Long-term fluctuations in distribution and populations of a threatened rodent (Pseudomys novaehollandiae) in coastal woodlands of the Otway Ranges, Victoria: a regional decline or extinction?
Barbara A. Wilson A B , Mandy Lock A and Mark J. Garkaklis AA School of Life and Environmental Sciences, Faculty of Science Engineering and Built Environment, Deakin University, Burwood, Vic. 3125, Australia.
B Corresponding author. Email: barbara.wilson@deakin.edu.au
Australian Mammalogy 40(2) 281-293 https://doi.org/10.1071/AM17036
Submitted: 3 July 2017 Accepted: 9 November 2017 Published: 18 December 2017
Abstract
Since European settlement Australian native rodents have experienced dramatic extinctions and declines. We investigated long-term population and distribution changes during 1981–2003, and known or potential causal factors of decline in the vulnerable New Holland mouse (Pseudomys novaehollandiae). We found that populations (n = 8) were extant for 1–6 years and were predominantly small, localised and extinction prone. High-density populations occurred after above-average rainfall but declined precipitously during drought. Wildfire resulted in the extirpation of some populations, while others survived in unburnt refugia. We propose that post-fire vegetation (3–7 years) contemporaneous with above-average rainfall delivered productive habitat resulting in both a population irruption, and recovery after wildfire. Population declines occurred in drought periods. Recent trapping at 42 sites (2013–17) failed to record any New Holland mice. The species has not been recorded since 2003. Recovery is unlikely without intensive management, focussed on remnant or reintroduced populations, including protection from habitat fragmentation and inappropriate fire regimes. Prevention of extinction of the species throughout its southern range will require similar management strategies.
Additional keywords: climate, fire, New Holland mouse, rainfall.
Introduction
Severe extinctions and declines of Australian mammals have occurred since European settlement (Burbidge and McKenzie 1989; Johnson 2006; McKenzie et al. 2007; Burbidge et al. 2008; Woinarski et al. 2014, 2015). Our understanding of how or why many species have declined is poor. The endemic conilurine rodents have been severely impacted, particularly in southern Australia, and more recently in northern Australia (Lee 1995; Smith and Quin 1996; Lawes et al. 2015; Wayne et al. 2017). Of 49 conilurine rodent species, eight are extinct and 35 are in decline (Smith and Quin 1996; IUCN 2010). Declines are high amongst Notomys (hopping mice) and Pseudomys (native mice) (Lawes et al. 2015). Declines have been correlated with ecosystem productivity (rainfall), grazing and fire, factors that result in declines in vegetation structure and increased vulnerability to fox and cat predation (Dickman et al. 1993; Smith and Quin 1996; Lawes et al. 2015).
Long-term studies of the dynamics of native rodents in Australian arid systems have found that rainfall has a significant impact on their distribution and population abundance (Dickman et al. 1999; Letnic et al. 2004, 2005, 2011; Letnic and Dickman 2005, 2006; Greenville et al. 2009). Species such as the plains mouse (Pseudomys australis), and the sandy inland mouse (P. hermannsburgensis) increased in numbers following significant rainfall events and underwent population crashes during drought (Masters 1993; Southgate and Masters 1996; Letnic and Dickman 2005, 2006; Letnic et al. 2005, 2011; Greenville et al. 2009, 2012). These population fluctuations related to increased food supply and resource depletion, respectively, impacting on reproduction and recruitment (Predavec 1994; Brandle and Moseby 1999; Letnic and Dickman 2006).
In temperate south-eastern Australia, rodent population dynamics have been strongly associated with the structure and floristic composition of habitats and with fire regimes (Catling 1986; Fox 1996; Fox and Monamy 2007; Arthur et. al. 2012). Different species recolonise recovering burnt areas at different rates and recovery has been related to variables such as fire intensity and species’ habitat requirements (Newsome et al. 1975; Catling and Newsome 1981; Fox and McKay 1981; Fox 1982, 1983, 1990; Catling 1991). The rate of recovery of vegetation, not time per se, has been proposed as the most important factor (Monamy and Fox 2000; Fox and Monamy 2007). Rainfall and drought also impact population dynamics in temperate communities. For example, Lunney et al. (1987) found significant suppression of rodent populations for the full extent of an intense drought in eucalypt forest, while Recher et al. (2009) recorded a milder response at another site. While evidence of ‘booms and busts’ characteristic of arid-zone rodents have not been common, the rare Pilliga mouse (Pseudomys pilligaensis) did exhibit a population irruption following high rainfall before suddenly declining to a low density (Tokushima et al. 2008).
The New Holland mouse (Pseudomys novaehollandiae), an Australian endemic, is a burrowing species found in small, disjunct coastal populations in Queensland, New South Wales, Victoria and Tasmania (Menkhorst et al. 2009; Threatened Species Scientific Committee 2010). The species occurs on vegetated sand dunes and in closed–open heathland, woodland and forest, and has a preference for floristically rich vegetation and habitat regenerating after disturbance, particularly fire (Braithwaite and Gullan 1978; Fox and Fox 1978, 1984; Kemper 1990; Wilson 1991, 1996b; Lock and Wilson 1999; Wilson and Laidlaw 2003; Wilson et al. 2005).
The New Holland mouse is classified as Vulnerable under the national Environment Protection and Biodiversity Conservation Act 1999 as its geographical distribution (~420 km2) is limited and it is facing a high risk of extinction (Threatened Species Scientific Committee 2010). In Victoria the species is considered to be critically endangered as it has been lost from six localities and its area of occupancy has declined by 45%, due to a combination of threats including altered fire regimes, habitat loss and fragmentation (Seebeck et al. 1996; Threatened Species Scientific Committee 2010).
Long-term research investigating the reproduction, population ecology and captive breeding of the New Holland mouse has been conducted in the eastern Otways since 1981; however, studies ceased in 2003 (Kentish 1982, 1983; Wilson et al. 1990; Wilson 1991, 1994, 1996a; Lock and Wilson 1999, 2017; Lock 2005). Trial reintroductions were undertaken in 2002 to develop techniques and protocols involving soft release from enclosed pens; however, studies on the species ceased after the trials (Tidey 2003; Gibson et al. 2004). In 2013 it was evident that the status of the species in the region was unclear and required reassessment. The aims of this paper were thus to: investigate the species’ current distribution and status compared with previous records, assess long-term population changes, and identify known or potential causal factors of decline to provide information for effective management and recovery.
Materials and methods
Study area
The study area was in the eastern Otway Ranges 100 km south-west of Melbourne, Victoria (Fig. 1). The area is predominantly public land including the Great Otway National Park and the Anglesea Heath, which is recognised for its high biodiversity values (McMahon and Brighton 2002). The vegetation communities comprise a mosaic of eucalypt forests, woodlands, heathlands, and shrublands (Land Conservation Council Victoria 1985). An area of 7500 ha was leased (1961–2015) by Alcoa of Australia Pty Ltd for brown coal extraction.
The most extensive recent fire, the ‘Ash Wednesday’ wildfire (1983), burnt 40 000 ha, leaving a few small unburnt patches (Wilson et al. 1990). Fuel-reduction burns have been undertaken since 1986 and an increase in burning is being implemented as a result of recommendations of the 2009 Victorian Bushfires Royal Commission (Parliament of Victoria 2010; Coates et al. 2013; Department of Environment and Primary Industries 2013, 2014). Regular fox baiting has been conducted since 2005 (Parks Victoria and DSE 2009).
Assessment of current distribution and abundance (2013–17)
We re-established trapping at sites that were originally studied between 1981 and 2003. Live-trapping was undertaken between May 2013 and April 2017 at 42 sites, nine of which where the species had been trapped previously (Fig. 1). Trapping was conducted annually (1–3 years per site) over a total of 6378 trap-nights at 21 sites in 2013–14, 28 sites in 2015, 13 sites in 2016 and eight sites in 2017. Camera trapping was conducted at three sites in 2013–14, 20 sites in 2015, five sites in 2016 and six sites in 2017.
Trapping was undertaken using Elliott traps (33 × 9 × 10 cm) baited with a bait mixture of rolled oats, honey and peanut butter. Thirty traps were set for three nights in transect formation in lines of 10 at 10–15-m intervals at most sites. Species, sex, reproductive condition, body weight and body measurements were recorded for each individual animal.
Two cameras (Reconyx HC600 Hyperfire Passive Infrared) were established ~100 m apart at 27 sites, for 1–5 weeks. Bait lures were not employed in order to avoid bias towards specific species. Cameras were set to high sensitivity with a 15-s interval between triggers and three images per trigger. They were secured vertically 0.2–1 m from ground level, and dense vegetation was cleared from the field of view. Photographs of individuals were identified to species level using reference photographs from the area and species descriptions (Van Dyck and Strahan 2008; Van Dyck et al. 2013).
Assessment of long-term abundance trends at repeat trapping sites (1981–2015)
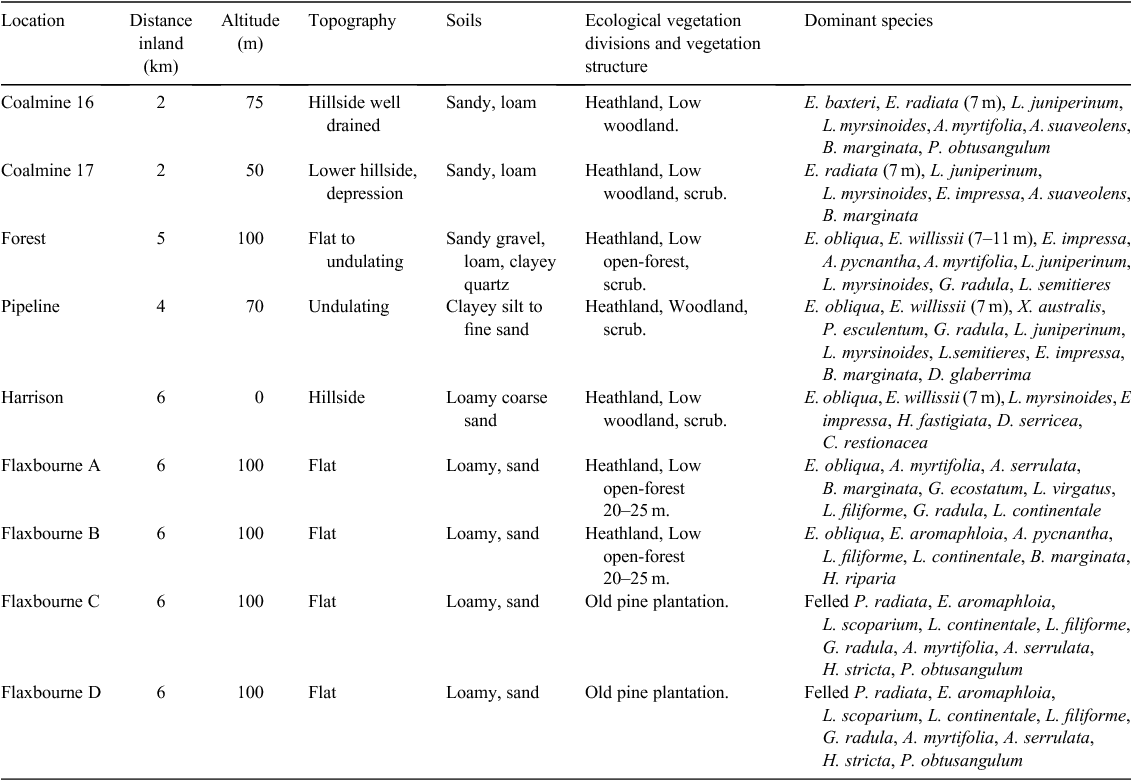
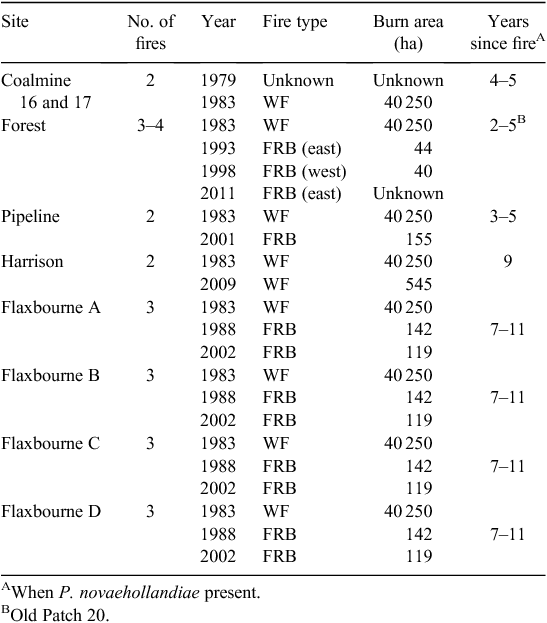
Long-term capture data were available for the New Holland mouse at nine sites (Table 1, Fig. 1). Trapping conducted in 1981 west of the Anglesea River resulted in the capture of the New Holland mouse at two heathy woodland sites (Coalmine 16 and 17) (Fig. 1, Table 1). One grid consisting of 100 trap sites at 20-m intervals (4 ha) was established at each site and trapping was undertaken (1981–82) on nine occasions at Coalmine 16 and twice at Coalmine 17. Both sites were burnt in the 1983 wildfire and trapping was then conducted annually during 1983–1988, 1991–1994 and in 1998. After 1998 the Coalmine 17 site was destroyed by mining, while Coalmine 16 was surveyed in 2001 before it too was mined.
|
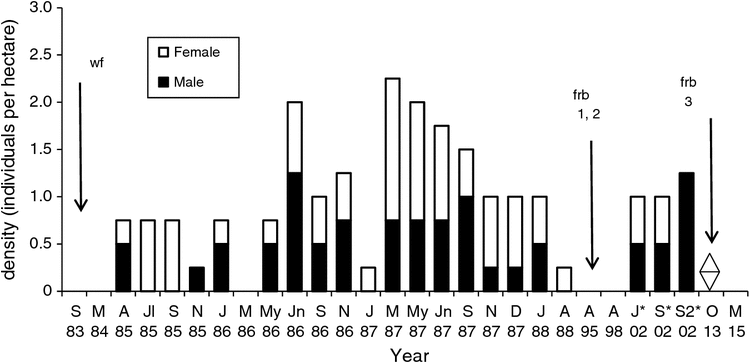
Following the 1983 wildfire the New Holland mouse was captured at two sites east of the Anglesea River in low forest (Forest; F in Fig. 1) and woodland (Pipeline; P in Fig. 1) (Table 1). The first captures of the species at the Forest site in 1985 were in a small unburnt patch ~20 years after fire and at Pipeline (1985) in burnt habitat. At the Forest site a grid (5 × 20) with 20-m trap spacing (4 ha) was trapped from July 1985 to August 1988 on 18 occasions. In all, 5% of the grid covered the unburnt patch and 95% the 1983 burnt area. At the Pipeline site a grid (3 × 20) with 20-m trap spacing (2.4 ha) was trapped from March 1986 to August 1988 on 13 occasions. Trapping was undertaken at the Forest site in 1995 and 1998 and during a trial reintroduction in July 2002 (2 males, 1 female) (Gibson et al. 2004). Recently, trapping was undertaken at the Forest site (October 2013, March 2015), and at the Pipeline site (April 2014, May 2015).
In 1991 the New Holland mouse was captured at a site east of the Anglesea River (Harrison north) (Table 1; H in Fig. 1). The site was burnt in the 1983 wildfire (nine years after fire). A grid consisting of 10 rows of 10 with 10-m trap spacing (1 ha) was established and was trapped from February to September 1992, on six occasions. Trapping was then conducted in 2002 (July, September, November) and 2003 (February) during trial reintroductions. The trials conducted in 2002 were undertaken in July (2 males, 1 female), September (5 males), October (6 males) and November (6 females). Recent trapping was conducted in June 2014 and May 2015.
The New Holland mouse was captured at four sites east of the Anglesea River (Flaxbourne Road) in 1995. The sites had been burnt during the 1983 wildfire and a fuel-reduction burn in 1988. Trapping grids were established, two (A and B) in heathy woodland and two (C and D) in vegetation regenerating after removal of a pine plantation (Table 1, Fig. 1). Each grid consisted of four transects (30 m apart), with 10 traps spaced at 20 m along transects (1.62 ha). Trapping was undertaken on 33 occasions (December 1995 – May 2000). Trapping was conducted in 2013 (April, July) and 2015 (March, April).
Population dynamics and demographics at long-term repeat trapping sites (1981–2015)
The total number of individuals and total number of captures of males and females were recorded, and captures used to calculate trapping success at each trapping session as:
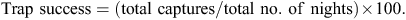
The population trapability (average proportion of marked individuals captured daily) was calculated.
The number of individual animals (males and females) known-to-be-alive (KTBA), was calculated for each trapping session and to calculate population density.
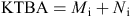
where Mi = the number of individuals captured at time i, and Ni = the number of individuals captured previously that were marked, released, not captured at time i, but subsequently recaptured. The density (KTBA per hectare) was calculated for each trapping session and population changes assessed.
Survival duration was estimated as the number of months over which an individual was captured. The breeding season was determined from observations of reproductive condition of females (vaginal condition, nipples) and males (scrotal testes), the presence of juveniles (<14 g), and the gestation period (32–39 days) (Kemper and Wilson 2008).
Fire history and rainfall patterns
The fire history at the New Holland mouse sites was acquired for time since last burn, number, area and type of burn; and the fire history of the major Ecological Vegetation Divisions was determined for area and percentage area burnt (Department of Environment and Primary Industry 2013, pers. comm.). Long-term rainfall data obtained from stations at Anglesea and Aireys Inlet were used to examine trends in annual and seasonal patterns (Bureau of Meteorology 2017).
Assessments of site-specific threats
Assessments of threats at the New Holland mouse sites were compared for the historical data and recent surveys. An estimate of the extent of fragmentation of sites was based on the habitat patch size delineated by roads or tracks. The presence of predators (fox, cat) together with human impacts (horse riders, or unaccompanied dogs) were based on site observations and camera results. The degree of threat was categorised as low or high.
Results
Summary of mammal survey results (2013–17)
In total, 352 mammal captures were recorded; however, the New Holland mouse was not captured at any site. The most commonly captured species were the southern bush rat (Rattus fuscipes), the swamp rat (Rattus lutreolus) and the agile antechinus (Antechinus agilis), with 186, 56 and 38 captures, respectively. The eastern pygmy possum (Cercartetus nanus) was captured at one site, photographed at one site, and observed in a nest at another. Twenty-two identifiable mammal species were recorded by cameras across 27 sites; however, there were no records of the New Holland mouse. The fox (Vulpes vulpes) was recorded at nine sites and the cat (Felis catus) at three sites.
Population dynamics of New Holland mouse at long-term repeat grid sites (1981–2015)
The New Holland mouse was present west of the Anglesea River on the Coalmine 16 grid between 1981 and 1982 at a density of 0.25–1 ha–1. Abundance ranged from 0 to 2.0 captures per 100 trap-nights (TN), and monthly KTBA from 1 to 4. The average (mean ± s.e.) captures of females was 2.0 ± 0.6 (n = 5), and males 1.8 ± 1.2 (n = 15). The population was male dominated (male : female = 2.0 : 1.0 to 4.0 : 0.0). Females were not captured during August–September 1981 or in February 1982. Minimum monthly survival rates of females and males ranged from 0 to 1.0. Based on the capture of lactating females and juveniles (December 1981 – January 1982) and the gestation period (32–39 days) the breeding season was from December to January. At the Coalmine 17 site only one individual was captured in July and December 1981. Following the 1983 wildfire the New Holland mouse was not recorded at either site, up to when the sites were mined in 1994 (Coalmine 17) and 2001 (Coalmine 16).
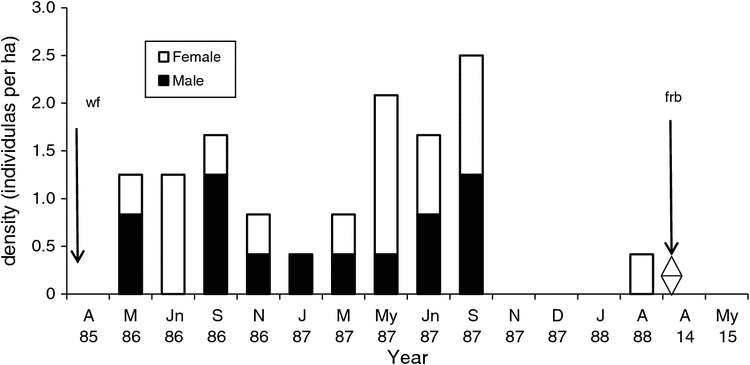
The New Holland mouse was consistently present on the Forest grid (1985–88) at a density of 0.25–2.25 ha–1 (Fig. 2). The population increased in autumn and winter but declined in spring. Abundance ranged from 0.33 to 5.6 captures per 100 TN, and monthly KTBA from 1 to 9. A total of 99 captures (27 individuals) was recorded. The sex ratio (male : female) varied from female-biased (1.0 : 3.0) to male-biased (2.0 : 1.0) and males were not captured during July–September 1985, or January 1987 (Fig. 2). No New Holland mice were captured in 1995 and 1998, or in 2013 and 2015 (Fig. 2). Individuals captured in 2002 were from reintroductions (Fig. 2). At the Pipeline site 23 individuals were recorded (38 captures). New Holland mouse were present in low numbers between 1986 and 1988 and none were captured from November 1987 to 1988 (Fig. 3). The sex ratio (male : female) varied from female-biased (1.0 : 3.0) to male-biased (2.0 : 1.0). No New Holland mice were captured in 2013 or 2015.
|
The New Holland mouse was trapped once or twice per session on the Forest and Pipeline grids and trapability was 0.5. Based on the capture of juveniles (January–March), pregnant and lactating females (December–January) and males with developed testes (November–January) the breeding season was December to January. The percentage of juveniles declined from 75% in the first breeding season to 50% in the second and 33% in the third.
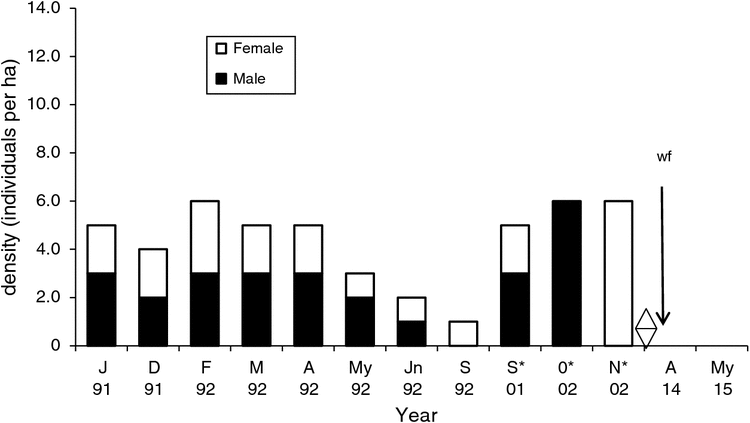
At the Harrison North site five individuals were captured in 1991 and six (107 captures) in 1992. Density ranged from 4.0 to 6.0 ha–1 (January–April), then declined (Fig. 4). The sex ratio (male : female) varied from female-biased (1.0 : 3.0) to male-biased (2.0 : 1.0). Juveniles were captured in January and February, indicating a breeding season from December to January. Reintroductions conducted in September 2001, and October and November 2002 resulted in the recapture of individuals (2001–02) (Fig. 5). No New Holland mice were captured in 2013 and 2015 (Fig. 4).
|
|
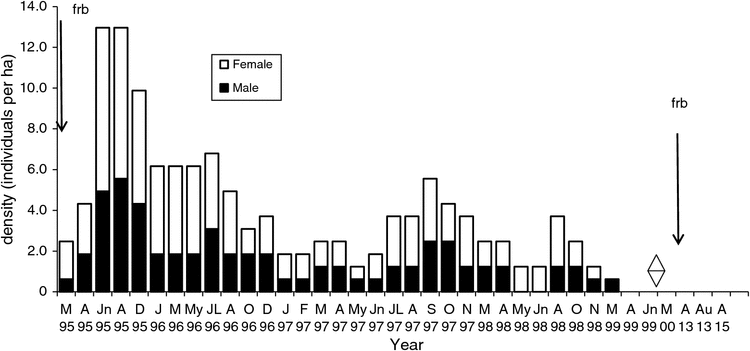
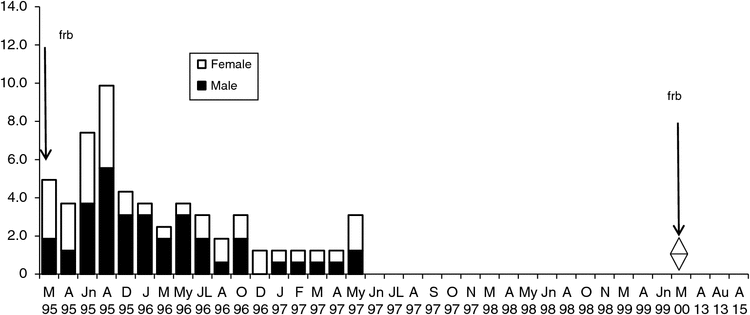
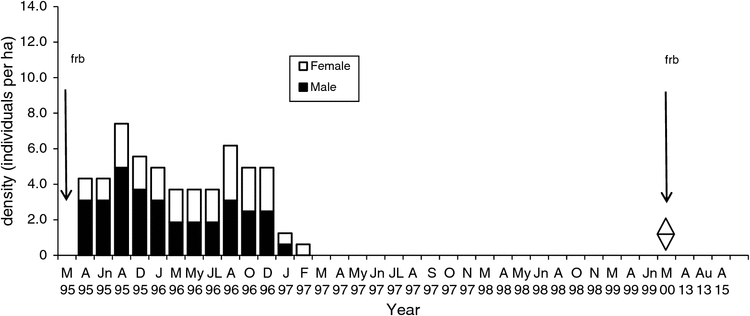
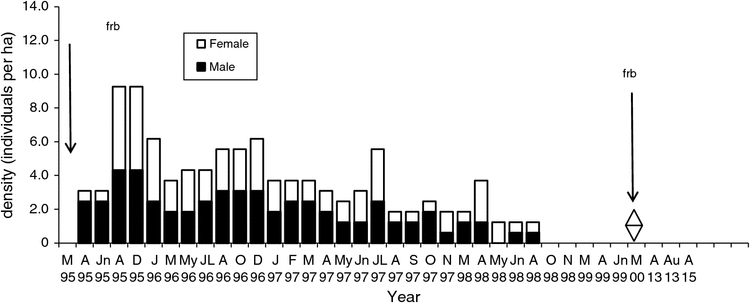
The density on the Flaxbourne Grids A–D (1995–2000) ranged from 0.1 to 13.0 ha–1 (Figs 5–8). The highest density was recorded in June–August 1995 followed by declines to <7 ha–1. Populations persisted on Grids A and D until 1999 and 1998 (Figs 5, 8) and on Grids B and C until 1997 (Figs 6, 7). The total abundance (KTBA) of the New Holland mouse across the grids was maximal in 1995 (n = 72) and declined each subsequent year: 42 in 1996, 26 in 1997, 12 in 1998, 2 in 1999. In total, 57 males (574 captures) and 52 females (624 captures) were recorded with trap success of 4.65 per 100 TN. In all, 22% of individuals were trapped only once, 55% were trapped for 2–12 months, and only four individuals were captured for longer than two years. Juveniles were captured in March, and subadults during March to April, indicating that breeding occurred from December to January. No New Holland mice were captured in 2013 and 2015.
|
|
|
Threatening processes
Fire history and rainfall patterns
All long-term New Holland mouse sites were burnt in the 1983 wildfire (Table 2). Fuel-reduction burns were conducted subsequently on the Forest site (1993, 1998, 2011), at the Pipeline site (2001) and at the Flaxbourne grids (1988, 2002). A wildfire burnt the Harrison site in 2009 (Table 2).
|
There was little burning of heathland vegetation between 1970 and 2005 apart from the 1983 wildfire when an area of ~3900 ha was burnt (Department of Environment and Primary Industries 2013). The total area burnt between 1987 and 2008 (21 years) was 2266 ha (mean = 107.9 ha per annum), and between 2009 and 2012 (3 years) was 3095 ha (mean = 1031.67 ha per annum) (Department of Environment and Primary Industries 2013), a 900% increase in the area burnt annually.
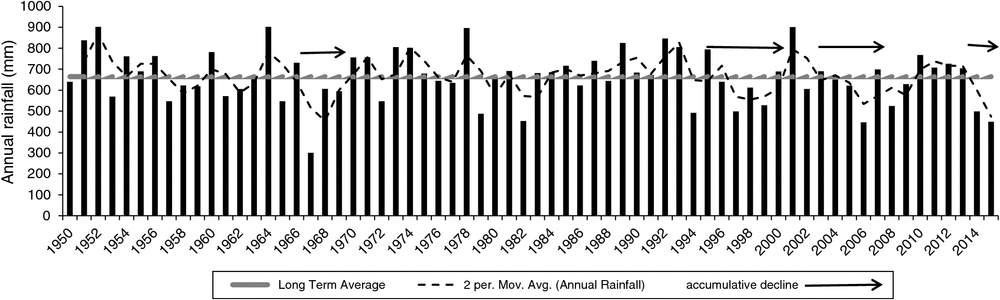
The long-term average annual rainfall was 664 mm (Fig. 9). Rainfall in 1982 (403 mm), the year before the 1983 wildfire, was the lowest recorded over a 36-year period (1968–2004) (Bureau of Meteorology 2015). Rainfall from one to eight months after the wildfire was higher than the monthly average (>80 mm), and in the first three years (1983–85) was greater than the annual mean. Above-average rainfall was recorded (1990–93), while record levels of annual deficit and accumulative declines occurred during the ‘millennium drought’ (1996–2010) (Fig. 9) (CSIRO and Bureau of Meteorology 2015). Between 2010 and 2013 annual rainfall was average; however, in 2014 and 2015 it was extremely low (Fig. 9).
|
Threats at long-term sites
The main threats and potential threats identified before 2003 were habitat clearing and fragmentation, inappropriate fire regimes and fox predation. This included fragmentation caused by agriculture (1850s), pine plantations (1920s), coal mining and infrastructure, e.g. aqueducts, reservoirs (1950s). Foxes were identified at all sites. During the recent surveys the potential threats were from historic clearing and habitat fragmentation, inappropriate fire regimes, introduced predators and human impacts, e.g. horse riding, off-leash dogs.
Discussion
Current status of P. novaehollandiae in the eastern Otways
The results of our recent surveys (2013–17) that failed to record the New Holland mouse were unexpected. The species was absent from seven extant sites (totalling 22 ha), even at sites that once supported high-density populations. The results are significant because they confirm that there have been no positive records of the New Holland mouse in the eastern Otways since 2002 when trial reintroductions of the species were undertaken (Tidey 2003; Gibson et al. 2004). Additional surveys are required to assess other areas of suitable habitat to determine whether the New Holland mouse is no longer extant in the eastern Otways. A habitat-suitability model that identifies potential optimal habitat provides important information to focus surveys for any unidentified populations (Wilson et al. 2015).
Population and demographic changes at trapping study sites – evidence of declines
The long-term population sites were established for specific studies into the ecology, life-history and reproduction of the species, not for long-term monitoring purposes. However, they have provided valuable information on the species’ long-term population dynamics across the landscape. Most populations were small, isolated and fragmented, and persisted for a limited time, from one to six years. There was evidence of high proportions of residents at the sites.
A feature of the populations was seasonal fluctuations in abundance, with recruitment of subadults from early autumn resulting in population increases, while declines occurred from spring to summer. Studies of other populations have recorded increases in autumn and declines in spring (Pye 1991; Lazenby 1999; Wilson et al. 2005). The declines may be related to increased stress during the breeding season, due to high energy requirements for gestation and lactation, and activities such as construction of burrows and nests (Lock and Wilson 2017). If food is limited or foraging distances large then higher mortality may result from these stressors.
The Flaxbourne population exhibited a sudden decline following the peak population in 1995. It is unclear how long the peak population density persisted for because no data were available before 1995. The peak and sudden decline was similar to that recorded for the species at Wilsons Promontory, Victoria, where populations attained maximum densities (~24 ha–1) before declining >50% (Wilson et al. 2005). The species thus appears to exhibit a life-history pattern characteristic of r-selected species, where population irruptions and declines can occur; however, these are not common and the species occurs predominantly in low-density populations.
The population declines in the study area were related to the loss of adults before and during breeding, and either low breeding rates, smaller litter sizes or increased juvenile mortality, as evidenced by declines of juvenile captures (75 to 33%) (Lock and Wilson 2017). Similarly, the high loss of animals was recorded before breeding at Wilsons Promontory (Wilson et al. 2005). Overall, the population declines are indicative of significant pressures such as resource deterioration or high-level predation.
Impacts of fire regimes and rainfall patterns on population dynamics
The New Holland mouse was lost from the two Coalmine sites following the 1983 wildfire, and was not recorded at one site for 10 years and the other for 17 years, at which time the sites were destroyed by mining. No populations have been located at any other location west of the Anglesea River since then, providing evidence that the extensive wildfire may have resulted in the demise of other populations. The species did survive east of the Anglesea River, where it was first located in a small unburnt patch in 1985. The persistence of the New Holland mouse elsewhere in the landscape is likely to have been dependent on the presence of small unburnt refuges, of which there were very few.
The post-fire age of the vegetation at sites in the eastern Otways where the New Holland mouse has been captured ranged from 2 to 20 years. Overall, the 3–7 years post-fire age supported the highest density and may represent optimum habitat. Twenty-year-old vegetation appears to represent refugia following wildfire, and is unlikely to be optimum. Similar responses to post-fire succession have been found elsewhere for the species where maximum abundance was recorded between ~2–8 years after fire, when floristically rich vegetation provides optimal food resources (Braithwaite and Gullan 1978; Fox and Fox 1978; Cockburn 1980). Significant differences recorded in the consumption of food items by the New Holland mouse between Victorian localities were related to successional age, together with floristic differences (Wilson and Bradtke 1999).
There is now strong evidence that rainfall fluctuations have impacted the New Holland mouse populations in the region. At the Flaxbourne site, where abundance peaked following six years of above-average rainfall, and declined precipitously when rainfall was well below average, abundance was positively correlated with rainfall (Lock and Wilson 2017). The recovery of the New Holland mouse populations at Forest and Pipeline after the 1983 wildfire benefitted from above-average monthly rain over the first eight months and annual rainfall for three years that resulted in rapid recovery of the vegetation (Wark et al. 1987; Wilson 1991).
It is likely that the productivity of the vegetation in the early stages of post-fire regeneration, together with concurrent periods of above-average rainfall, contributed to the provision of favourable food resources and habitat for the New Holland mouse leading to population recovery (after 1983) and irruption (1995). At the Flaxbourne site vegetation changes were recorded between 1995 and 2000 as the populations declined, including significant declines in vegetation density, species richness and diversity (Lock 2005). The changes indicate a decline in vegetation growth and productivity after 1995, seven years after fire. This study highlights the complexity of interactions of fire, succession and rainfall patterns with the population dynamics of the New Holland mouse.
Similar population dynamics have been described for the Pilliga mouse, which exhibited a population irruption to a maximum density (~33 ha–1) following high rainfall, before suddenly declining to a low density of ~2 ha–1 (Tokushima and Jarman 2008; Tokushima et al. 2008). During the irruption females had maximum reproductive success and ate significantly more protein-rich foods such as invertebrates (Tokushima and Jarman 2010). Subsequently, they consumed a lower proportion of invertebrates, which was associated with declines in female reproductive success, survival and growth. There was evidence that the irruption of populations following high rainfall was also facilitated by a fire that occurred 22 months previously (Tokushima et al. 2008).
While the impact of wildfire on the New Holland mouse populations in the Otways was instantaneous and easily discernible the impacts of post-fire succession, rainfall and drought occur over a longer term, and their impact is difficult to predict. The identification of these factors and their interactions would not have been possible without the availability of long-term data.
Management implications for P. novaehollandiae in a drying climate
In south-west Victoria the wet decades of the 1950s and 1970s were followed by the significant ‘millennium drought’ (1996–2010) (CSIRO and Bureau of Meteorology 2015) and rainfall is projected to decrease by 25–45% under high-carbon-emission scenarios by 2090 (Hope et al. 2015). This vulnerable species thus faces significant threats from climate change and modelling of rainfall changes on the distribution of the New Holland mouse predicted a decline of up to 50% (Brereton et al. 1995).
It is unclear if the New Holland mouse is extant in the eastern Otways and it is likely that recovery will not be possible without reintroductions. There remains a need to implement habitat management either for recovery of remnant or reintroduced populations. There is an opportunity to mitigate threats with appropriate management such as prevention of fragmentation, establishment and protection of ecological linkages, baiting control of predators and implementation of evidence-based fire regimes.
Suitable habitat for the species is fragmented, with only 45% occurring in patches larger than 30 ha (Wilson et al. 2015). Unsuitable fire ages may also contribute to fragmentation of the habitat for the species. Management to prevent further fragmentation either through clearing or inappropriate fire ages should be implemented, together with developing linkages between habitat and suitable succession ages (Driscoll et al. 2010).
Although there is no direct evidence of fox or cat predation on the New Holland mouse, even a limited predation rate would be significant for declining small populations (Wilson and Wolrige 2000). While general fox baiting currently occurs four times a year (Parks Victoria 2015) it is unclear to what extent foxes impact the New Holland mouse, or how effective baiting is in its habitat. The impacts of predators and predator control on the species require further study. Recent studies in the area have found that, after fire, fox occurrence increased in burnt patches, feral cat occurrence increased across the whole burn area, and the consumption of medium-sized mammals by foxes tripled (Hradsky et al. 2017). Integrated management of predators and fire are thus strongly recommended.
Implementation of burning regimes to ensure access to appropriate age classes for persistence of the New Holland mouse populations needs to be given high priority. An increase in burning has been implemented since the 2009 Victorian Bushfires Royal Commission (Parliament of Victoria 2010). The increase in the annual rate of burning of heathlands between 1987 and 2008 (21 years) to the three-year period between 2009 and 2012 was 900%. Analyses of spatial and temporal fire history is required to determine whether burning has been at appropriate scales and in areas suitable for the species (Driscoll et al. 2010). Comprehensive information of vegetation structure and floristics of optimal habitat to assess whether burning is achieving ideal results is available (Wilson 1991; Lock 2005). It is important that the timing of burning takes into account the impacts of rainfall, and that burning not be undertaken during drought, as the likelihood of extinctions is significant.
While the New Holland mouse is considered to be critically endangered in Victoria, it may be more precarious than previously estimated. The recent trapping confirming the loss of this species from known locations means that regional extinction cannot be ruled out. Captive breeding and reintroductions of the New Holland mouse in the eastern Otways were implemented previously (Tidey 2003; Gibson et al. 2004; Lock 2005). There is a need to reassess these programs and evaluate the likelihood of their success to improve the species’ survival.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We gratefully acknowledge the legacy of Mr John Seebeck (1939–2003), who discovered the New Holland mouse in Victoria, instigated the New Holland mouse Recovery Team (1999–2000), and inspired many to continue his work. The work, contributed data and support of our colleagues and students (Dr L. Gibson, K. Kentish, Dr J. Mcleod, Dr D. Mills, T. Reichl, A. Williamson, J. Wolrige, L. Zhuang-Griffin) is appreciatively acknowledged. Many thanks to the following for their assistance and support: Dr J. Aberton, Dr K. Annett, Dr M. Antos (Parks Victoria), Dr M. Cullen, E. Danby (Parks Vic), A. Garkaklis, Dr K. Hicks, D. Hopper, Dr M. Ibbett (DELWP), Dr W. S. Laidlaw, J. Naylor-Pratt, J. Stump, and R. Sydenham. The work was supported by grants from Alcoa Australia, Australian Research Council, Deakin Foundation, Deakin University, Department of Conservation, Forests and Lands, Holsworth Wildlife Research Endowment, M. A. Ingram Trust, National Estate Grants Program, Parks Victoria. Research was carried out under scientific permits issued by the Victorian Department of Environment, Land, Water and Parks and its antecedents, under the Wildlife Act and National Parks Act Permit, Victoria (Nos 87–45, RP-91-103, RP-92-016, RP-95-006, RP-96-013, RP-96-015, 10001132, 10006714, 10007838) and ethics approval from the Deakin University Animal Ethics Committee A7/91, A20/96, A22/99, B05-2013, B01-2016.
References
Arthur, A., Catling, P. C., and Reid, A. (2012). Relative influence of habitat structure, species interactions and rainfall on the post-fire population dynamics of ground-dwelling vertebrates. Austral Ecology 37, 958–970.| Relative influence of habitat structure, species interactions and rainfall on the post-fire population dynamics of ground-dwelling vertebrates.Crossref | GoogleScholarGoogle Scholar |
Braithwaite, R. W., and Gullan, P. (1978). Habitat selection by small mammals in a Victorian heathland. Australian Journal of Ecology 3, 109–127.
| Habitat selection by small mammals in a Victorian heathland.Crossref | GoogleScholarGoogle Scholar |
Brandle, R., and Moseby, K. E. (1999). Comparative ecology of two populations of Pseudomys australis in northern South Australia. Wildlife Research 26, 541–564.
| Comparative ecology of two populations of Pseudomys australis in northern South Australia.Crossref | GoogleScholarGoogle Scholar |
Brereton, R., Bennett, S., and Mansergh, I. (1995). Enhanced greenhouse climate change and its potential effect on selected fauna of south-eastern Australia: a trend analysis. Biological Conservation 72, 339–354.
| Enhanced greenhouse climate change and its potential effect on selected fauna of south-eastern Australia: a trend analysis.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., and McKenzie, N. L. (1989). Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., McKenzie, N. L., Brennan, K. E. C., Woinarski, J. C. Z., Dickman, C. R., Baynes, A., Gordon, G., Menkhorst, P. W., and Robinson, A. C. (2008). Conservation status and biogeography of Australia’s terrestrial mammals. Australian Journal of Zoology 56, 411–422.
| Conservation status and biogeography of Australia’s terrestrial mammals.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2015). Recent rainfall, drought and southern Australia’s long-term rainfall decline. Australian Bureau of Meteorology, Canberra.
Bureau of Meteorology (2017). Australian Bureau of Meteorology climate data on-line. Commonwealth of Australia, Canberra. Available at: www.bom.gov.au/climate
Catling, P. C. (1986). Rattus lutreolus, colonizer of heathland after fire in the absence of Pseudomys species? Australian Wildlife Research 13, 127–139.
| Rattus lutreolus, colonizer of heathland after fire in the absence of Pseudomys species?Crossref | GoogleScholarGoogle Scholar |
Catling, P. C. (1991). Ecological effects of prescribed burning practices on the mammals of southeastern Australia. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D. Lunney.) pp. 353–363. (Royal Zoological Society of New South Wales: Sydney.)
Catling, P. C., and Newsome, A. (1981). Responses of the Australian vertebrate fauna to fire: an evolutionary approach. In ‘ Fire and the Australian Biota’. (Eds A. M. Gill, R. H. Groves and I. R. Noble.) pp. 273–310. (Australian Academy of Science: Canberra.)
Cheal, D. (2010). Growth stages and tolerated fire intervals for Victorian native vegetation data. Fire and Adaptive Management Report No. 84, Department of Sustainability and Environment, Melbourne.
Coates, F., Loyn, R., Ibbett, M., and Brown, G. (2013). Progress report, Otways Hawkeye – biodiversity monitoring for improved fire management. Arthur Rylah Institute for Environmental Research. Unpublished report of the Department of Environment and Primary Industries, Melbourne.
Cockburn, A. (1980). The diet of the New Holland mouse (Pseudomys novaehollandiae) and the house mouse (Mus musculus) in Victorian coastal heathland. Australian Mammalogy 3, 31–34.
CSIRO and Bureau of Meteorology (2015). Climate change in Australia – information for Australia’s natural resource management regions: technical report. CSIRO and Bureau of Meteorology, Australia.
Department of Environment and Primary Industries (2013). Bushfire science strategy. Victorian Fire Management Policy Division, Melbourne.
Department of Environment and Primary Industries (2014). Strategic bushfire management plan, Barwon Otway bushfire risk landscape. Victorian Government, Department of Environment and Primary Industries, Melbourne.
Dickman, C. R., Pressey, R. L., Lim, L., and Parnaby, H. E. (1993). Mammals of particular conservation concern in the western division of New South Wales. Biological Conservation 65, 219–248.
| Mammals of particular conservation concern in the western division of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Dickman, C. R., Mahon, P. S., Masters, P., and Gibson, D. F. (1999). Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. Wildlife Research 26, 389–403.
| Long-term dynamics of rodent populations in arid Australia: the influence of rainfall.Crossref | GoogleScholarGoogle Scholar |
Driscoll, D., Lindenmayer, D., Bennett, A. F., Bode, M., Brdstock, R. A., Cary, G. J., Clarke, M. F., Dexter, N., Fensham, R., Friend, G., Gill, M., James, S., Kay, G., Keith, D. A., MacGregor, C., Russell-Smith, J., Salt, D., Watson, J. E. M., Williams, R. J., and York, A. (2010). Fire management for biodiversity conservation: key research questions and our capacity to answer them’. Biological Conservation 143, 1928–1939.
| Fire management for biodiversity conservation: key research questions and our capacity to answer them’.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J. (1982). Fire and mammalian secondary succession in an Australian coastal heath. Ecology 63, 1332–1341.
| Fire and mammalian secondary succession in an Australian coastal heath.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J. (1983). Mammal species diversity in Australian heathland: the importance of pyric succession and habitat diversity. In ‘Mediterranean Type Ecosystems – the Role of Nutrients’. (Eds F. J. Kruger, D. Mitchell, and J. U. M. Jarvis.) pp. 473–489. (Springer-Verlag: Berlin.)
Fox, B. J. (1990). Changes in the structure of mammal communities over successional time scales. Oikos 59, 321–329.
| Changes in the structure of mammal communities over successional time scales.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J. (1996). Long-term studies of small mammal communities from disturbed habitats in eastern Australia. In ‘Long-term Studies of Vertebrate Communities’. (Eds M. L. Cody, and J. A. Smallwood.) pp. 467–501. (Academic Press: San Diego, CA.)
Fox, B. J., and Fox, M. D. (1978). Recolonization of coastal heath by Pseudomys novaehollandiae (Muridae) following sand mining. Australian Journal of Ecology 3, 447–465.
| Recolonization of coastal heath by Pseudomys novaehollandiae (Muridae) following sand mining.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J., and Fox, M. D. (1984). Small mammal recolonization of open-forest following sand mining. Australian Journal of Ecology 9, 241–252.
| Small mammal recolonization of open-forest following sand mining.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J., and McKay, G. M. (1981). Small mammal responses to pyric successional changes in eucalypt forest. Australian Journal of Ecology 6, 29–41.
| Small mammal responses to pyric successional changes in eucalypt forest.Crossref | GoogleScholarGoogle Scholar |
Fox, B. J., and Monamy, V. (2007). A review of habitat selection by the swamp rat, Rattus lutreolus (Rodentia: Muridae). Austral Ecology 32, 837–849.
| A review of habitat selection by the swamp rat, Rattus lutreolus (Rodentia: Muridae).Crossref | GoogleScholarGoogle Scholar |
Gibson, L., Wilson, B. A., and Cahill, D. (2004). Restoration and management of habitats of threatened species on manufacturing land of high conservation value – Anglesea Heath. Report to Australian Research Council, Canberra.
Greenville, A. C., Dickman, C. R., Wardle, G. M., and Letnic, M. (2009). The fire history of an arid grassland: the influence of antecedent rainfall and ENSO. International Journal of Wildland Fire 18, 631–639.
| The fire history of an arid grassland: the influence of antecedent rainfall and ENSO.Crossref | GoogleScholarGoogle Scholar |
Greenville, A. C., Wardle, G. M., and Dickman, C. R. (2012). Extreme climatic events drive mammal irruptions: regression analysis of 100-year trends in desert rainfall and temperature. Ecology and Evolution 2, 2645–2658.
| Extreme climatic events drive mammal irruptions: regression analysis of 100-year trends in desert rainfall and temperature.Crossref | GoogleScholarGoogle Scholar |
Hope, P., Grose, M. R., Timbal, B., Dowdy, A. J., Jonas, B., Katzfey, J. J., Bedin, T., Wilson, L., and Whetton, P. H. (2015). Seasonal and regional signature of the projected southern Australian rainfall reduction. Australian Meteorological and Oceanographic Journal 65, 54–71.
| Seasonal and regional signature of the projected southern Australian rainfall reduction.Crossref | GoogleScholarGoogle Scholar |
Hradsky, B. A. K., Mildwaters, C., Ritchie, E. G., Christie, F., and Di Stefano, J. (2017). Responses of invasive predators and native prey to a prescribed forest fire. Journal of Mammalogy 98, 835–847.
| Responses of invasive predators and native prey to a prescribed forest fire.Crossref | GoogleScholarGoogle Scholar |
IUCN (2010). IUCN Red List of threatened species. Species Survival Commission, Gland, Switzerland. Available at: http:// www.iucnredlist.org/
Johnson, C. N. (2006). ‘Australia’s Mammal Extinctions: A 50 000 Year History.’ (Cambridge University Press: Cambridge.)
Kemper, C. M. (1990). Small mammals and habitat disturbance in open forest of coastal New South Wales. 1. Population parameters. Australian Wildlife Research 17, 195–206.
| Small mammals and habitat disturbance in open forest of coastal New South Wales. 1. Population parameters.Crossref | GoogleScholarGoogle Scholar |
Kemper, C. M., and Wilson, B. A. (2008). Pseudomys novaehollandiae (New Holland mouse). In ‘The Mammals of Australia’. 3rd edn. (Eds S. Van Dyck and R. Strahan.) pp. 643–644. (New Holland Publishers: Sydney.)
Kentish, K. M. (1982). A new record of New Holland mouse (Pseudomys novaehollandiae) from Anglesea, Victoria. Victorian Naturalist 99, 128–129.
Kentish, K. M. (1983). Mine rehabilitation: a study of revegetation and fauna return at Anglesea, Victoria (1980–1982). M.Sc. Thesis, Deakin University, Geelong.
Land Conservation Council Victoria (1985). Report on the Melbourne Area, District 1 review. Land Conservation Council, Melbourne.
Lawes, M. J., Fisher, D. O., Johnson, C. N., Blomberg, S. P., Frank, A. S. K., and Fritz, S. A. (2015). Correlates of recent declines of rodents in northern and southern Australia: habitat structure is critical. PLoS One 10, e0130626.
| Correlates of recent declines of rodents in northern and southern Australia: habitat structure is critical.Crossref | GoogleScholarGoogle Scholar |
Lazenby, B. T. (1999). Vegetation associations and spatial relations in the New Holland mouse, Pseudomys novaehollandiae (Rodentia: Muridae) in Tasmania. B.Sc.(Honours) Thesis, University of Tasmania, Hobart.
Lee, A. K. (1995). The action plan for Australian rodents. Environment Australia – Biodiversity Group, Threatened Species & Communities Section, Canberra.
Letnic, M., and Dickman, C. R. (2005). The responses of small mammals to patches regenerating after fire and rainfall in the Simpson Desert, central Australia. Austral Ecology 30, 24–39.
| The responses of small mammals to patches regenerating after fire and rainfall in the Simpson Desert, central Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., and Dickman, C. R. (2006). Boom means bust: interactions between the El Nino/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodiversity and Conservation 15, 3847–3880.
| Boom means bust: interactions between the El Nino/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Dickman, C. R., Tischler, M. K., Tamayo, B., and Beh, L. (2004). The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. Journal of Arid Environments 59, 85–114.
| The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Tamayo, B., and Dickman, C. R. (2005). The response of mammals to La Nina (El Nino Southern Oscillation)-associated rainfall, predation and wildfire in central Australia. Journal of Mammalogy 86, 689–703.
| The response of mammals to La Nina (El Nino Southern Oscillation)-associated rainfall, predation and wildfire in central Australia.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Story, P., Story, G., Field, J., Brown, O., and Dickman, C. R. (2011). Resource pulses, switching trophic control, and the dynamics of small mammal assemblages in arid Australia. Journal of Mammalogy 92, 1210–1222.
| Resource pulses, switching trophic control, and the dynamics of small mammal assemblages in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Lock, M. L. (2005). Conservation and recovery of the New Holland mouse (Pseudomys novaehollandiae) at Anglesea, Victoria. Ph.D. Thesis, Deakin University, Geelong.
Lock, M. L., and Wilson, B. A. (1999). The distribution of the New Holland mouse (Pseudomys novaehollandiae) with respect to vegetation near Anglesea, Victoria. Wildlife Research 26, 565–577.
| The distribution of the New Holland mouse (Pseudomys novaehollandiae) with respect to vegetation near Anglesea, Victoria.Crossref | GoogleScholarGoogle Scholar |
Lock, M. L., and Wilson, B. A. (2017). Influence of rainfall on population dynamics and survival of a threatened rodent (Pseudomys novaehollandiae) under a drying climate in coastal woodlands of south-eastern Australia. Australian Journal of Zoology 65, 60–70.
| Influence of rainfall on population dynamics and survival of a threatened rodent (Pseudomys novaehollandiae) under a drying climate in coastal woodlands of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., Cullis, B., and Eby, P. (1987). Effects of logging and fire on small mammals in Mumbulla State Forest, near Bega, New South Wales. Australian Wildlife Research 14, 163–181.
| Effects of logging and fire on small mammals in Mumbulla State Forest, near Bega, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Masters, P. (1993). The effects of fire-driven succession and rainfall on small mammals in spinifex grassland at Uluru National Park, Northern Territory. Wildlife Research 20, 803–813.
| The effects of fire-driven succession and rainfall on small mammals in spinifex grassland at Uluru National Park, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
McKenzie, N. L., Burbidge, A., Baynes, A., Brereton, R. N., Dickman, C. R., Gordon, G., Gibson, L. A., Menkhorst, A. C., Robinson, A. C., Williams, M. R., and Woinarski, J. C. Z. (2007). Analysis of factors implicated in the recent decline of Australia’s mammal fauna. Journal of Biogeography 34, 597–611.
| Analysis of factors implicated in the recent decline of Australia’s mammal fauna.Crossref | GoogleScholarGoogle Scholar |
McMahon, K., and Brighton, M. (2002). Anglesea Heath management plan November 2002. Parks Victoria, Melbourne.
Menkhorst, P., Dickman, C., Denny, M., Aplin, K., Lunney, D., and Ellis, M. (2009). Pseudomys novaehollandiae. In ‘IUCN Red List of Threatened Species. Version 2009.2, Gland, Switzerland’. Available at: www.iucnredlist.org
Monamy, V., and Fox, B. J. (2000). Small mammal succession is determined by vegetation density rather than time elapsed since disturbance. Austral Ecology 25, 580–587.
| Small mammal succession is determined by vegetation density rather than time elapsed since disturbance.Crossref | GoogleScholarGoogle Scholar |
Newsome, A., McIlroy, J., and Catling, P. (1975). The effects of an extensive wildfire on populations of twenty ground vertebrates in south-east Australia. Proceedings of the Ecological Society of Australia 9, 107–123.
Parks Victoria (2015). Eastern Otways Fox Control Program report (2011–2015). Parks Victoria, Anglesea, Victoria.
Parks Victoria and DSE (2009). Caring for country – The Otways and you. Great Otway National Park and Otway Forest Park Management Plan. Parks Victoria and DSE, Melbourne.
Parliament of Victoria (2010). 2009 Victorian Bushfires Royal Commission, Final Report; Summary. Parliament of Victoria, Melbourne.
Predavec, M. (1994). Population dynamics and environmental changes during natural irruptions of Australian desert rodents. Wildlife Research 21, 569–582.
| Population dynamics and environmental changes during natural irruptions of Australian desert rodents.Crossref | GoogleScholarGoogle Scholar |
Pye, T. (1991). The New Holland mouse (Pseudomys novaehollandiae) (Rodentia: Muridae) in Tasmania: a field study. Wildlife Research 18, 521–531.
| The New Holland mouse (Pseudomys novaehollandiae) (Rodentia: Muridae) in Tasmania: a field study.Crossref | GoogleScholarGoogle Scholar |
Recher, H. F., Lunney, D., and Matthews, A. (2009). Small mammal populations in a eucalypt forest affected by fire and drought. I. Long-term patterns in an era of climate change. Wildlife Research 36, 143–158.
| Small mammal populations in a eucalypt forest affected by fire and drought. I. Long-term patterns in an era of climate change.Crossref | GoogleScholarGoogle Scholar |
Seebeck, J., Menkhorst, P., Wilson, B. A., and Lowe, K. M. (1996). Flora and Fauna Guarantee Action Statement No. 74, New Holland mouse Pseudomys novaehollandiae. Department of Natural Resources and Environment, Victoria.
Smith, A. P., and Quin, D. G. (1996). Patterns and causes of extinction and decline in Australian conilurine rodents. Biological Conservation 77, 243–267.
| Patterns and causes of extinction and decline in Australian conilurine rodents.Crossref | GoogleScholarGoogle Scholar |
Southgate, R., and Masters, P. (1996). Fluctuations of rodent populations in response to rainfall and fire in central Australian hummock grassland dominated by Plectranche schinzii. Wildlife Research 23, 289–303.
| Fluctuations of rodent populations in response to rainfall and fire in central Australian hummock grassland dominated by Plectranche schinzii.Crossref | GoogleScholarGoogle Scholar |
Specht, R. L. (1981). Structural attributes – foliage projective cover and standing biomass. In ‘Vegetation Classification in the Australian Region’. (Eds A. N. Gillison and D. J. Anderson.) pp. 10–21. (CSIRO and Australian National University Press: Canberra.)
Threatened Species Scientific Committee (TSSC) (2010). Conservation advice for Pseudomys novaehollandiae New Holland Mouse. Threatened Species Scientific Committee, Canberra.
Tidey, D. L. (2003). Re-introduction trials of the New Holland mouse, Pseudomys novaehollandiae at Anglesea, Victoria. B.Sc.(Honours) Thesis, Deakin University, Geelong.
Tokushima, H., and Jarman, P. J. (2008). Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. II. Demography, home range and dispersal. Australian Journal of Zoology 56, 375–387.
| Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. II. Demography, home range and dispersal.Crossref | GoogleScholarGoogle Scholar |
Tokushima, H., and Jarman, P. J. (2010). Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. III. Dietary ecology. Australian Journal of Zoology 58, 85–89.
| Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. III. Dietary ecology.Crossref | GoogleScholarGoogle Scholar |
Tokushima, H., Green, S. W., and Jarman, P. J. (2008). Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. I. Population fluctuation and breeding season. Australian Journal of Zoology 56, 363–373.
| Ecology of the rare but irruptive Pilliga mouse, Pseudomys pilligaensis. I. Population fluctuation and breeding season.Crossref | GoogleScholarGoogle Scholar |
Van Dyck, S., and Strahan, R. (Eds) (2008). ‘The Mammals of Australia.’ (New Holland Publishers: Sydney.)
Van Dyck, S., Gynther, I., and Baker, A. (Eds) (2013). ‘Field Companion to The Mammals of Australia.’ (New Holland Publishers: Sydney.)
Wark, M. C., White, M. D., Robertson, D. J., and Marriott, P. F. (1987). Regeneration of heath and heath woodland in the north-eastern Otway ranges following the wildfire of February 1983. Proceedings of the Royal Society of Victoria 99, 51–88.
Wayne, A. F., Wilson, B. A., and Woinarski, J. C. Z. (2017). Falling apart? Insights and lessons from three recent studies documenting rapid and severe decline in terrestrial mammal assemblages of northern, south-eastern and south-western Australia. Wildlife Research 44, 114–126.
| Falling apart? Insights and lessons from three recent studies documenting rapid and severe decline in terrestrial mammal assemblages of northern, south-eastern and south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A. (1991). The ecology of Pseudomys novaehollandiae (Waterhouse 1843) in the eastern Otway Ranges, Victoria. Wildlife Research 18, 233–247.
| The ecology of Pseudomys novaehollandiae (Waterhouse 1843) in the eastern Otway Ranges, Victoria.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A. (1994). The distribution of Pseudomys novaehollandiae (Waterhouse 1843) in the eastern Otway Ranges, Victoria. Victorian Naturalist 111, 46–53.
Wilson, B. A. (1996a). The distribution and status of (New Holland Mouse) Pseudomys novaehollandiae (Waterhouse 1843) in Victoria. Australian Mammalogy 19, 31–46.
Wilson, B. A. (1996b). Fire effects on vertebrate fauna and implications for fuel reduction burning and management. In ‘Fire and Biodiversity: the Effects and Effectiveness of Fire Management’. (Ed. Victorian National Parks Association.) pp. 131–147. (Biodiversity Unit, Department Of Environment, Sport and Territory: Canberra.)
Wilson, B. A., and Bradtke, E. L. (1999). The diet of the New Holland mouse (Pseudomys novaehollandiae) in Victoria. Wildlife Research 26, 439–451.
| The diet of the New Holland mouse (Pseudomys novaehollandiae) in Victoria.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A., and Laidlaw, W. S. L. (2003). Habitat characteristics for New Holland mouse Pseudomys novaehollandiae in Victoria. Australian Mammalogy 25, 1–11.
| Habitat characteristics for New Holland mouse Pseudomys novaehollandiae in Victoria.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A., and Wolrige, J. (2000). Assessment of the diet of the fox, Canis vulpes in habitats of the eastern Otway Ranges, Victoria. Australian Mammalogy 22, 201–211.
Wilson, B. A., Robertson, D., Moloney, D. J., Newell, G. R., and Laidlaw, W. S. (1990). Factors affecting small mammal distribution and abundance in the eastern Otway Ranges, Victoria. Proceedings of the Ecological Society of Australia 16, 379–396.
Wilson, B. A., White, N. M., Hanley, A., and Tidey, D. L. (2005). Population fluctuations of the New Holland mouse Pseudomys novaehollandiae at Wilson’s Promontory National Park, Victoria. Australian Mammalogy 27, 49–60.
| Population fluctuations of the New Holland mouse Pseudomys novaehollandiae at Wilson’s Promontory National Park, Victoria.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A., Millie, S., and Aberton, J. (2015). Identification of optimal habitat for the recovery and management of New Holland mouse (Pseudomys novaehollandiae) in the eastern Otways, southern Victoria. Report to Parks Victoria. School of Life & Environmental Sciences, Deakin University, Victoria.
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2014). ‘The Action Plan for Australian Mammals 2012.’ (CSIRO Publishing: Melbourne.)
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2015). The ongoing unravelling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences of the United States of America 112, 4531–4540.
| The ongoing unravelling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitlagsbg%3D&md5=da7ece5323de2e1810285b7f5d06faf3CAS |


 ) survey sites ● and trapping grids □: Coalmine 16 (16) Coalmine 17 (17), Forest (F), Pipeline (P), Harrisson (H), Flaxbourne – Grid A (A), Grid B (B), Grid C (C), Grid D (D).
) survey sites ● and trapping grids □: Coalmine 16 (16) Coalmine 17 (17), Forest (F), Pipeline (P), Harrisson (H), Flaxbourne – Grid A (A), Grid B (B), Grid C (C), Grid D (D).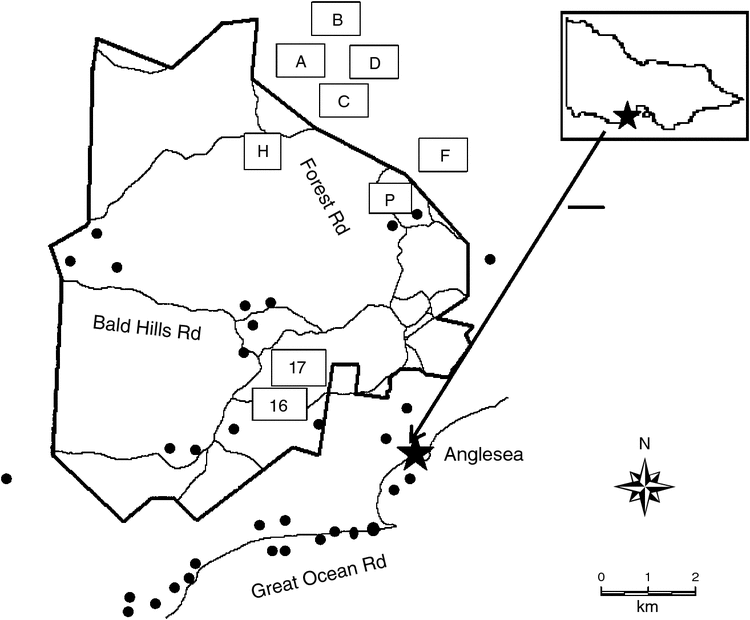
 , not trapped 2003–12.
, not trapped 2003–12.