Observations of breeding behaviour and possible infanticide in a wild population of Tasmanian echidnas (Tachyglossus aculeatus setosus)
Rachel L. Harris A B and Stewart C. Nicol AA School of Zoology, University of Tasmania, Churchill Avenue, Hobart, Tas. 7001, Australia.
B Corresponding author. Email: rachel.harris@utas.edu.au
Australian Mammalogy 36(1) 108-112 https://doi.org/10.1071/AM13011
Submitted: 26 April 2013 Accepted: 9 December 2013 Published: 4 March 2014
Abstract
We describe field observations of Tasmanian echidna behaviour, including possible infanticide, where males damaged and entered nursery burrows. We also present the second report of a female producing a second offspring within a single reproductive season after the loss of her first young at an early stage.
Additional keywords: Monotreme, reproductive behaviour, short-beaked echidna.
Introduction
The short-beaked echidna (Tachyglossus aculeatus) is the most common extant species of monotreme and has a near ubiquitous distribution throughout most of mainland Australia, Tasmania, New Guinea and several offshore islands (Griffiths 1968; Augee 2008). Despite being common, details of echidna life history, reproduction and breeding behaviour are quite patchy. Echidnas are usually solitary, cryptic, semifossorial animals (Griffiths 1978) and mating often occurs in shelters (Nicol et al. 2005; Morrow et al. 2009), which makes observing breeding behaviour difficult. Mating occurs between June and September throughout Australia (Morrow et al. 2009) and is characterised by intense male–male competition for access to females (Griffiths 1978; Rismiller and Seymour 1991; Morrow et al. 2009). However, there are several differences in activity patterns, courtship and maternal behaviour between the geographic subspecies (reviewed by Morrow et al. 2009). For example, courtship is relatively prolonged in Kangaroo Island echidnas (T. a. multiaculeatus), where males follow females in ‘trains’ for several days or weeks before the formation of mating ‘ruts’ (Rismiller 1992; Rismiller and McKelvey 2000), while in Tasmania, courtship appears to be minimal during the early part of the breeding season, as males seek out and mate with females before the female’s final emergence from hibernation (Morrow and Nicol 2009). Females typically produce a single young in years in which they have mated (Rismiller and McKelvey 2000; Morrow et al. 2009). Beard and Grigg (2000) report a single female producing a second offspring within a single mating season after the apparent loss of the first young, but it is unclear whether this behaviour also occurs in other subspecies. Females enter purpose-built nursery burrows before egg-laying (Griffiths 1978; Morrow and Nicol 2012), while males provide no parental care. The period of maternal burrow confinement and age at weaning differs between subspecies (reviewed by Morrow et al. 2009). In Tasmania, females construct and enter a single-chambered nursery burrow approximately three days before egg-laying and remain in the nursery until young are ~37 days old. The female then begins to leave the burrow on foraging trips until the young is weaned at ~150 days (Morrow and Nicol 2012).
Echidnas have only recently been successfully and consistently bred in captivity (Ferguson and Turner 2013). This may be due to high levels of offspring mortality during the early stages of egg incubation and lactation, as observed in our Tasmanian study population (Morrow and Nicol 2012). Females may move their nursery burrows to new locations, but offspring often do not survive (Morrow and Nicol 2012) and the causes are unclear, although one possible explanation is male harassment. Female Tasmanian echidnas are promiscuous (Morrow et al. 2009; Morrow and Nicol 2009) and continue to attract males and even mate during pregnancy (Morrow 2013). Pregnant females have been found with males only a few days prior to burrow construction and egg-laying (Morrow 2013; Harris, unpubl. data). It is unknown whether females also continue to attract males after entering nursery burrows, as cameras have not yet been used to monitor burrows at such an early stage of maternal confinement. Here, we used camera traps, external temperature loggers and behavioural observations to intensively monitor several females during the 2012 mating season, in order to describe female behaviour and that of conspecifics around the time of nursery-burrow construction and egg-laying. Specifically, we asked whether females continue to experience harassment by males after burrow construction and around the time of egg-laying, and whether females consequently move to new nurseries.
Materials and methods
Field site and animals
Observations reported here were made as part of a long-term study on ecological and physiological aspects of Tasmanian echidnas (T. a. setosus) at our field site in the southern midlands (42°28′S, 142°14′E), located ~55 km north of Hobart (for details see Morrow et al. 2009; Nicol et al. 2011). Between January 1996 and December 2013, 276 individual echidnas have been captured and fitted with passive implantable (PIT) tags for identification. Selected individuals had radio-frequency transmitters (Holohil Systems, Ontario, Canada) glued to the spines on the lower back, allowing us to monitor mating activity and maternal behaviour up to three times per week during the mating season. Twenty-four adults (15 females, 9 males) had radio-transmitters attached during the 2012 field season. The records from external temperature loggers (Thermochron iButtons, DS1922 L, Maxim/Dallas Semiconductor, TX, USA) attached to the radio-transmitters allow accurate dating of events such as arousal from hibernation, entry into nursery burrows and timing of egg-laying (Nicol and Andersen 2002, 2006; Morrow and Nicol 2009). Camera traps (Scoutguard SG550, HuntingCamOnline, Gadsden, SC, USA; Reconyx PC800, Holmen, WI, USA) were set up outside female hibernacula before the mating period (Morrow and Nicol 2009) and over nursery burrows (Morrow and Nicol 2012), but we set up cameras immediately after the female was first tracked to a plugged burrow following mating activity. The positions of radio-transmitters and colour-coded plastic tubing attached to the spines allowed us to identify individuals on camera footage. Images were downloaded from cameras and batteries replaced at least weekly. We checked for the presence of spermatozoa (confirming recent mating activity) in females’ reproductive tracts by collecting urogenital smears while the animals were under light inhalation anaesthesia (Morrow and Nicol 2009).
Results and discussion
Echidna activity at nursery burrows
During the 2012 breeding season, seven female echidnas were observed in mating aggregations and subsequently entered nursery burrows. Camera traps were set up over 10 separate nurseries between 19 July and 21 September, usually within 2–3 days of burrow construction. The number of nursery burrows exceeds the number of females because one female produced two eggs within the same reproductive season and moved her nursery to different locations several times (details below); we have counted these as separate burrows. At eight nurseries, cameras recorded a combined total of 18 instances of echidna activity (excluding the mother), involving sniffing, digging and probing the soil. This activity was concentrated around the burrow entrance and above the chamber where the female was located, often resulting in substantial damage (Fig. 1). Diggings and new ‘entrances’ were also found before the camera had been set up, indicating high levels of echidna activity in the days immediately after burrow construction. At one nursery burrow, eight instances of intense digging activity were recorded over a three-day period. One female moved her nurseries three times (details below), each time coinciding with intense digging activity and damage at the previous burrow. Known males were recorded outside the nursery burrows of females with which they had previously been found in mating groups (n = 3; echidnas 1B75, 5036, 6D18). The identities and sexes of the remaining animals recorded disturbing nursery burrows (n = 15) are unknown and at least some of these could have been the same individual multiple times. It is likely that these animals were also males, as most reproductive females are confined to nursery burrows by this time of year (Morrow et al. 2009; Morrow and Nicol 2012) and non-reproductive females usually hibernate until mid-September (Nicol and Morrow 2012).
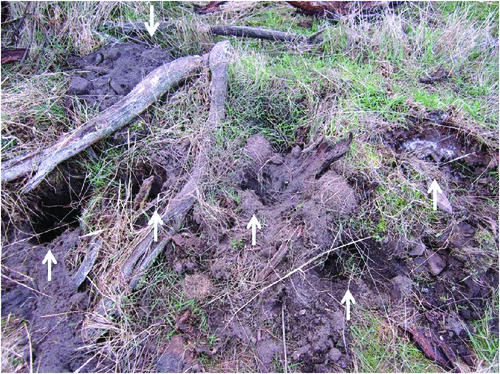
|
Echidnas other than the mother were recorded entering five nursery burrows: three times while females were inside and twice after burrows had been abandoned (Video S1, available as supplementary material to this paper). Known males that had been found with the female in mating aggregations appeared to enter nurseries only after burrows had already been disturbed by other echidnas (n = 2; echidnas 6D18, 5036). The entry of a second echidna was associated with a sudden, prolonged change in temperature recorded by the logger attached to the female’s transmitter (e.g. female 060A, see Table 1, Fig. 2).

|
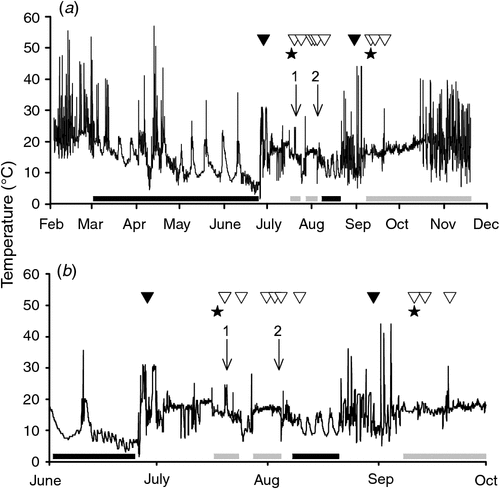
|
Our results show that females attract conspecifics and experience continued disturbance even after entering nursery burrows. Echidnas have occasionally been recorded outside nursery burrows in previous studies, including one instance of an unidentified echidna entering a nursery while the lactating female was away foraging (G. Morrow, pers. comm.), but this is the first time we have set up cameras immediately after burrow construction. Female echidnas in our study population often move to a new nursery burrow early in the incubation or lactation stages and this was previously suggested to be in response to burrow collapse, flooding, or to regulate burrow temperature (Morrow and Nicol 2012). New nursery burrow entrances have also been observed (G. Morrow, pers. comm.), but were thought to be caused by the female abandoning or rebuilding the nursery (Morrow and Nicol 2012). Our observations indicate that females may move nurseries in response to harassment by males and damage caused to the burrow. Male interest in females is suggested to decline after mating has occurred (Rismiller and Seymour 1991; Ferguson and Turner 2013); however, females in our study population continue to attract males during pregnancy (Morrow 2013), and even after entering a nursery (this study). Males probably use sex-specific differences in olfactory cues to locate females during the mating season (Harris et al. 2012) and it is possible that males continue to be attracted to female scent even after she has entered a nursery. The intensity of echidna activity at least indicates that conspecifics detect the presence of females within nursery burrows.
Second female breeding event within a single season
One female (060A) had an egg in her pouch on 23 July (confirmed by visual inspection) and lost her young ~12 days later, possibly as a result of repeated disturbance by males while in two separate nurseries (Table 1). No egg or young was found, but loss of young was confirmed when she abandoned her second nursery and re-entered hibernation. Female 060A emerged from hibernation and was later found with a male (echidna 5036) on 30 August, with no egg or young in her pouch (Table 1, Fig. 2). A 210 g young was later found in her fourth nursery burrow in November. Based on the offspring’s size and development (Morrow and Nicol 2012), we can estimate that fertilisation had occurred in late August. Another female (echidna 2957) also re-entered hibernation after losing her egg (confirmed by visual inspection), and was found in further mating aggregations, but did not produce a second young in 2012.
This is only the second report of a wild female echidna producing a second offspring within a single reproductive season. Beard and Grigg (2000) reported an ~27-day interval between loss of the first young and remating, compared with the ~26-day interval observed here. However, female 060A was often inaccessible after emerging from hibernation, so fertilisation could have occurred before she was found on 30 August. Nevertheless, these timings are consistent with the suggested length of the echidna’s oestrous cycle of 33 days (Higgins et al. 2004). Although we did not recover sperm from 060A after the loss of her first young or see the second egg, successful remating must have taken place as we later found a young in her nursery burrow. Beard and Grigg (2000) state that producing a second offspring within a single season ‘may be more likely to occur in Queensland than in cooler parts of Australia because of the longer time available for young to develop and for the female to regain condition before the onset of a comparatively shorter, milder winter’. Our observations demonstrate that female echidnas are capable of producing a second offspring, even in colder areas such as Tasmania. The loss of an offspring before hatching, or shortly after, would represent a small energetic investment by the mother relative to the major energetic costs of early arousal from hibernation and time spent euthermic with minimal feeding during winter (Nicol and Andersen 2002). Females have been found near our study site with eggs in their pouches as late as October (Morrow et al. 2009), which could also be the result of a second mating after the loss of the first offspring. One female at Philadelphia Zoo produced three eggs over a five-month period, although no young were successfully weaned (T. Sinander, unpubl. obs.).
Infanticide in echidnas?
The observations that females are capable of producing a second offspring following the loss of the first young within a single reproductive season and that males disturb and enter nursery burrows suggest the possibility of sexually selected infanticide in echidnas. Possible infanticide behaviour has been described in captivity (T. Sinander, unpubl. obs.), but not in a wild population. Infanticide (the killing of offspring by conspecifics other than the parents) has been widely documented in mammals (Hrdy 1979; Ebensperger 1998) and may increase male reproductive success, provided (1) the infanticidal male is not related to the young; (2) the mother’s subsequent interbirth interval is shortened; and (3) the male has access to the female when she becomes receptive again (Borries 1997; Ebensperger 1998). Females that lose their offspring are more likely to breed in the following year (Borries 1997; Morrow and Nicol 2012) or even within the same season (this study). Morrow and Nicol (2012) reported that in our study population only 20% of young survived to weaning, while 60% of young died before hatching or in the first two weeks of lactation and it is possible that this is at least partly due to males disturbing females in nursery burrows. Whether this behaviour is truly sexually selected infanticide cannot be verified without genetic information and further monitoring, thereby confirming whether potentially infanticidal males are related to the young, whether they have previously mated with the female and whether they are successful in siring subsequent offspring. The success of the captive breeding program described by Ferguson and Turner (2013) may be partly due to females being housed alone after mating, preventing any opportunity for nursery burrow damage to occur.
Acknowledgements
Funding support for this project was provided by the Holsworth Wildlife Research Endowment, the M. A. Ingram Trust and the National Geographic Committee for Research and Exploration. We thank the McShane family for allowing us access to their property and two anonymous reviewers for their comments. This research was carried out under University of Tasmania Animal Ethics Approval A12320 and the Tasmanian Department of Primary Industries, Water and the Environment Permit FA12069.
References
Augee, M. L. (2008). Short-beaked echidna. In ‘The Mammals of Australia’. 3rd edn. (Eds S. Van Dyck and R. Strahan.) pp. 30–31. (New Holland Publishers: Sydney.)Beard, L. A., and Grigg, G. C. (2000). Reproduction in the short-beaked echidna, Tachyglossus aculeatus: field observations at an elevated site in south-east Queensland. Proceedings of the Linnean Society of New South Wales 122, 89–99.
Borries, C. (1997). Infanticide in seasonally breeding multimale groups of Hanuman langurs (Presbytis entellus) in Ramnagar (South Nepal). Behavioral Ecology and Sociobiology 41, 139–150.
| Infanticide in seasonally breeding multimale groups of Hanuman langurs (Presbytis entellus) in Ramnagar (South Nepal).Crossref | GoogleScholarGoogle Scholar |
Ebensperger, L. A. (1998). Strategies and counterstrategies to infanticide in mammals. Biological Reviews of the Cambridge Philosophical Society 73, 321–346.
| Strategies and counterstrategies to infanticide in mammals.Crossref | GoogleScholarGoogle Scholar |
Ferguson, A., and Turner, B. (2013). Reproductive parameters and behaviour of captive short-beaked echidna (Tachyglossus aculeatus acanthion) at Perth Zoo. Australian Mammalogy 35, 84–92.
| Reproductive parameters and behaviour of captive short-beaked echidna (Tachyglossus aculeatus acanthion) at Perth Zoo.Crossref | GoogleScholarGoogle Scholar |
Griffiths, M. (1968). ‘Echidnas.’ (Pergamon Press: Oxford.)
Griffiths, M. (1978). ‘The Biology of the Monotremes.’ (Academic Press: London.)
Harris, R. L., Davies, N. W., and Nicol, S. C. (2012). Chemical composition of odorous secretions in the Tasmanian short-beaked echidna (Tachyglossus aculeatus setosus). Chemical Senses 37, 819–836.
| Chemical composition of odorous secretions in the Tasmanian short-beaked echidna (Tachyglossus aculeatus setosus).Crossref | GoogleScholarGoogle Scholar | 22871649PubMed |
Higgins, D. P., Tobias, G., and Stone, G. M. (2004). Excretion profiles of some reproductive steroids in the faeces of captive female short-beaked echidna (Tachyglossus aculeatus) and long-beaked echidna (Zaglossus sp.). Proceedings of the Linnean Society of New South Wales 125, 279–286.
Hrdy, S. B. (1979). Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethology and Sociobiology 1, 13–40.
| Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females.Crossref | GoogleScholarGoogle Scholar |
Morrow, G. E. (2013). Optimising reproduction in the Tasmanian echidna Tachyglossus aculeatus setosus: the influence of an obligatory hibernation period and intense sexual conflict. Ph.D. Thesis, University of Tasmania, Hobart.
Morrow, G., and Nicol, S. C. (2009). Cool sex? Hibernation and reproduction overlap in the echidna. PLoS ONE 4, e6070.
| Cool sex? Hibernation and reproduction overlap in the echidna.Crossref | GoogleScholarGoogle Scholar | 19562080PubMed |
Morrow, G. E., and Nicol, S. C. (2012). Maternal care in the Tasmanian echidna (Tachyglossus aculeatus setosus). Australian Journal of Zoology 60, 289–298.
| Maternal care in the Tasmanian echidna (Tachyglossus aculeatus setosus).Crossref | GoogleScholarGoogle Scholar |
Morrow, G., Andersen, N. A., and Nicol, S. (2009). Reproductive strategies of the short-beaked echidna – a review with new data from a long-term study on the Tasmanian subspecies (Tachyglossus aculeatus setosus). Australian Journal of Zoology 57, 275–282.
| Reproductive strategies of the short-beaked echidna – a review with new data from a long-term study on the Tasmanian subspecies (Tachyglossus aculeatus setosus).Crossref | GoogleScholarGoogle Scholar |
Nicol, S. C., and Andersen, N. A. (2002). The timing of hibernation in Tasmanian echidnas: why do they do it when they do? Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology 131, 603–611.
| The timing of hibernation in Tasmanian echidnas: why do they do it when they do?Crossref | GoogleScholarGoogle Scholar |
Nicol, S. C., and Andersen, N. A. (2006). Body temperature as an indicator of egg-laying in the echidna, Tachyglossus aculeatus. Journal of Thermal Biology 31, 483–490.
| Body temperature as an indicator of egg-laying in the echidna, Tachyglossus aculeatus.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. C., and Morrow, G. (2012). Sex and seasonality: reproduction in the echidna (Tachylossus aculeatus). In ‘Living in a Seasonal World: Thermoregulatory and Metabolic Adaptations’. (Eds T. Ruf, C. Bieber, W. Arnold and E. Millesi.) pp. 143–153. (Springer: Heidelberg.)
Nicol, S. C., Andersen, N. A., and Jones, S. M. (2005). Seasonal variations in reproductive hormones in free-ranging echidnas (Tachyglossus aculeatus): interaction between reproduction and hibernation. General and Comparative Endocrinology 144, 204–210.
| Seasonal variations in reproductive hormones in free-ranging echidnas (Tachyglossus aculeatus): interaction between reproduction and hibernation.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. C., Vanpé, C., Sprent, J. A., Morrow, G., and Andersen, N. A. (2011). Spatial ecology of a ubiquitous Australian anteater, the short-beaked echidna (Tachyglossus aculeatus). Journal of Mammalogy 92, 101–110.
| Spatial ecology of a ubiquitous Australian anteater, the short-beaked echidna (Tachyglossus aculeatus).Crossref | GoogleScholarGoogle Scholar |
Rismiller, P. D. (1992). Field observations on Kangaroo Island echidnas (Tachyglossus aculeatus multiaculeatus) during the breeding season. In ‘Platypus and echid.nas’. (Ed. M. L. Augee.) pp. 101–105. (The Royal Zoological Society of New South Wales: Sydney.)
Rismiller, P. D., and McKelvey, M. W. (2000). Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus. Journal of Mammalogy 81, 1–17.
| Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus.Crossref | GoogleScholarGoogle Scholar |
Rismiller, P. D., and Seymour, R. S. (1991). The echidna. Scientific American 264, 96–103.
| The echidna.Crossref | GoogleScholarGoogle Scholar |


