Activity patterns and roosting of the eastern blossom-bat (Syconycteris australis)
Rebecca L. Drury A and Fritz Geiser A BA Centre for Behavioural and Physiological Ecology, Zoology, University of New England, Armidale, NSW 2351, Australia.
B Corresponding author. Email: fgeiser@une.edu.au
Australian Mammalogy 36(1) 29-34 https://doi.org/10.1071/AM13025
Submitted: 12 July 2013 Accepted: 3 August 2013 Published: 30 September 2013
Abstract
We quantified activity patterns, foraging times and roost selection in the eastern blossom-bat (Syconycteris australis) (body mass 17.6 g) in coastal northern New South Wales in winter using radio-telemetry. Bats roosted either in rainforest near their foraging site of flowering coast banksia (Banksia integrifolia) and commuted only 0.3 ± 0.1 km (n = 8), whereas others roosted 2.0 ± 0.2 km (n = 4) away in wet sclerophyll forest. Most bats roosted in rainforest foliage, but in the wet sclerophyll forest cabbage palm leaves (Livistonia australis) were preferred roosts, which likely reflects behavioural thermoregulation by bats. Foraging commenced 44 ± 22 min after sunset in rainforest-roosting bats, whereas bats that roosted further away and likely flew over canopies/open ground to reach their foraging site left later, especially a female roosting with her likely young (~4 h after sunset). Bats returned to their roosts 64 ± 12 min before sunrise. Our study shows that S. australis is capable of commuting considerable distances between appropriate roost and foraging sites when nectar is abundant. Bats appear to vary foraging times appropriately to minimise exposure to predators and to undertake parental care.
Additional keywords: commuting distance, foraging times, roost fidelity.
Introduction
Roost selection and timing of activity of bats are of utmost importance for minimising exposure to predators and climatic extremes, and maximising the time available for feeding so they can fuel their high metabolic requirements (Kunz and Lumsden 2003; Speakman and Thomas 2003). This is especially critical for small nocturnal species that risk predation if they forage too early during the evening, but, on the other hand, will have to cope with increased energy loss due to low night temperatures if foraging commences too late.
The eastern blossom-bat (Syconycteris australis) is one of the smallest members of the Megachiroptera (Nowak 1999). The species is distributed in tropical areas throughout Papua New Guinea and along the north-east coast of Australia as far south as 32°S at Myall Lakes, New South Wales (Law 1994; Winkelmann et al. 2000). In its southern distribution range this bat is known to have one the highest field metabolic rates reported for endotherms (Geiser and Coburn 1999). On the other extreme, like other small subtropical bats (Geiser and Stawski 2011), S. australis employs torpor to minimise energy expenditure even during summer (Geiser et al. 1996; Coburn and Geiser 1998), and it therefore appears that the species must carefully balance energy uptake and requirements by employing optimal or at least appropriate foraging strategies.
Syconycteris australis mainly feeds on nectar and pollen, although in the northern part of its range it also may feed on fruit and foliage (Law 1993; Law and Spencer 2008). In New South Wales, S. australis roosts primarily in littoral rainforest while foraging in nearby coast banksia (Banksia integrifolia) heathland (Law 1993). Fragmentation and/or loss of these two habitats, which are required by this bat for roosting and foraging, may have detrimental effects on blossom-bat populations. For instance, a bat may have to fly further to its foraging site, which may have negative implications on its daily energy budget (Law 1993) and increase the risk of predation.
Available information about foraging, roosting and activity patterns of S. australis is largely limited to the work by Law (1993, 1996, 1997), who showed that adult bats are active for 45% of the night and, in northern Australia, activity patterns are affected by the lunar cycle. Because roosting and activity form such a pivotal part in the life of bats, the purpose of our study was to gain new information specifically about these topics. In particular, we aimed to quantify distance and commuting times between foraging sites and to provide further information about roosts, roost selection and roost fidelity.
Methods
Bats were netted in Iluka Nature Reserve (29°24′S, 153°22′E), situated on the north coast of New South Wales, in early June 2002. Bats were captured using mist nets (9–12 m long, 2.5 m high) erected beneath the canopy in their foraging habitat of open Banksia integrifolia woodland. Banksias were flowering abundantly at the time of trapping. Radio-transmitters (Holohil, LB-2NT, Carp, Ontario, Canada; mass 0.6–0.7 g; ~4% of the animals’ body mass) were attached to each bat’s dorsal surface between the shoulder blades. The fur was clipped close to the skin surface and the transmitter attached using glue (Skinbond, Smith and Nephew, Australia). Bats were then released at the trapping site, ~2 h later. Radio-tracking was conducted from 4 to 13 June.
Bats were tracked to their roost site on the following day, and on each day that they carried the transmitter and could be found their roost location was recorded. The roost sites of some individuals were not found for several days after release. Individual roost trees were identified where possible. A receiver/logger was placed near the roost of each bat to record transmitter signals at 10-min intervals (details in Körtner and Geiser 2000). The first and last time a signal was detected by the receiver/logger station was taken to be the time of arrival and departure from the roost, when the same roost was used on consecutive days. Loggers were checked periodically to ensure the bat was still roosting within reception range (~20 m). After sunset, each transmitter frequency was monitored opportunistically at the trapping site to determine whether bats returned to the same foraging area; presence or absence at that time was recorded. Over the study period sunset occurred at ~16:55 hours and sunrise at ~06:36 hours. The lunar phase over the study period progressed from last quarter to several days following the new moon, so there was no moonlight at dusk. On most days there was some cloud cover.
A hand-held GPS (Garmin GPS 12) was used to determine and record the geographic position of roosts to an accuracy of 10 m. Distances between consecutive roosts were calculated. Distances travelled between roost sites and foraging sites were measured between: (1) the point of capture/nightly radio-tracking and the roost site used the next day; and (2) the last roost site used and the site where the transmitter was shed if found in a banksia feeding area. Numerical data are presented as mean ± 1 standard deviation. ‘N’ represents the number of observations and ‘n’ the number of individuals.
Results
Roosts
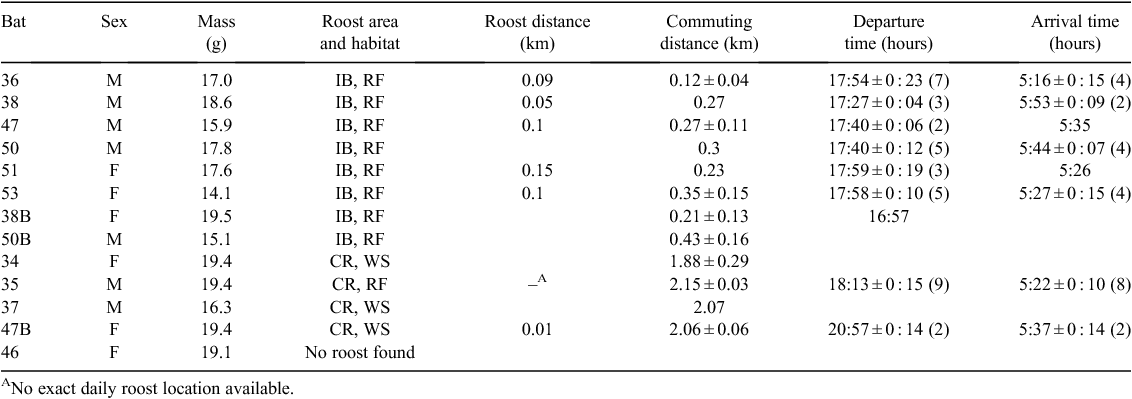
A total of 13 bats, average mass 17.6 ± 1.8 g, were captured, equipped with transmitters, and tracked for 1–10 days to 18 different roost sites. Roost sites of 12 individuals were located; the roost of one bat was never found. After locating the roosts, we were able to visually confirm the presence of bats in 50% of roosts. Bats roosted in two general areas. Most bats (n = 8) roosted in the littoral rainforest inland from Iluka Bluff, within 700 m of the trapping/foraging site. These bats all roosted within a localised area of 6.1 ha, with six individuals all roosting within a core area of only 1.4 ha (Fig. 1). The other bats (n = 4) roosted over 2 km away in wet sclerophyll forest on the western side of the Iluka peninsula near the Clarence River (Clarence River forest). One of these four bats roosted in littoral rainforest; the other three bats roosted in an area of wet sclerophyll forest dominated by flooded gums (Eucalyptus grandis) and cabbage palms (Livistonia australis).
|
Roosts were all situated below the canopy, less than 5 m above the ground (2–5 m), with bats hanging among the dense foliage of small trees, larger trees and overhanging vines. All bats roosted solitarily, with the exception of two individuals (see below). One bat was seen roosting in a small lillypilly (Acmena spp.), covered heavily with a vine (Smilax australis), only 2 m above the ground; this roost was used on two consecutive days, although different leaves were used on each day. Two bats were observed roosting in large clumps of dense, dead leaves hanging beneath the canopy in which the bats were well camouflaged. The three bats roosting in the Clarence River forest were all seen at the base of cabbage palm leaves; two were seen using dead palm leaves as well as live ones. One of these bats was seen under a live cabbage palm leaf, the next day it was 10 m away under a dead palm leaf and two days later was, again, under the first palm leaf. This bat (female #47B) was seen twice huddling with a smaller bat (wing around smaller bat), possibly a dependent young (Fig. 2).

|
Six bats were observed using multiple roosts, with an average distance between roosts of 90 ± 50 m. However, one bat moved from one known roost to another roost that was never found, possibly much further away.
Activity patterns
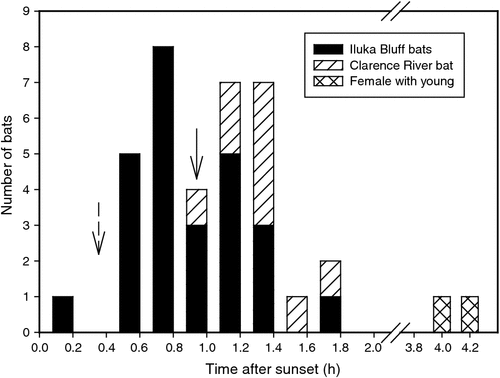
Roost departure and arrival times were recorded for nine bats over periods lasting 1–8 days. All bats left the roost after sunset (Fig. 3; Table 1) with a mean departure time of 17 : 53 hours ± 22 min (N = 35, n = 8), 58 min after sunset (excluding departure times of female #47B with an apparently dependent young, which left its roost considerably later than all other bats: 20 : 57 hours ± 14 min, N = 2; 4 h 2 min after sunset). One bat roosting in the rainforest left its roost only 2 min after sunset, well before full darkness (civil twilight occurred 26 min after sunset), whereas all other bats left after complete darkness had fallen. The only bat monitored for multiple days from the Clarence River forest (bat #35) left the roost later (18 : 13 hours ± 15 min, N = 9; 1 h 18 min after sunset) than the bats roosting at Iluka Bluff (17 : 46 hours ± 20 min, N = 26, n = 7; 51 min after sunset). All bats from all areas returned to their roosts between 36 min and 1 h 30 min before sunrise (Table 1) at a mean time of 05 : 29 hours ± 15 min (N = 26, n = 8), 1 h 7 min before sunrise.
|
The distance travelled from roost to foraging site fell into two groups according to their roost area (Table 1). Bats roosting in the Clarence River forest travelled 2.03 ± 0.17 km (N = 6, n = 4) to their foraging sites, while bats roosting in the Iluka Bluff rainforest moved only 0.27 ± 0.13 km (N = 13, n = 8). All bats returned to the original foraging site at least once over the study period, with an average return rate of 78%. Eight bats returned on every night of tracking; however, four of these bats were tracked for only 1–3 nights before the transmitters fell off. The other four bats showed strong fidelity to their original foraging site, being recorded at the site every night for 6–8 nights. Of these four bats, two adult males were roosting over 2 km away while an adult male and subadult female were roosting 90–400 m from the foraging site. Specifically, male #35 roosting in the Clarence River forest, every night for 8 nights travelled the 2.15 km distance to the foraging area and back to its roost in the morning. On one night the signal from this bat was heard near the foraging site only 3 min after the last time a signal was recorded on the data logger at its roost, a flight speed of ~40 km h−1, while on other nights this interval was between 15 and 34 min (mean = 18.5 ± 12.9 min).
Transmitters from nine bats were found in or underneath B. integrifolia, i.e. at banksia foraging sites; only one transmitter was found beneath the roost site of a bat (Fig. 1). Five transmitters were shed in the original foraging area within 300 m of the trapping site. Two bats shed their transmitters at a separate foraging site, 700 m west of the trapping site. This was an inland sand dune, with a tall stand of B. integrifolia bordered by littoral rainforest and dry sclerophyll woodland. Another bat shed its transmitter 500 m north-west of the trapping site, on a B. integrifolia–covered dune that was continuous with the dune where the trapping site was located.
Discussion
The roosts of Syconycteris australis previously recorded during winter at Iluka Nature Reserve were mainly clustered in the rainforest along the edge of the Iluka Bluff road (Fig. 1) (Law 1993). Similarly, we found the majority of bats roosting in a 1.4-ha area at the edge of the same road, with no bats found roosting further into the rainforest along the walking track. However, in addition we found bats using a separate habitat in the Clarence River forest on the western side of the Iluka Peninsula. All bats roosted amongst rainforest foliage or cabbage palms, although some bats roosted, not in littoral rainforest, but in wet sclerophyll forest that makes up the transition zone between rainforest and Melaleuca swamp, an area dominated by E. grandis (Nicolls 1966). The roosts described by Law (1993) at Harrington, south of Iluka where the rainforest is patchily distributed, were similar to those found in this transition zone, with roosts under large cabbage palm leaves.
Twelve of our tracked bats were roosting solitarily, as has been found in previous studies (Law 1993). However, one female shared a roost with a visibly smaller bat. Little is known on the reproduction of S. australis, although they may give birth twice a year with one time occurring between February and April; lactation may last for up to 3 months (Law and Spencer 2008). It is likely that this bat was a mother with a dependent young born during the second breeding season of the year. It is interesting that this was the only bat to leave its roost long (4 h 2 min) after sunset, ~3 h after the other bats, suggesting that the bat may have been involved in some form of parental care.
The tracked bats showed a high fidelity to their original foraging site, although their presence at other foraging sites was also shown by the location of shed transmitters, with two other foraging sites identified. Law (1993) found that several bats visited foraging sites on the way to their main foraging site and it is possible these other sites we found were used in a similar way.
In a fragmented landscape S. australis commuted longer distances to its foraging sites (Law 1993), but Iluka is not highly fragmented, with large areas of seemingly suitable roosting habitat close to foraging habitat. So why would the bats roost so far away from their banksia foraging site? It is possible that food resources abound in Clarence River forest at other times of the year than when our study was conducted and that bats continue to show area fidelity in roost selection year round. Alternatively, it may be related to the ambient temperature differentials within the forest because bats during winter prefer to roost on the edge of the rainforest where temperatures are higher (Law 1993). The sclerophyll Clarence River forest is a much more open habitat than the rainforest near the foraging site so sun can penetrate and ambient temperatures in the former will be higher and provide preferable roost sites in winter. Moreover, roosting under cabbage palm leaves exposed to sun (Fig. 3) may have further increased ambient temperatures and positively contributed to behavioural thermoregulation (Stawski et al. 2009), as well as protecting the bats from rain.
It is likely that roost departure time is related to the location of the bats’ roost site and exposure to light during commuting. Bat #35 roosting in Clarence River forest regularly left later (27 ± 20 min) than the mean time of all bats roosting at Iluka Bluff to travel 2 km to the same foraging site. The observed maximum speed with which this bat was able to travel the distance implies that it would have flown directly to the foraging site above the canopy, much of this across the open ground of a golf course. Conversely, the bat that departed earliest from its roost left only 2 min after sunset, well before civil twilight. This bat was roosting beneath the rainforest canopy only 200 m from the banksia foraging site and was probably able to remain beneath the canopy for its nightly commute. Blossom bats tracked in the rainforests of north Queensland were found to vary their departure time according to the lunar phase, or, more specifically, the brightness of the sky at dusk (Law 1997). Bats delayed their departure times by 1.5–4.0 h after sunset on brightly lit nights. This delay possibly reflects predation pressure from several species of owls found in the area (Law 1997). Owl species that are known to prey on bats are also present in the Iluka area (Majnep et al. 1977; Debus and Chafer 1994; Kavanagh et al. 1995; Rose 1996; S. Debus, pers. comm.). We observed a southern boobook owl (Ninox novaeseelandiae) regularly roosting low (~1 m above the ground) in a bush in the rainforest site not far from the roosting bats. Possibly this predation pressure and visibility of bats flying in the open before night are responsible for the differing roost departure times between the two roost areas. For the similarly sized, frugivorous bat Carollia perspicillata, roost departure time is closely related to ambient light levels, with bats roosting in exposed roosts leaving up to an hour later than those roosting in the forest (Heithaus and Fleming 1978) and in the tube-nosed bat Nyctimene robinsoni, nocturnal activity is reduced on moonlit nights (Riek et al. 2010).
What are the implications for these delays in terms of the daily energy budget of bats that leave roosts later? The insectivorous bat Eptesicus fuscus was found to roost up to 4.4 km from feeding grounds, even though roosts were plentiful nearby, perhaps because energetic costs of commuting are trivial in relation to total daily energy budget (Brigham 1991). Whereas the higher cost of a longer commute may also be small in S. australis, loss in foraging time could have potential energetic consequences. On average, Syconycteris australis spends 45% (6.45 h in winter) of the night active (Law 1993). Daily energy expenditure of S. australis in winter is 76.9 kJ day–1 (Geiser and Coburn 1999) and of this ~57 kJ is required for flight as predicted for a 18-g bat (Speakman and Racey 1991; Winter and von Helversen 1998; Geiser and Coburn 1999). A 18.5-min commute flight, as observed on average for bat #35, would require ~2.6 kJ, which is less than the energy saved by an average 5.5-h torpor bout observed in S. australis in winter (Coburn and Geiser 1998; Geiser and Coburn 1999). So purely from an energetic point of view, the bats should be able to compensate for the loss of energy intake. If time for foraging is considered, a 27-min delay in foraging results in less than 10% reduction in foraging time, which should be of little consequence since bats are not active throughout the night and the late arrival simply will shift the onset of foraging. As nectar is abundant in winter (Law 1996), the loss in foraging time and extra commuting cost should not adversely affect the bats’ ability to balance their daily energy budget at that time of the year.
Acknowledgements
We thank Gerhard Körtner for constructive comments on the manuscript and for producing the map, and Christopher Turbill for comments on the manuscript. The Australian Research Council provided financial support for the study. The work was conducted under a permit provided by the University of New England Animal Ethics Committee and the NSW National Parks and Wildlife Service.
References
Brigham, R. M. (1991). Flexibility in foraging and roosting behaviour by the big brown bat (Eptesicus fuscus). Canadian Journal of Zoology 69, 117–121.| Flexibility in foraging and roosting behaviour by the big brown bat (Eptesicus fuscus).Crossref | GoogleScholarGoogle Scholar |
Coburn, D. K., and Geiser, F. (1998). Seasonal changes in energetics and torpor patterns in the sub-tropical blossom-bat Syconycteris australis (Megachiroptera). Oecologia 113, 467–473.
| Seasonal changes in energetics and torpor patterns in the sub-tropical blossom-bat Syconycteris australis (Megachiroptera).Crossref | GoogleScholarGoogle Scholar |
Debus, S. J. S., and Chafer, C. J. (1994). The powerful owl Ninox strenua in New South Wales. Australian Birds 28, 21–38.
Geiser, F., and Coburn, D. K. (1999). Field metabolic rates and water uptake in the blossom-bat Syconycteris australis (Megachiroptera). Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 169, 133–138.
| Field metabolic rates and water uptake in the blossom-bat Syconycteris australis (Megachiroptera).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3ktlOksQ%3D%3D&md5=1466dfa881003fa7c03874d898e7c8a5CAS | 10227186PubMed |
Geiser, F., and Stawski, C. (2011). Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integrative and Comparative Biology 51, 337–348.
| Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy.Crossref | GoogleScholarGoogle Scholar | 21700575PubMed |
Geiser, F., Coburn, D. K., Körtner, G., and Law, B. S. (1996). Thermoregulation, energy metabolism, and torpor in blossom-bats, Syconycteris australis (Megachiroptera). Journal of Zoology 239, 583–590.
| Thermoregulation, energy metabolism, and torpor in blossom-bats, Syconycteris australis (Megachiroptera).Crossref | GoogleScholarGoogle Scholar |
Heithaus, E. R., and Fleming, T. H. (1978). Foraging movements of a frugivorous bat, Carollia perspicillata (Phyllostomatidae). Ecological Monographs 48, 127–143.
| Foraging movements of a frugivorous bat, Carollia perspicillata (Phyllostomatidae).Crossref | GoogleScholarGoogle Scholar |
Kavanagh, R. P., Debus, S. J. S., Rose, A. B., and Turner, R. J. (1995). Diet and habitat of the barking owl Ninox connivens in New South Wales. Australian Bird Watcher 16, 137–144.
Körtner, G., and Geiser, F. (2000). Torpor and activity patterns in free-ranging sugar gliders Petaurus breviceps (Marsupialia). Oecologia 123, 350–357.
| Torpor and activity patterns in free-ranging sugar gliders Petaurus breviceps (Marsupialia).Crossref | GoogleScholarGoogle Scholar |
Kunz, T. H., and Lumsden, L. F. (2003). Ecology of cavity and foliage roosting bats. In ‘Bat Ecology’. (Eds T. H Kunz and M. B. Fenton). pp. 3–89. (University of Chicago Press: Chicago.)
Law, B. S. (1993). Roosting and foraging ecology of the Queensland blossom bat (Syconycteris australis) in northern New South Wales: flexibility in response to seasonal variation. Wildlife Research 20, 419–431.
| Roosting and foraging ecology of the Queensland blossom bat (Syconycteris australis) in northern New South Wales: flexibility in response to seasonal variation.Crossref | GoogleScholarGoogle Scholar |
Law, B. S. (1994). Climatic limitation of the southern distribution of the common blossom bat Syconycteris australis in New South Wales. Australian Journal of Ecology 19, 366–374.
| Climatic limitation of the southern distribution of the common blossom bat Syconycteris australis in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Law, B. S. (1996). Residency and site fidelity of marked populations of the common blossom-bat Syconycteris australis in relation to the availability of Banksia inflorescences in New South Wales, Australia. Oikos 77, 447–458.
| Residency and site fidelity of marked populations of the common blossom-bat Syconycteris australis in relation to the availability of Banksia inflorescences in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Law, B. S. (1997). The lunar cycle influences time of roost departure in the common blossom bat, Syconycteris australis. Australian Mammalogy 20, 21–24.
Law, B. S., and Spencer, H. J. (2008). Eastern blossom-bat Syconycteris australis. In ‘The Mammals of Australia’. (Eds S. Van Dyck and R. Strahan.) pp. 428–429. (Reed New Holland: Sydney.)
Majnep. I.S., Bulmer, R.N.H. and Healey, C.J. (1977). ‘Birds of My Kalam Country.’ (University of Auckland Press: Auckland.)
Nicolls, A. O. (1966). A report on the vegetation of the coastal sands of the Iluka region, north coast NSW. B.Sc. Honours Thesis, University of New England, Armidale.
Nowak, R. M. (1999). ‘Walker’s Mammals of the World.’ (Johns Hopkins University Press: Baltimore and London.)
Riek, A., Körtner, G., and Geiser, F. (2010). Thermobiology, energetics and activity patterns of the Eastern tube-nosed bat (Nyctimene robinsoni) in the Australian tropics: effect of temperature and lunar cycle. Journal of Experimental Biology 213, 2557–2568.
| 20639416PubMed |
Rose, A. B. (1996). Notes on the diet of the southern boobook Ninox novaeseelandiae in New South Wales. Australian Bird Watcher 16, 339–343.
Speakman, J. R., and Racey, P. A. (1991). No cost of echolocation for bats in flight. Nature 350, 421–423.
| No cost of echolocation for bats in flight.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M7oslWisw%3D%3D&md5=92b19c615d4f12dafd504da5124a5c3fCAS | 2011191PubMed |
Speakman, J. R., and Thomas, D. W. (2003). Physiological ecology and energetics of bats. In ‘Bat Ecology’. (Eds T. H. Kunz, and M. B. Fenton.) pp. 430–490. (University of Chicago Press: Chicago.)
Stawski, C., Turbill, C., and Geiser, F. (2009). Hibernation by a free-ranging subtropical bat (Nyctophilus bifax). Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 179, 433–441.
| Hibernation by a free-ranging subtropical bat (Nyctophilus bifax).Crossref | GoogleScholarGoogle Scholar | 19112568PubMed |
Winkelmann, J. R., Bonaccorso, F. J., and Strickler, T. L. (2000). Home range of the southern blossom bat, Syconycteris australis, in Papua New Guinea. Journal of Mammalogy 81, 408–414.
| Home range of the southern blossom bat, Syconycteris australis, in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Winter, Y., and von Helversen, O. (1998). The energy costs of flight: do small bats fly more cheaply than birds? Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology 168, 105–111.
| The energy costs of flight: do small bats fly more cheaply than birds?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3gtlamsw%3D%3D&md5=8d4082da2b341c5381cba75c75914a15CAS | 9542147PubMed |