Monitoring of small mammal populations in the Brindabella Ranges after fire
Micah J. Davies A C and Alex Drew BA CSIRO Ecosystem Sciences, Black Mountain Laboratories, Clunies Ross Street, Black Mountain, ACT 2601, Australia.
B Australian National Wildlife Collection, CSIRO Ecosystem Sciences, Gungahlin, ACT 2601, Australia.
C Corresponding author. Email: micah.davies@csiro.au
Australian Mammalogy 36(1) 103-107 https://doi.org/10.1071/AM13017
Submitted: 21 May 2013 Accepted: 28 November 2013 Published: 13 January 2014
Abstract
We live-trapped small mammals in the Brindabella Ranges west of Canberra, Australian Capital Territory from April 2009 until October 2011 to assess population recovery after an intense and widespread fire that occurred across the region in 2003. Three native mammals (agile antechinus, Antechinus agilis; dusky antechinus, Antechinus swainsonii; bush rat, Rattus fuscipes) were encountered. Trapping records and spool-and-line movement patterns suggested a strong association of these small mammals with moist gully vegetation that had survived the fire.
Additional keywords: Antechinus, Brindabellas, Elliott, Rattus, road-crossing.
Introduction
In 2003 a bushfire burnt much of the Brindabella Ranges. The impact of this fire on small mammal populations was largely unknown. In order to assess this impact a short-term small-mammal trapping project was conducted. This work builds on previous research on small mammals in the mountains of the Australian Capital Territory, some of which was very brief and not in a post-fire context (Woolley 1966; Stewart 1979a, 1979b; Dickman 1982, 1983, 1986; Lawrence 1986; Mayo 1987; Mayo et al. 1987; Osborne and Preece 1987; especially Site 1: Lintermans 1988). Here we present trapping data over three consecutive years from a single site for three small mammal species. We also present data on survival and movements, including road crossing.
Methods
Study area
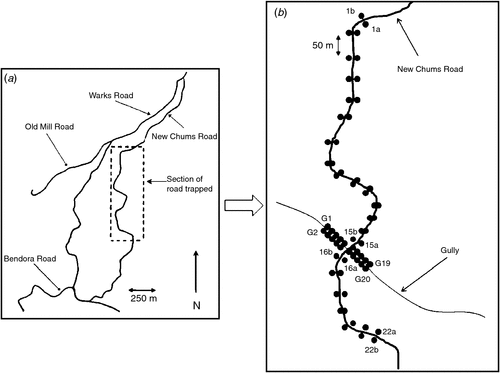
Trapping was conducted along a 1200-m section of New Chums Road, a short (<3 km) management-only access track within Namadgi National Park (35°23′14.17″S, 148°49′58.36″E) (Fig. 1a). The vegetation is classified as ‘Southern Escarpment Wet Sclerophyll Forest’ (Keith 2004). Mean annual rainfall is ~1140 mm per year. A wildfire occurred in 2003 across large parts of the Australian Capital Territory, burning much of the site. In November 2007 the road was cleared of dense post-fire regeneration to allow vehicle access for managers and researchers only. Since then only the northern half of the road has regularly been kept clear of vegetation.
|
Trapping and handling
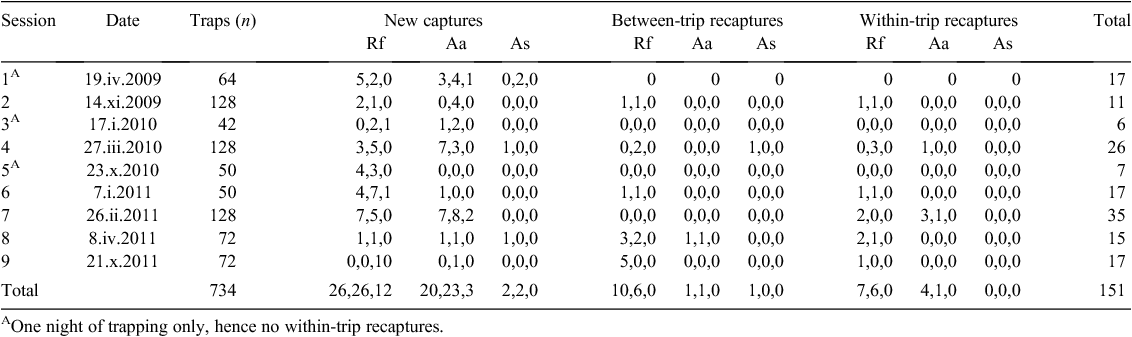
Live-trapping was conducted approximately every two months from April 2009 until October 2011 (excluding winter months to avoid trap deaths due to exposure), resulting in nine trapping sessions (Table 1). We used Elliott folding box traps (100 × 90 × 330 mm, Elliott Scientific Equipment, Upwey, Victoria), baited with a mixture of rolled oats and peanut butter. A wad of non-absorbent Dacron cotton-wool was added to each trap for warmth and each trap was placed inside a thin clear plastic bag for protection from the weather.
Trapping effort varied (both in number of traps and number of nights) between sessions. Fig. 1b shows the typical trapping configuration centred on the gully through which ran an intermittent stream and which comprised a denser and moister habitat than the surrounding forest. The maximum number of traps set per night for any trip was 64 (44 traps in the roadside vegetation and 20 traps in the gully) (Table 1).
Captured animals were transferred from the trap to a cloth bag in which they were weighed. A passive integrated transponder (PIT) tag (11 mm ISP microchip, Allflex Australia) was inserted under the skin between the shoulder blades. The PIT tag number was then scanned using an Allflex Compact Reader. At first capture, and at subsequent recaptures between trips, measurements of head–body length, tail length, ear length and hind foot length were taken using a clear plastic 300-mm ruler. Males and females were assessed for age and breeding condition. Males were classified as adults if their testes were scrotal. Females were assessed as adults if their vaginas were perforate or if their teats showed evidence of lactation. Adult females were also assessed for pregnancy via palpation. Within-trip recaptures were not measured but PIT tag number and trap location were recorded. Occasionally it was not possible to record measurements (e.g. due to damaged tails). All animals were released at point of capture.
Movements as revealed by trapping
Trap location data from recaptures were used to estimate an average distance moved between captures for each species. As traps were set along the edge of this winding road and evidence for road-crossing was found only in and around the gully that contained an under-road pipe (see Results below), movement measurements from recapture events that occurred along the road were taken as a path along the roadside, rather than as a straight line that crossed a bend in the road. Measurements from gully to roadside recaptures (or vice versa) from the same side of the road were taken as a straight line. Measurements were taken using the measuring tool in Google Earth™ and known GPS locations.
Results and discussion
Trapping results
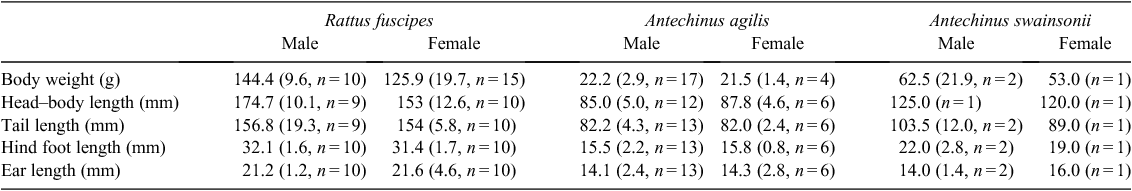
Overall trap success was 20.6%. There were 114 individual animals, with 18 within-trip recaptures and 19 between-trip recaptures (Table 1). There were 64 captures of Rattus fuscipes, 46 Antechinus agilis, and 4 Antechinus swainsonii. No feral small mammal species were observed or trapped during this study. Average adult measurements are presented in Table 2.
A majority of the R.fuscipes captures were from the gully (58 of 93 captures; 62.3% of captures), with the remainder captured from the roadside vegetation. In comparison, 83% (44 of 53) of captures for A.agilis were from the roadside vegetation and only 17% were from the gully. All of these gully captures were within 10 m of the road (i.e. the first two pairs of traps on either side of the road). All captures of A. swainsonii (5 of 5), including one recapture, were from the gully.
The 2003 bush fires did not appear to cause the local extinction of any of these species. The small mammals identified during this study were typical for the location and consistent with previous studies listed above. This study commenced approximately six years after the fire, which was widespread and intense, destroying most of the vegetation and leaving only small pockets of unburnt refugia in the moister gullies. It is likely that animals survived the fire in these refugia and recolonised the surrounding areas when conditions became favourable, which is consistent with other studies that have investigated the effects of fire on small mammal populations (Leonard 1972; Heislers 1974; Recher et al. 1974; Catling 1991).
The relatively low number of captures of A.swainsonii could be due to several factors. Most of the trapping effort was focussed on the roadside vegetation, which is not preferred habitat for this species (Leonard 1970; Hall and Lee 1982). It is also possible that these animals are still in a post-fire recovery stage as they are known to recover slowly after fires (Catling 1991).
Recaptures
There were five within-trip recaptures of A. agilis and all occurred in the same habitat type (either roadside or gully). There were two between-trip recaptures (separated by six weeks), both involving traps around the road/gully intersection (Table 3).
There were 14 within-trip recapture events (8 males, 6 males) of 11 individual R. fuscipes (6 males, 5 females). Of these, only one (#6951) was recaptured in a different habitat (gully to roadside trap closest to gully). There were 15 between-trip recapture events (10 males, 5 females) of 11 individuals (7 males, 4 females). Of these, only two, both male, were caught in a habitat different from their original capture – both were initially captured in roadside vegetation and subsequently recaptured in the gully.
Between-trip recapture data for R. fuscipes using first and last capture dates did not reveal any animals surviving more than 12 months. The maximum interval between recaptures was 34 weeks. Although limited, these data are comparable to those presented by Carron (1985) and Stewart (1979b).
There were no within-trip recaptures of A. swainsonii. There was one between-trip recapture of a female, with an interval of 49 weeks. All captures of A. swainsonii occurred in the gully habitat.
Movements as revealed by trapping
Movement data, combining within- and between-trip recaptures are presented in Table 3. The ‘average distance moved’ figure is derived from successive captures. The average distance moved by A. agilis was 27.5 m (s.d. = 24.7 m, n = 2). One of these (#5311) showed evidence of two road-crossing events involving the gully area.
Only one A swainsonii was recaptured, giving a movement length of ~40 m. Both of these capture events occurred in the gully on either side of the road, also revealing a road-crossing event.
Thirteen R. fuscipes provided movement data via trapping. The average movement distance between successive recaptures for males was 18 m (s.d. = 16 m, n = 7) and for females 36 m (s.d. = 35.8 m, n = 6). The average total distance travelled was 36 m (s.d. = 31 m, n = 7) for males and 48 m (s.d. = 45.5 m, n = 6) for females. Three individuals (animals #1569, #9301 and #9250, all in the gully) provided evidence for road-crossing.
Estimates of home range and movements for small mammals are problematic (Stickel 1954; Wood 1970; Statham 1982). Trapping for this study was not conducted using a grid pattern, trapping sessions were irregularly timed using varying numbers of traps, traps were set with varying gaps in between and trapping alongside a winding road made straight-line measurements difficult. Therefore we have not presented formal estimates of home range length. Instead, we present average distances moved between successive captures and total distance moved. These figures for R. fuscipes are lower than those presented in other studies but our data obviously suffer from a low number of recaptures. Barnett et al. (1978) in northern New South Wales showed that male R. fuscipes moved an average of 95 m between successive captures, and females moved 42 m. Leonard (1972) found that males from the Daylesford area in the non-breeding season had a restricted movement range of ~40 m but in the breeding season this expanded to an average of ~115 m between successive captures.
There were too few recaptures of A. agilis to give any reliable estimates of movement range. The figures given in Table 3 are consistent with two studies close to New Chums Road. Woolley (1966) gives movement data for female A. agilis of 50 yards (45 m) and 200 yards (182 m) and Dickman (1983) gives figures of 50 m for males and 37 m for females.
Trapping results showed that several animals crossed from one side of the road to the other. All of these road-crossing events occurred where the road passes through a gully containing a drainage pipe. During the study the creek in the gully was dry, possibly allowing for easy passage. There was no direct evidence that these animals used the pipe to cross from one side to the other but it was possible and may warrant further investigation.
Acknowledgements
This work was conducted under Australian Capital Territory Government Territory and Municipal Services Scientific Licences LT2009351, LT2010429 and LT2011518. Animal Ethics approval was granted by the CSIRO Sustainable Ecosystems Animal Ethics Committee (CSEAEC) under Application #09-02. We received funding from an ACT Government Environment Grant. We are very grateful to the CSIRO staff, friends and family who have generously and voluntarily given their time and effort – it is much appreciated. Thanks to the ACT Parks and Conservation Service staff, particularly Michael Maconachie and Bob Burdick, for providing ongoing support, advice and accommodation. Thanks to Jacqui Stol and Michael Doherty for the vegetation survey work. Thanks to Dr Peter Brown and Dr Veronica Doerr who provided comments on an early draft of this manuscript.
References
Barnett, J. L., How, R. A., and Humphreys, W. F. (1978). Use of habitat components by small mammals in eastern Australia. Australian Journal of Ecology 3, 277–285.| Use of habitat components by small mammals in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Carron, P. L. (1985). The ecology of three species of small mammals in sub-alpine habitat. Ph.D. Thesis. Australian National University, Canberra.
Catling, P. C. (1991). Ecological effects of prescribed burning practices on the mammals of southeastern Australia. In ‘Conservation of Australia’s Forest Fauna’ . (Ed. D. Lunney.) pp. 353–364. (Royal Zoological Society of New South Wales: Sydney.)
Dickman, C. R. (1982). Some ecological aspects of seasonal breeding in Antechinus (Dasyuridae, Marsupialia). In ‘Carnivorous Marsupials’ . (Ed. M. Archer.) Volume 1, pp. 139–150. (Royal Zoological Society of New South Wales: Sydney.)
Dickman, C. R. (1983). Competition between the dasyurid marsupials Antechinus stuartii and Antechinus swainsonii. Ph. D. Thesis. Australian National University, Canberra.
Dickman, C. R. (1986). An experimental study of competition between two species of dasyurid marsupials. Ecological Monographs 56, 221–241.
| An experimental study of competition between two species of dasyurid marsupials.Crossref | GoogleScholarGoogle Scholar |
Hall, S., and Lee, A. K. (1982). Habitat use by two species of Antechinus and Rattus fuscipes in tall open forest in southern Victoria. In ‘Carnivorous Marsupials’ . (Ed. M. Archer.) Volume 1, pp. 209–220. (Royal Zoological Society of New South Wales: Sydney.)
Heislers, A. (1974). Effects of wildfire on mammals of the mixed species forest of western Victoria. In ‘3rd Fire Ecology Symposium, Monash University, Melbourne, Australia’.
Keith, D. A. (2004). ‘Ocean Shores to Desert Dunes: the Native Vegetation of New South Wales and the ACT.’ (Department of Environment and Conservation (NSW): Hurstville, NSW.)
Lawrence, J. (1986). A survey of the Bulls Head area in the Australian Capital Territory, for Pseudomys fumeus. Unpublished report to Wildlife Research Unit, ACT Parks and Conservation Service, Canberra.
Leonard, B. (1970). Effects of control burning on the ecology of small mammal populations. In ‘2nd Fire Ecology Symposium, Monash University, Melbourne, Australia’.
Leonard, B. (1972). The effect of fire upon selected small mammals and leaf litter fauna in sclerophyll forest in southern Australia. Ph.D. Thesis. Monash University, Melbourne.
Lintermans, M. (1988). A survey of Mt Kelly for Pseudomys fumeus. Unpublished report to Wildlife Research Unit, ACT Parks and Conservation Service, Canberra.
Mayo, G. (1987). The smoky mouse (Pseudomys fumeus) outside Victoria. Victorian Naturalist 104, 188.
Mayo, G., Fisher, J., Fisher, B., and Bollen, R. (1987). A search for the mountain pygmy-possum (Burramys parvus) Broom in Namadgi National Park, ACT. Unpublished Report.
Osborne, W. S., and Preece, M. A. (1987). Extension of the range of the smoky mouse, Pseudomys fumeus (Rodentia: Muridae), into the Australian Capital Territory. Australian Mammalogy 10, 35–36.
Recher, H. F., Lunney, D., and Posamentier, H. G. (1974). Effects of high intensity wildfire on small mammals at Nadgee Nature Reserve, N.S.W. In ‘3rd Fire Ecology Symposium, Monash University, Melbourne, Australia’.
Statham, H. L. (1982). Trappability of Antechinus stuartii (Dasyuridae, Marsupialia) at Petroi, northeastern New South Wales. In ‘Carnivorous Marsupials’ . (Ed. M. Archer.) Volume 1, pp. 333–344. (Royal Zoological Society of New South Wales: Sydney.)
Stewart, A. P. (1979a). Trapping success in relation to trap placement with three species of small mammals, Rattus fuscipes, Antechinus swainsonii and Antechinus stuartii. Australian Wildlife Research 6, 165–172.
| Trapping success in relation to trap placement with three species of small mammals, Rattus fuscipes, Antechinus swainsonii and Antechinus stuartii.Crossref | GoogleScholarGoogle Scholar |
Stewart, A. P. (1979b). Winter adaptation and the bush rat (Rattus fuscipes). Ph.D. Thesis. Australian National University, Canberra.
Stickel, L. F. (1954). A comparison of certain methods of measuring ranges of small mammals. Journal of Mammalogy 35, 1–15.
| A comparison of certain methods of measuring ranges of small mammals.Crossref | GoogleScholarGoogle Scholar |
Wood, D. H. (1970). An ecological study of Antechinus stuartii (Marsupialia) in a south-east Queensland rain forest. Australian Journal of Zoology 18, 185–207.
| An ecological study of Antechinus stuartii (Marsupialia) in a south-east Queensland rain forest.Crossref | GoogleScholarGoogle Scholar |
Woolley, P. (1966). Reproductive biology of Antechinus stuartii Macleay. Ph.D. Thesis. Australian National University, Canberra.