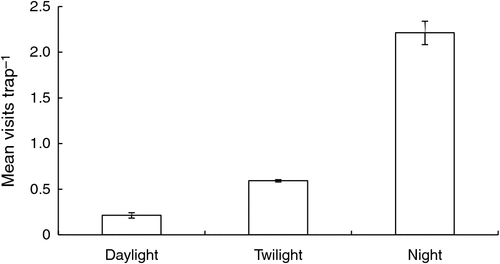
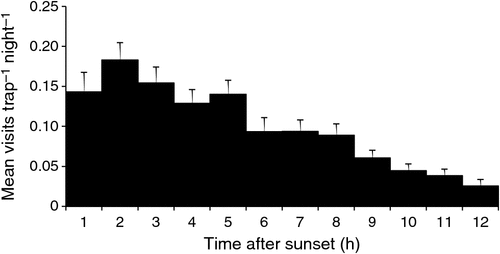
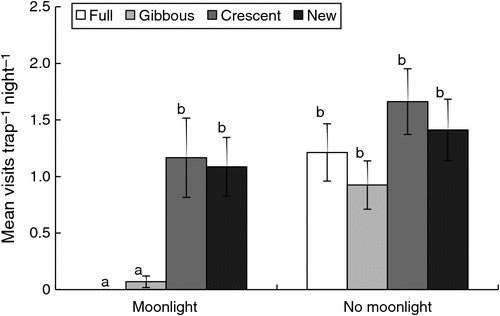
Trap success of swamp wallabies was low (9.4%), we captured just 23 individual swamp wallabies and had 38 subsequent recaptures over 620 trap-nights. The camera traps detected 1317 swamp wallaby visits at a mean (±s.e.) rate of 1.39 ± 0.14 per day (24 h). The temperature during visits ranged from –2 to 19°C (mean ± s.e. = 6.2 ± 0.1°C), but was not related to the visit rate (F1,543 = 0.10, P = 0.753). The majority (84%) of visits occurred at night (F2,122 = 60.28, P < 0.001) (Fig. 1), but the frequency of visits decreased throughout the night (F1,766 = 107.58, P < 0.001) (Fig. 2). Nocturnal visit rate was also influenced by the phase of the moon (F3,60 = 10.69, P < 0.001), being highest around crescent and new moon, but moon phase did not affect the duration of these visits (F3,58 = 0.39, P = 0.759). In addition, visits were also more frequent when the moon was set (F1,60 = 82.67, P < 0.001), and moon phase and moonlight interacted (F3,60 = 5.92, P = 0.001), such that almost all visits under moonlight occurred during crescent and new moons (Fig. 3).
|
|
|
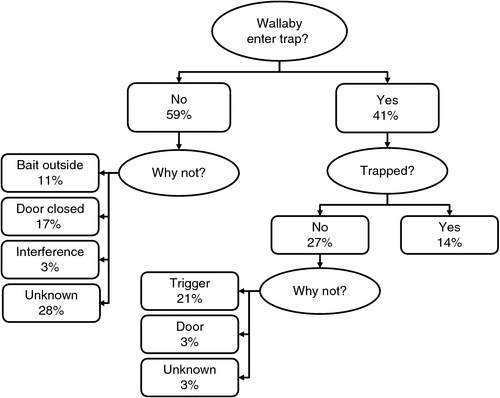
The outcome of swamp wallaby visits to traps that were set (n = 340) is summarised in Fig. 4. Wallabies did not enter traps on 59% of visits. We could not discern why wallabies did not enter traps for 28% of visits, but some reasons were evident: wallabies consumed bait outside the trap then left the area on 11% of visits, and the door was already closed on 17% of visits, due to other animals, including swamp wallabies (7% of visits), bobucks (6%) and wombats (<1%), as well as some unknown causes (3%). Bobucks were the most frequent non-target species to visit the traps, but were generally able to escape through a small gap in the wool bags that form the trap walls (Fig. 5a), so we had to release only two individuals. We did not quantify the activity of bobucks, except when they coincided with swamp wallaby visits to traps, but bobucks were generally early visitors to traps each evening and preferentially consumed bait outside traps before moving into the traps. When bobucks entered traps, they commonly took one piece of carrot, often without triggering the trap, then left the trap to consume it before returning for another. Common wombats occasionally entered, but only two triggered the traps, and then escaped by digging at the floor until they broke a cable tie holding the outer bag in place. Interference accounted for the remaining 3% of visits when the wallaby did not enter the trap: bait was either removed by other animals during the night, or another swamp wallaby or bobuck inside the trap prevented access to it by physical obstruction or aggression towards the new visitor (Fig. 5b). Other non-target species visited the traps and consumed bait but usually did not trigger the traps: bush rats, Rattus fuscipes, European rabbits, Oryctolagus cuniculus, and satin bower birds, Ptilonorhynchus violaceus. Nonetheless, we largely prevented bush rats removing bait from traps by using bigger pieces of carrot so they could not be easily carried, and because satin bower birds are active only during the day we closed the door of traps that attracted particularly large numbers of these birds during the day.
|

|
Swamp wallabies entered traps on 41% of visits, but were trapped in only 14% of visits. The unsuccessful captures following entry (27% of trap visits) could be attributed to three causes. Failure to trigger the trap on 21% of visits encompassed occasions when the wallaby stood just inside the trap and reached in to obtain bait without disturbing the trigger mechanism (14% of visits) (Fig. 5c), and times when the trigger did not release despite wallaby activity inside the trap (7%). Door failures (3% of visits) occurred when the hinges had rusted and did not swing freely to close the door, or when a wallaby triggered the trap when reaching inside but its tail or rump blocked the door from closing, so was able to escape before the lock bar could fall into place (Fig. 5d).
Discussion
Swamp wallaby trap success in the Yarra Ranges National Park during 2011 was low (9.4%), although this is probably not unusual; Di Stefano (2007) reported a similar trap success of 11.4%. Swamp wallabies at our site visited traps most frequently during darkness, declining steadily by ~13% per hour after sunset. We checked traps 2–3 h after sunset; however, future programs could schedule trap checks and closures more closely aligned with the declining visit rate found in this study. Visits to traps were strongly influenced by lunar phase and presence of moonlight at this site, with the wallabies favouring the low-light conditions of new and crescent moon or when the moon had set. The influence of moonlight on foraging behaviour of small mammals is well documented (e.g. Daly et al. 1992; Kotler et al. 2010), but there has been limited study of the effects of moonlight on larger mammals. Kie et al. (1991) showed that mule deer, Odocoileus hemionus, altered feeding patterns, and Carter and Goldizen (2003) found increased vigilance of brush-tailed rock-wallabies, Petrogale penicillata, on bright moonlit nights. These behavioural responses to bright moonlight have been attributed to increased susceptibility to predation because the light enhanced the ability of predators to detect prey. In swamp wallabies, Osawa (1989) reported a trend towards fewer road-kills, suggesting reduced feeding activity on road verges, on full and gibbous moon nights. Our data are consistent with these studies and suggest that a fear of predators at this site reduced visitation to traps by swamp wallabies under bright moonlit conditions.
When swamp wallabies visited the traps, they still did not enter them on more than half of these visits. In most cases we were unable to determine why this occurred; however, in some cases the wallabies simply consumed the bait outside the trap, then left, or the door had already been closed, primarily by other swamp wallabies or bobucks. The crepuscular to nocturnal habits of bobucks were similar to those of our target species, so we were unable to deter bobucks from the traps without impacting on potential swamp wallaby captures. Had we not placed a few carrots outside the trap we suspect more entries and subsequent door closures by this non-target species would have occurred. By also ensuring that there were large quantities of bait inside the trap, there was greater likelihood that bait would still be present should a swamp wallaby visit the trap.
When swamp wallabies entered traps but did not trigger the door mechanism, the wallaby usually stood just inside the door and reached in to obtain bait, despite our placing bait in the rear corners of the trap. To combat this problem, three changes could be made to traps: (1) increase the length of the pit beneath the trap, (2) fasten bait to the rear of the trap, perhaps in a mesh pocket to avoid entanglement, or (3) increase the length of traps. The first two changes are easily implemented and should be thoroughly tested before considering the third option, which would make traps more difficult to construct and more cumbersome to operate.
The use of camera traps in this study allowed us to explore potential relationships between a set of environmental variables and the rate of visits to traps by our target species, the swamp wallaby. Once wallabies had visited the traps, the camera traps then allowed us to identify aspects of trap design, in addition to behavioural responses of both target and non-target species, which further influenced trap success. Although our primary goal was to identify the most influential environmental and intrinsic factors in order to improve the efficiency of our trapping program, our findings also provide insights for other researchers working with swamp wallabies. Future trapping programs should be scheduled with consideration for time of day, moon phase, and moon rise and set times. Some simple steps can also be taken to reduce the impact of non-target species, and modifications to the trap itself could be made to enhance capture rates. This study highlights the potential of using cameras on traps to better understand the environmental and intrinsic factors that can influence trap success for a wide range of species.
Acknowledgements
This research was supported by Melbourne Water and conducted under University of Melbourne Animal Ethics approval No. 1011809.3 and Department of Sustainability and Environment research permit No. 10005790. We thank John Bennett for the design and construction of the folding traps for this study, Julian Di Stefano and two anonymous reviewers for helpful comments on the draft of this manuscript, and Julian Di Stefano and Sarah Garnick, University of Melbourne, for sharing their experience with wallaby trapping.
References
Albanese, S., Rodríguez, D., and Ojeda, R. A. (2011). Differential use of vertical space by small mammals in the Monte Desert, Argentina. Journal of Mammalogy 92, 1270–1277.| Differential use of vertical space by small mammals in the Monte Desert, Argentina.Crossref | GoogleScholarGoogle Scholar |
Bennett, A. (2008). The impacts of sambar (Cervus unicolor) in the Yarra Ranges National Park. Ph.D. Thesis, The University of Melbourne.
Bluff, L. A., Clausen, L., Hill, A., and Bramwell, M. D. (2011). A decade of monitoring the remnant Victorian population of the brush-tailed rock-wallaby (Petrogale penicillata). Australian Mammalogy 33, 195–201.
| A decade of monitoring the remnant Victorian population of the brush-tailed rock-wallaby (Petrogale penicillata).Crossref | GoogleScholarGoogle Scholar |
Bureau of Meterology (2013). Rainfall ranges. Australian Government, Melbourne. Available at http://www.bom.gov.au/climate/data/ [accessed 14 January 2013].
Caley, P. (1994). Factors affecting the success rate of traps for catching feral pigs in a tropical habitat. Wildlife Research 21, 287–292.
| Factors affecting the success rate of traps for catching feral pigs in a tropical habitat.Crossref | GoogleScholarGoogle Scholar |
Carter, K., and Goldizen, A. W. (2003). Habitat choice and vigilance behaviour of brush-tailed rock-wallabies (Petrogale penicillata) within their nocturnal foraging ranges. Wildlife Research 30, 355–364.
| Habitat choice and vigilance behaviour of brush-tailed rock-wallabies (Petrogale penicillata) within their nocturnal foraging ranges.Crossref | GoogleScholarGoogle Scholar |
Coulson, G. (1996). A safe and selective draw-string trap to capture kangaroos moving under fences. Wildlife Research 23, 621–627.
| A safe and selective draw-string trap to capture kangaroos moving under fences.Crossref | GoogleScholarGoogle Scholar |
Cunningham, R. B., Lindenmayer, D. B., MacGregor, C., Barry, S., and Welsh, A. (2005). Effects of trap position, trap history, microhabitat and season on capture probabilities of small mammals in a wet eucalypt forest. Wildlife Research 32, 657–671.
| Effects of trap position, trap history, microhabitat and season on capture probabilities of small mammals in a wet eucalypt forest.Crossref | GoogleScholarGoogle Scholar |
Daly, M., Behrends, P. R., Wilson, M. I., and Jacobs, L. F. (1992). Behavioural modulation of predation risk: moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Animal Behaviour 44, 1–9.
| Behavioural modulation of predation risk: moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami.Crossref | GoogleScholarGoogle Scholar |
Davis, N. E., Coulson, G., and Forsyth, D. M. (2008). Diets of native and introduced mammalian herbivores in shrub-encroached grassy woodland, south-eastern Australia. Wildlife Research 35, 684–694.
| Diets of native and introduced mammalian herbivores in shrub-encroached grassy woodland, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Department of Sustainability and Environment (2008). Ecological Vegetation Class (EVC) benchmarks for each bioregion. Department of Sustainability and Environment Victoria, Melbourne. Available at http://www.dse.vic.gov.au/conservation-and-environment/native-vegetation-groups-for-victoria/ecological-vegetation-class-evc-benchmarks-by-bioregion [accessed March 2008].
Di Stefano, J. (2007). Home range size and resource selection by the swamp wallaby, Wallabia bicolor, in a landscape modified by timber harvesting. Ph.D. Thesis, The University of Melbourne.
Di Stefano, J., and Newell, G. R. (2008). Diet selection by the swamp wallaby (Wallabia bicolor): feeding strategies under conditions of changed food availability. Journal of Mammalogy 89, 1540–1549.
| Diet selection by the swamp wallaby (Wallabia bicolor): feeding strategies under conditions of changed food availability.Crossref | GoogleScholarGoogle Scholar |
Di Stefano, J., Moyle, R., and Coulson, G. (2005). A soft-walled double-layered trap for capture of swamp wallabies Wallabia bicolor. Australian Mammalogy 27, 235–238.
Geoscience Australia (2011). Astronomical information. Australian Government, Canberra. Available at http://www.ga.gov.au/earth-monitoring/astronomical-information.html [accessed May 2011].
Glen, A. S., Fay, A. R., and Dickman, C. R. (2006). Diets of sympatric red foxes Vulpes vulpes and wild dogs Canis lupus in the Northern Rivers region, New South Wales. Australian Mammalogy 28, 101–104.
| Diets of sympatric red foxes Vulpes vulpes and wild dogs Canis lupus in the Northern Rivers region, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Greenberg, C. H., Neary, D. G., and Harris, L. D. (1994). A comparison of herpetofaunal sampling effectiveness of pitfall, single-ended, and double-ended funnel traps used with drift fences. Journal of Herpetology 28, 319–324.
| A comparison of herpetofaunal sampling effectiveness of pitfall, single-ended, and double-ended funnel traps used with drift fences.Crossref | GoogleScholarGoogle Scholar |
Hollis, C. J., Robertshaw, J. D., and Harden, R. H. (1986). Ecology of the swamp wallaby (Wallabia bicolor) in north-eastern New South Wales. I. Diet. Australian Wildlife Research 13, 355–365.
| Ecology of the swamp wallaby (Wallabia bicolor) in north-eastern New South Wales. I. Diet.Crossref | GoogleScholarGoogle Scholar |
Jury, S. H., Howell, H., O’Grady, D. F., and Watson, W. H. (2001). Lobster trap video: in situ video surveillance of the behaviour of Homarus americanus in and around traps. Marine and Freshwater Research 52, 1125–1132.
| Lobster trap video: in situ video surveillance of the behaviour of Homarus americanus in and around traps.Crossref | GoogleScholarGoogle Scholar |
Kie, J. G., Evans, C. J., Loft, E. R., and Menke, J. W. (1991). Foraging behavior by mule deer: the influence of cattle grazing. The Journal of Wildlife Management 55, 665–674.
| Foraging behavior by mule deer: the influence of cattle grazing.Crossref | GoogleScholarGoogle Scholar |
Kotler, B. P., Brown, J., Mukherjee, S., Berger-Tal, O., and Bouskila, A. (2010). Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proceedings. Biological Sciences 277, 1469–1474.
| Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging.Crossref | GoogleScholarGoogle Scholar |
Le Mar, K., and McArthur, C. (2005). Comparison of habitat selection by two sympatric macropods, Thylogale billardierii and Macropus rufogriseus rufogriseus, in a patchy eucalypt-forestry environment. Austral Ecology 30, 674–683.
| Comparison of habitat selection by two sympatric macropods, Thylogale billardierii and Macropus rufogriseus rufogriseus, in a patchy eucalypt-forestry environment.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., Triggs, B., Eby, P., and Ashby, E. (1990). Analysis of scats of dogs Canis familiaris and foxes Vulpes vulpes (Canidae, Carnivora) in coastal forests near Bega, New South Wales. Wildlife Research 17, 61–68.
| Analysis of scats of dogs Canis familiaris and foxes Vulpes vulpes (Canidae, Carnivora) in coastal forests near Bega, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Merchant, J. C. (2008). Swamp wallaby. In ‘The Mammals of Australia’. 3rd edn. (Eds S. Van Dyck and R. Strahan.) pp. 404–406. (New Holland Publishers (Australia) Pty Ltd: Sydney.)
Morrant, D. S., Petit, S., and Schumann, R. (2010). Floral nectar sugar composition and flowering phenology of the food plants used by the western pygmy possum, Cercartetus concinnus, at Innes National Park, South Australia. Ecological Research 25, 579–589.
| Floral nectar sugar composition and flowering phenology of the food plants used by the western pygmy possum, Cercartetus concinnus, at Innes National Park, South Australia.Crossref | GoogleScholarGoogle Scholar |
Osawa, R. (1989). Road-kills of the swamp wallaby, Wallabia bicolor, on North Stradbroke Island, southeast Queensland. Wildlife Research 16, 95–104.
| Road-kills of the swamp wallaby, Wallabia bicolor, on North Stradbroke Island, southeast Queensland.Crossref | GoogleScholarGoogle Scholar |
Ough, K. (2001). Regeneration of Wet Forest flora a decade after clear-felling or wildfire – is there a difference? Australian Journal of Botany 49, 645–664.
| Regeneration of Wet Forest flora a decade after clear-felling or wildfire – is there a difference?Crossref | GoogleScholarGoogle Scholar |
Pollock, D., and Montague, T. (1991). A new trap trigger mechanism for the capture of swamp wallabies, Wallabia bicolor (Marsupialia: Macropodidae). Wildlife Research 18, 459–461.
| A new trap trigger mechanism for the capture of swamp wallabies, Wallabia bicolor (Marsupialia: Macropodidae).Crossref | GoogleScholarGoogle Scholar |
Robertshaw, J. D., and Harden, R. H. (1986). The ecology of the dingo in northeastern New South Wales. IV. Prey selection by dingoes, and its effect on the major prey species, the swamp wallaby, Wallabia bicolor (Desmarest). Wildlife Research 13, 141–163.
| The ecology of the dingo in northeastern New South Wales. IV. Prey selection by dingoes, and its effect on the major prey species, the swamp wallaby, Wallabia bicolor (Desmarest).Crossref | GoogleScholarGoogle Scholar |
Ruette, S., Stahl, P., and Albaret, M. (2003). Factors affecting trapping success of red fox Vulpes vulpes, stone marten Martes foina and pine marten M. martes in France. Wildlife Biology 9, 11–19.
Swan, M., Di Stefano, J., Greenfield, A., and Coulson, G. (2008). Fine-scale habitat selection by adult female swamp wallabies (Wallabia bicolor). Australian Journal of Zoology 56, 305–309.
| Fine-scale habitat selection by adult female swamp wallabies (Wallabia bicolor).Crossref | GoogleScholarGoogle Scholar |
Triggs, B., Brunner, H., and Cullen, J. (1984). The food of fox, dog and cat in Croajingalong National Park, south-eastern Victoria. Wildlife Research 11, 491–499.
| The food of fox, dog and cat in Croajingalong National Park, south-eastern Victoria.Crossref | GoogleScholarGoogle Scholar |
van Hensbergen, H. J., and Martin, S. C. (1993). Climatic factors affecting trapping success of some South African small mammals. South African Journal of Wildlife Research 23, 87–94.
Vernes, K., and Pope, L. C. (2006). Capture success and population density of the northern bettong Bettongia tropica in northeastern Queensland. Australian Mammalogy 28, 87–92.
| Capture success and population density of the northern bettong Bettongia tropica in northeastern Queensland.Crossref | GoogleScholarGoogle Scholar |
Wiggins, N. L., Williamson, G. J., McCallum, H. I., McMahon, C. R., and Bowman, D. M. J. S. (2010). Shifts in macropod home ranges in response to wildlife management interventions. Wildlife Research 37, 379–391.
| Shifts in macropod home ranges in response to wildlife management interventions.Crossref | GoogleScholarGoogle Scholar |
Willers, N., Mawson, P., Morris, K., and Bencini, R. (2011). Biology and population dynamics of the black-flanked rock-wallaby (Petrogale lateralis lateralis) in the central wheatbelt of Western Australia. Australian Mammalogy 33, 117–127.
| Biology and population dynamics of the black-flanked rock-wallaby (Petrogale lateralis lateralis) in the central wheatbelt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Milne, D. J., and Wanganeen, G. (2001). Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia. Austral Ecology 26, 360–370.
| Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |


