Breeding in wild populations of a small dasyurid marsupial, Planigale ingrami, in north-western Queensland using a novel method for collection of specimens
P. A. Woolley A B and C. Elliott AA Department of Zoology, La Trobe University, Melbourne, Vic. 3086, Australia.
B Corresponding author. Email: p.woolley@latrobe.edu.au
Australian Mammalogy 36(1) 81-89 https://doi.org/10.1071/AM13027
Submitted: 18 July 2013 Accepted: 7 November 2013 Published: 19 December 2013
Abstract
The reproductive biology of the long-tailed planigale (Planigale ingrami) is less well known than that of its congeners P. gilesi and P. tenuirostris. Aspects of the anatomy of reproductive structures and the pattern of reproduction of P. ingrami were established by examination of specimens extracted from the stomachs of feral cats shot in north-western Queensland. This species has an extended breeding season that commences in August and probably ends in December, and both males and females may live to breed in more than one season, providing support for the similarity of the reproductive strategies of these three species of Planigale. Females of P. ingrami have twelve nipples in the pouch, the form of which may differ from that of other planigales. Pouch morphology may prove useful in the identification of species of Planigale providing observations are made on the appearance of the pouch throughout the breeding cycle.
Additional keywords: life-history strategy, Planigale gilesi, Planigale ingrami, Planigale maculata, Planigale tenuirostris, pouch morphology.
Introduction
Comparatively little is known of the reproductive biology of the long-tailed planigale, Planigale ingrami, one of the four currently recognised Australian species in this genus of very small dasyurid marsupials. Archer (1976) recorded observations made on each of the three previously recognised forms of P. ingrami, namely the typical form ingrami, the subtilissima form and the brunnea form. Based largely on this information, Lee et al. (1982) tentatively placed P. ingrami among species of dasyurid marsupials exhibiting life-history Strategy V. In this strategy, based on reproductive traits, the breeding season is extended but seasonal, the females are polyoestrous and males may breed in more than one season. Krajewski et al. (2000) followed this assessment.
Studies on reproduction in wild populations of small dasyurid marsupials (e.g. Sminthopsis crassicaudata: Morton 1978; Planigale gilesi and P. tenuirostris: Read 1984; Sminthopsis dolichura: Friend et al. 1997; Sminthopsis youngsoni, Ningaui ridei and Dasycercus cristicauda: Dickman et al. 2001), generally report observations made on individuals trapped and released at intervals throughout the year. The information that can be obtained from such studies is largely restricted to seasonal changes in external features such as pouch condition of females and scrotal size of males, sometimes correlated with body weight. Additional information on reproductive cycles and changes in the internal reproductive organs of both males and females can be obtained from species studied in the laboratory (e.g. Antechinus stuartii: Woolley 1966; Sminthopsis macroura: Woolley 1990a, 1990b; Dasykaluta rosamondae: Woolley 1991). Data obtained from both field and laboratory studies have been used to define the reproductive strategy of many species of dasyurid marsupials (Lee et al. 1982; Krajewski et al. 2000).
Carcasses recovered from the stomachs of feral cats shot in three areas in the course of another study (Mifsud and Woolley 2012) provided an opportunity to investigate aspects of reproduction in wild populations of Planigale ingrami. This material has allowed for the assessment of reproductive condition of many of the carcasses from observations made on both external features and the reproductive organs without the need to maintain animals in captivity. The observations made on the planigale specimens, together with some made on a smaller number of individuals live-trapped in areas where the cats were shot in north-western Queensland (Woolley 1992; Mifsud and Woolley, unpubl. data), are presented here. The specific identity of the specimens studied, previously based largely on the known distribution of the species, in particular that of P. ingrami brunneus described by Troughton (1928), is discussed.
Materials and methods
Examination of planigales
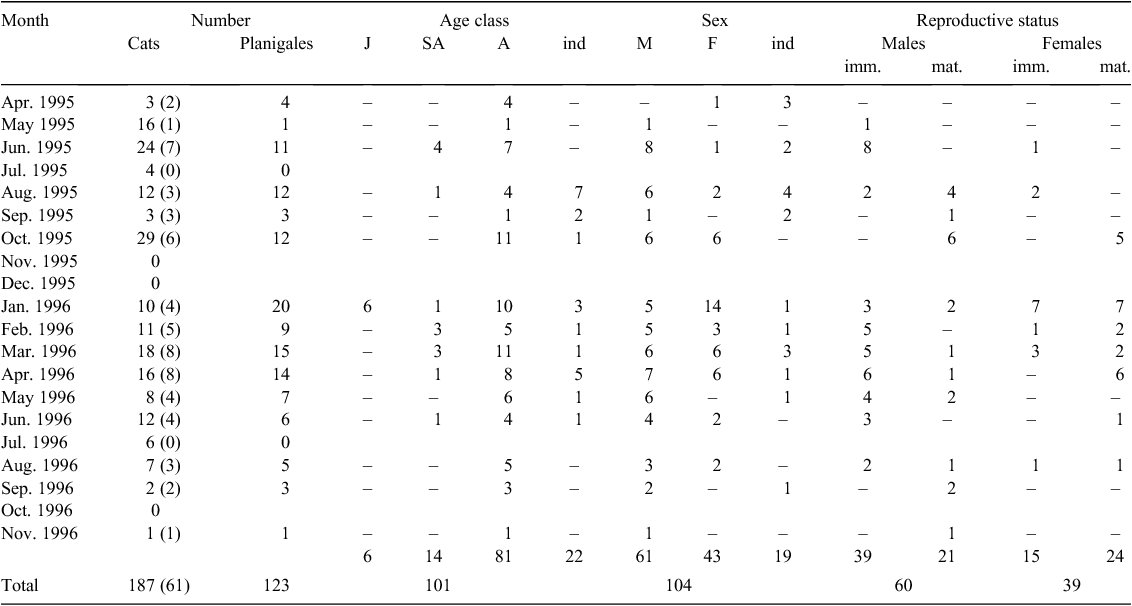
In total, 123 planigales were recovered from 61 of 187 cat stomachs and were preserved in 70% ethanol. The number of planigales per stomach ranged from 1 to 11: 34 stomachs contained one, 14 contained two, 5 (three), 4 (four), 1 (five), 1 (six), 1 (eight) and 1 (eleven). About half the specimens were whole, with a variable amount of damage inflicted by the teeth of the predator; others were reconstructed from clearly matching pieces. Skulls were extracted and individuals assigned to one of three age classes based on stage of tooth eruption: (1) juvenile (first upper incisor not fully erupted, deciduous third premolar present, molars 1–3 erupted); (2) subadult (permanent third premolar erupting, molars 1–4 erupted); and (3) adult (all teeth fully erupted). Scrotal width was measured and notes made on the pouch area, including size, hairiness, pigmentation of skin, number and size of nipples, presence of young and, after removal of the pouch skin from the carcass, the size of the mammary tissue. The reproductive tract was dissected out of each specimen. Measurements were made of the length and width of one testis and the prostate. The prostate was examined for zonation (semitranslucent upper zone and opaque lower zone), the size of the bulbo-urethral (Cowpers) glands and the size, and presence or absence of the sigmoid flexure, of the penis noted. Epididymal smears were prepared and examined for the presence of spermatozoa. The size of the vaginal complex and uteri of each female were noted. Observations made on the live-trapped planigales were limited to measurement of scrotal width and appearance of the pouch.
Assessment of reproductive condition
With knowledge gained from studies on other dasyurids (e.g. Woolley 1966, 1974, 1990a, 1990b, 1991) of the changes that occur in the reproductive structures in relation to breeding, males and females could be classed as either sexually immature or mature. The signs upon which sexual maturity of males was based included a large scrotum, testes, prostate, bulbo-urethral glands and penis; zonation of the prostate, sigmoid flexure of the penis and spermatozoa in the epididymis. In other species of dasyurid marsupials (Woolley 1966, 1990b) enlargement of the testes (and scrotum) is known to precede that of the prostate and bulbo-urethral glands as males mature. The prostate, which develops as a thickening of the wall of the membranous urethra, reaches maximal size with zonation evident shortly before the onset of the breeding season. Some regression in size of the testes (and scrotum), prostate and bulbo-urethral glands occurs with the cessation of breeding, but never to the pre-breeding condition, and the zonation of the prostate becomes less obvious. Spermatozoa are found in the epididymis of males in breeding condition. As males mature the penis becomes larger and assumes a sigmoid flexure when retracted.
Assessment of the reproductive condition of females was based on the appearance of the pouch area and reproductive tract. The pouch area, nipples and reproductive tract of immature animals are small but become larger during pregnancy and lactation. The lateral vaginae are enlarged during oestrus and the uteri become enlarged after ovulation has occurred. After birth of the young the tract regresses and, following weaning of the young, the pouch area and suckled nipples regress, but never to the prebreeding condition.
Results
Planigales collected by cats
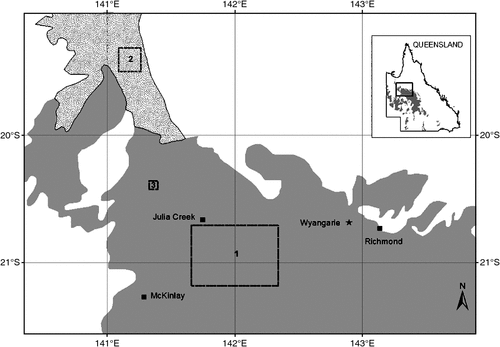
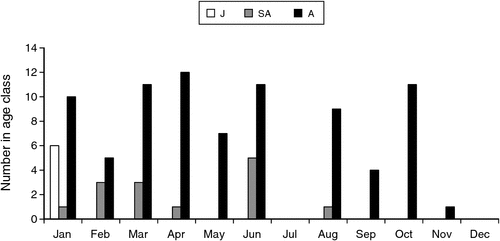
The cats were shot between 24 April 1995 and 18 November 1996: 54 in Area 1, 6 in Area 2 and 1 in Area 3 (see Fig. 1). No shooting was done in November and December 1995 and October 1996, and no planigales were found in the stomachs of cats shot in July of 1995 and 1996. Their absence from the sample in July may perhaps be due to decreased activity, at least above ground, of these crevice-dwelling animals in what is the coldest month of the year in Area 1 where the cats shot in that month were obtained. Most planigales came from Area 1, the exceptions being 12 individuals (6 male, 6 female) obtained in October 1995 from Area 2, and one female obtained in February 1996 from Area 3. Age class based on dentition was established for 101 planigales (6 juveniles, 14 subadults and 81 adults) (Fig. 2, data from specimens obtained in the same month in two consecutive years combined for the 12-month period from January to December). Adult animals, both male and female, were found in all months in which a sample was obtained, and subadults from January to August. The observation that the first upper incisor teeth were not fully erupted in the juveniles, found only in January, suggested that they might still be suckling. The sex of 104 specimens (61 male, 43 female) and the reproductive status (sexually immature or mature) of 99 specimens (60 male and 39 female) was established. Age class, sex and reproductive status could not all always be determined for each specimen (see Appendix 1).
|
|
Reproductive condition of males
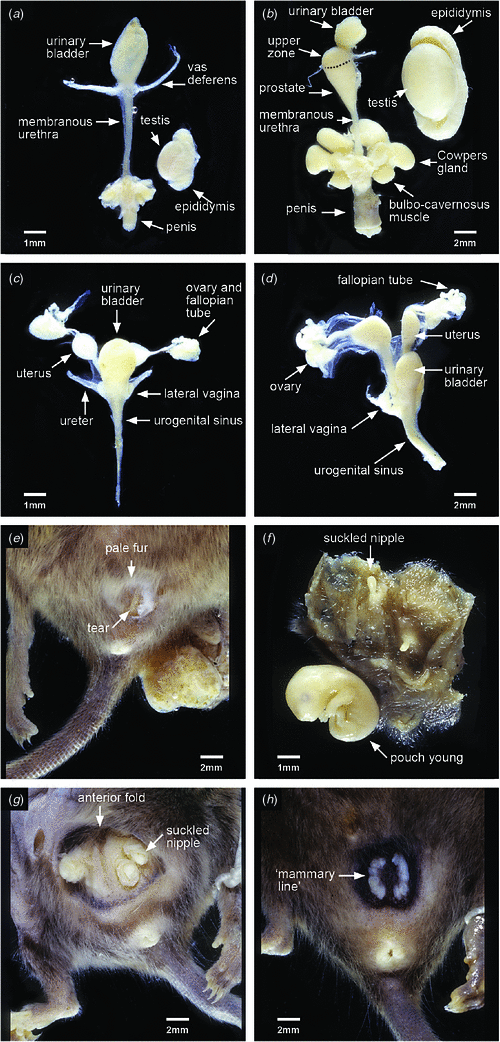
The reproductive status (immature or mature) of each individual was assessed using all available information. All juvenile and subadult males were sexually immature, as were some males with adult dentition. The relative sizes of the organs can be seen in Fig. 3a (juvenile, immature male) and Fig. 3b (adult, mature male).
|
The scrotum was intact in only 22 males and it ranged in width in immature males (juveniles, subadults and adults) from 2 to 6 mm and in mature males (adults) from 5.5 to 8 mm (Fig. 4a). Testis width (Fig. 4b) and length (Fig. 4c) were obtained for 32 individuals and it ranged from 0.5 × 1.0 mm to 3.5 × 5.2 mm (19 immature males) and from 2.5 × 4.0 mm to 4.0 × 5.5 mm (13 mature males). Prostate width (Fig. 4d) and length (Fig. 4e) was obtained for 20 individuals and it ranged from 1.0–2.5 mm (width) to 3.5–6.5 mm (length) in males obtained from January to April and from August to November. Zonation of the prostate was evident only in males taken from late August to November. No prostatic thickening of the membranous urethra was seen in another 33 males obtained from February to August (Fig. 4f). Epididymal smears were obtained from 46 individuals and spermatozoa were found in males taken in January and from late August to November (Fig. 4g). Males judged to be in breeding condition (presence of spermatozoa in the epididymis together with maximal development, including zonation, of the prostate) were found only from late August to November. Six mature males (prostate developed but lacking zonation, one with epididymal spermatozoa) taken in January–May were judged to be mature individuals no longer in breeding condition rather than males approaching maturity. Their maturity was supported by observations on other attributes (e.g. dimensions of testis, penis size and presence of sigmoid flexure, size of the bulbo-urethral glands). Sexually immature individuals (including juveniles, subadults and adults), in which development of the prostate had not begun, were found from January to August.
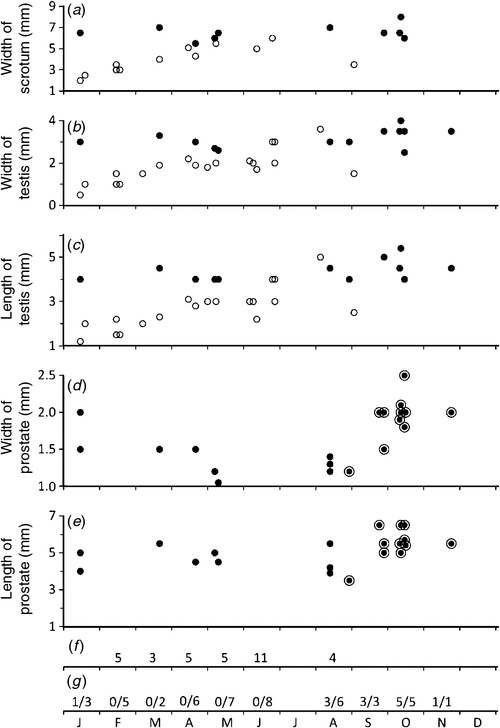
|
Reproductive condition of females
The relative sizes of the reproductive tract can be seen in Fig. 3c (juvenile, immature female) and Fig. 3d (adult, mature female). The pouch area of immature planigales is small and covered with pale fur (Fig. 3e) that conceals minute nipples surrounded by very lightly pigmented skin. In very early lactation the pouch area is enlarged, the skin granular in appearance and suckled nipples elongated (Fig. 3f). In late lactation, when young are no longer permanently attached to the nipples, a shallow antero-lateral skin fold may be present and the suckled nipples and associated mammary glands are enlarged (Fig. 3g). The skin peripheral to the mammary area is pigmented. After the young are weaned regression of the pouch area and nipples occurs and the pouch of mature females that have suckled young is characterised by deeply pigmented skin surrounding slightly enlarged, pale nipples on ‘mammary lines’ (Fig. 3h). One female collected in August 1996, assessed as mature on appearance of the pouch, was apparently breeding for at least the second time; enlargement of the lateral vaginae indicated that she was in oestrus but had not yet ovulated as no eggs were found in the uteri. The pouch condition of 36 of the 39 females whose reproductive status was established is shown by month of collection in Fig. 5. Immature females (juveniles and subadults) were present in the sample from January to August and lactating females with hairless pouch young in October. The crown–rump length (CRL) of young associated with three of these females, two obtained on 7 October and one on 10 October, ranged from 6 to 6.5 mm (see Fig. 3f for appearance of young at this size). Other lactating females were present from January to March. These females were at a late stage of lactation and it is possible that those in the January sample were suckling the juveniles found with them in cat stomachs.
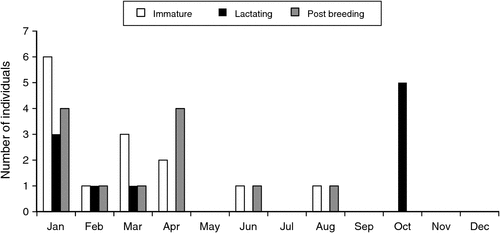
|
Live-trapped planigales
Eighteen males and three females were trapped in Elliott traps in Area 1 in 1995, 1996 and 1997 and four males and two females in Area 2 in 1992, 1994 and 1995. Scrotal width of males ranged from 4.5 mm for one collected in May and two collected in April to 7.5–9.0 mm for 19 collected in August–October. The pouch of a female captured in February was immature and another, in May, mature (post-breeding). Females with pouch young were trapped on 12 October (one with six young, CRL 10 mm) and two, one on 4 and one on 5 November (both with six young, CRL 7 mm).
Nipple number
Nipple number could not be determined for all females collected by cats; in some the pouch area was not intact and, in those that were lactating, enlarged mammary tissue made it difficult to see small, unsuckled nipples. Twelve nipples were found in each of 10 females from Area 1 and in the one from Area 3. All six females from Area 2 were lactating and enlarged mammary tissue, together with damage to the pouch area, precluded accurate assessment of nipple number but seven were seen in two individuals.
Number of pouch young
One of the Area 1 females with 12 nipples was a postbreeding individual in which nine of the 12 nipples were elongated, suggesting that nine young had been reared. Three live-trapped females, in which a count of the nipples could not be made, each had six pouch young.
Seasonality of breeding
On the basis of the presence of males in breeding condition, breeding appears to commence in late August and continue until at least November. Commencement of breeding in August is supported by the finding of one female in oestrus late in that month. The months in which females with young attached to the nipples gave birth have been estimated, by comparison of CRL measurements with those of known-age young of Planigale tenuirostris (Read 1985), to be mid-September for the one obtained on 12 October, late September for females obtained on 7 and 10 October, and mid-October for females obtained on 4 and 5 November. These females probably mated between early September and early October if the gestation period is of the order of 18.5–19.5 days, as given by Read (1984) for P. tenuirostris. Females in late lactation (young no longer permanently attached to the nipples) were found in the first three months of the year, when males were no longer in breeding condition. If the young become independent around 75–90 days, as found for laboratory-reared P. tenuirostris by Read (1984), the P. ingrami found in late lactation on 19 March can be estimated to have given birth in late December, and to have mated earlier in that month. Provided the duration of pregnancy and lactation, and growth rates of the young, are similar in P. ingrami and P. tenuirostris, the breeding season (season of mating) of P. ingrami appears to extend from August to December.
Discussion
Planigale ingrami has an extended breeding season similar in length to that of its arid-zone congeners, P. gilesi and P. tenuirostris. Read (1984) established that breeding (conception) occurs in the wild from the first week in August to the last week in December for P. gilesi and from the second week in August to the second week in January for P. tenuirostris, with a peak in births in September–early October for P. gilesi and September for P. tenuirostris. A few individuals of both species were found by Read to live for two breeding seasons. It seems that P. ingrami may also do so. One female in the P. ingrami sample, in oestrus in August at the start of the breeding season, had bred previously. In addition, the presence of sexually mature males, no longer in breeding condition during January–May, suggests that some may survive to breed in a second season. Read also found instances of females of each species producing a second litter during the breeding season. While it is not known if females of P. ingrami are polyoestrous they may, given that the length of the breeding season is comparable to that found for the species studied by Read, also attempt to breed twice in a season. The absence of juvenile and subadult animals in the sample during the breeding season suggests that the young do not mature until the following breeding season. These observations lend support to the similarity of the reproductive strategies of P. ingrami, P. tenuirostris and P. gilesi, all classed as Strategy V by Lee et al. (1982) and Krajewski et al. (2000).
The P. ingrami examined in this study have a Type 1 pouch (Woolley 1974), in which the nipples are exposed in immature and postbreeding females and an anterior skin fold is present when young are being suckled. The maximum extent to which the skin fold covers the young while they are attached to the nipples is not known. They have 12 nipples in the pouch; however, live-trapped females were never found with more than six pouch young or, in lactating females found in the cat stomachs, a maximum of nine elongated nipples, indicative of the number of young suckled. P. gilesi and P. tenuirostris also have 12 nipples and litter sizes of 3–10 and 4–9 respectively (Read 1984). Read’s description of the pouch morphology of these species suggests that they may have a Type 2 pouch (i.e. mammary area partially covered by a crescentic antero-lateral fold of skin (Woolley 1974)).
The genus Planigale was erected by Troughton (1928) for species of small dasyurids exhibiting marked flattening of the skull. They included Phascogale ingrami Thomas, 1906, Phascogale subtilissima Lönnberg, 1913, a new subspecies Planigale ingrami brunneus and a new species, Planigale tenuirostris. In the most recent revision of Planigale, Archer (1976) recognised five species (ingrami, tenuirostris, gilesi, maculata and novaeguineae) and he considered subtilissima Lönnberg, 1913 and brunneus Troughton, 1928 to be forms of Planigale ingrami. The form of the pouch and number of nipples cannot be determined for the nominate form, five specimens of which (including the male holotype and two females, 1906.3.9.78 and 1906.3.9.80 collected at Alexandria in the Northern Territory) are held in the Natural History Museum (London). From the description of the pouch given by Troughton (1928) for Planigale ingrami brunneus Woolley (1974) incorrectly assessed the pouch as Type 2. A recent examination by one of us (PAW) of the specimen, a lactating female (Australian Museum M2174) with one associated young collected at Wyangarie, Queensland (Fig. 1), established that the pouch conforms to the Type 1 description. Troughton (1928) recorded the nipple number as six, apparently having failed to see unsuckled nipples in the pouch of the adult specimen. Two minute and three elongated nipples were located on the right, and three minute and three elongated nipples on the left. The associated, lightly haired, female pouch young was found to have 12 nipples. The pouch of the subtilissima form of P. ingrami (referred to as Planigale subtilissima by Woolley 1974, following its inclusion within Planigale by Troughton) was found to differ from all other dasyurid marsupials for which information was available at the time. The nipples were located within two well defined, anteriorly directed pockets, with five nipples counted in each pocket and it was described (Woolley 1974) as a Type 4 pouch from two specimens now held in the Western Australian Museum (M11069 and M11070). One of the two was known to be suckling young when collected. Archer (1976: 352–353) commented ‘Pouch morphology may distinguish the subtilissima form from other forms (as suggested by Woolley 1974) although subtilissima form not unique in possession of accessory anterior pockets. Less well developed pockets occur in P. ingrami from Richmond and may occur in all forms but possibility at present cannot be checked’. It is clear from the present study that anterior pockets are not found in postbreeding specimens of the Planigale ingrami studied and thus specific distinction of the subtilissima form on this basis may be warranted. The identity of the Richmond specimen referred to by Archer (Queensland Museum JM824) requires further investigation, as does that of three particular specimens (Queensland Museum JM 1485–87) currently identified as P. maculata. Observations made on these animals in captivity at La Trobe University before lodgement in the Queensland Museum revealed that the pouches of two (JM 1485 and JM 1487) had anterior pockets but JM 1486 did not. The latter was assessed (by PAW) as not having bred (i.e. the pockets had not yet developed).
Blacket et al. (2000) have established the genetic similarity of the holotype of Planigale ingrami brunneus (Australian Museum M2174) with a specimen (Queensland Museum JM 18677) from Lyrian Station, which is in Area 2 where other specimens examined in the present study were obtained, providing support for their identification as P. ingrami. By using specimens that had been characterised genetically, Blacket et al. (2008) were able to find morphological characters that distinguished P. ingrami from other species of Planigale, which led to an extension of the range of P. ingrami into South Australia. They found that the arrangement of the nipples in South Australian specimens, which were most easily counted in postbreeding females in the museum specimens studied, was as in a Type 1 pouch, in agreement with the findings in the present study.
For pouch morphology to be used as a reliable diagnostic character in Planigale, it is essential for observations to be made on changes in the pouch throughout the breeding cycle, to avoid misinterpretation of pouch type which might arise in the course of examination of museum specimens such as the P. maculata (JM 1485–87) referred to above. In his species account, Fisher (2008) states that P. ingrami have ‘a well developed, rear opening pouch with eight to 10 teats’, in contrast to the findings of Blacket et al. (2000) and the present study, highlighting the need, first expressed by Archer (1976), for revisionary studies to investigate changes in pouch morphology as a function of reproductive condition. The report by Armstrong et al. (2011) of multiple cryptic taxa of Planigale (a five-fold increase across northern Australia) further highlights the need for revision of the genus.
Acknowledgements
We thank Heather Janetzki (Queensland Museum), Sandy Ingleby and Anja Divljan (Australian Museum) and Claire Stephenson (Western Australian Museum) for access to museum specimens. Anja Divljan assisted in establishing nipple number of M2174 and young. Paula Jenkins kindly examined the specimens in the Natural History Museum (London). We are indebted to Trevor Phillips for photography of reproductive organs, to Angie Haslem for preparation of Fig. 1, and Steve Leonard for Fig. 4. Cats were shot and live trapping carried out with approval from the La Trobe University Ethics Committee under permits LSB 95/2 and 97/4 and the Queensland Department of Environment and Heritage permit 922/97/SA.
References
Archer, M. (1976). Revision of the marsupial genus Planigale Troughton (Dasyuridae). Memoirs of the Queensland Museum 17, 341–365.Armstrong, K. N., Ford, F., Aplin, K. P., Donnellan, S. C., and Cooper, A. (2011). Using ancient DNA methods on informative museum specimens to realise the true mammalian diversity of Australia. Newsletter of the Australian Mammal Society. October 2011, 28.
Blacket, M. J., Adams, M., Krajewski, C., and Westerman, M. (2000). Genetic variation within the dasyurid marsupial genus Planigale. Australian Journal of Zoology 48, 443–459.
| Genetic variation within the dasyurid marsupial genus Planigale.Crossref | GoogleScholarGoogle Scholar |
Blacket, M. J., Kemper, C., and Brandle, R. (2008). Planigales (Marsupialia: Dasyuridae) of eastern Australia’s interior: a comparison of morphology, distributions and habitat preferences, with particular emphasis on South Australia. Australian Journal of Zoology 56, 195–205.
| Planigales (Marsupialia: Dasyuridae) of eastern Australia’s interior: a comparison of morphology, distributions and habitat preferences, with particular emphasis on South Australia.Crossref | GoogleScholarGoogle Scholar |
Dickman, C. R., Haythornthwaite, A. S., McNaught, G. H., Mahon, P. S., Tamayo, B., and Lentic, M. (2001). Population dynamics of three species of dasyurid marsupials in arid central Australia: a ten-year study. Wildlife Research 28, 493–506.
| Population dynamics of three species of dasyurid marsupials in arid central Australia: a ten-year study.Crossref | GoogleScholarGoogle Scholar |
Fisher, A. (2008). Long-tailed planigale, Planigale ingrami. In ‘The Mammals of Australia’. (Eds S. Van Dyck and R. Strahan.) pp. 110–111. (New Holland Publishers: Australia.)
Friend, G. R., Johnson, B. W., Mitchell, D. S., and Smith, G. T. (1997). Breeding, population dynamics and habitat relationships of Sminthopsis dolichura (Marsupialia: Dasyuridae) in semi-arid shrublands of Western Australia. Wildlife Research 24, 245–262.
| Breeding, population dynamics and habitat relationships of Sminthopsis dolichura (Marsupialia: Dasyuridae) in semi-arid shrublands of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Krajewski, C., Woolley, P. A., and Westerman, M. (2000). The evolution of reproductive strategies in dasyurid marsupials: implications of molecular phylogeny. Biological Journal of the Linnean Society. Linnean Society of London 71, 417–435.
| The evolution of reproductive strategies in dasyurid marsupials: implications of molecular phylogeny.Crossref | GoogleScholarGoogle Scholar |
Lee, A. K., Woolley, P., and Braithwaite, R. W. (1982). Life history strategies of dasyurid marsupials. In ‘Carnivorous Marsupials’. (Ed. M. Archer.) pp. 1–11. (Royal Zoological Society of New South Wales: Sydney.)
Mifsud, G., and Woolley, P. A. (2012). Predation of the Julia Creek dunnart (Sminthopsis douglasi) and other native fauna by cats and foxes on Mitchell grass downs in Queensland. Australian Mammalogy 34, 188–195.
| Predation of the Julia Creek dunnart (Sminthopsis douglasi) and other native fauna by cats and foxes on Mitchell grass downs in Queensland.Crossref | GoogleScholarGoogle Scholar |
Morton, S. R. (1978). An ecological study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) III. Reproduction and life history. Australian Wildlife Research 5, 183–211.
| An ecological study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) III. Reproduction and life history.Crossref | GoogleScholarGoogle Scholar |
Read, D. G. (1984). Reproduction and breeding season of Planigale gilesi and P. tenuirostris (Marsupialia: Dasyuridae). Australian Mammalogy 7, 161–173.
Read, D. G. (1985). Development and growth of Planigale tenuirostris (Marsupialia: Dasyuridae) in the laboratory. Australian Mammalogy 8, 69–78.
Troughton, E. Le G. (1928). A new genus, species, and subspecies of marsupial mice (Family Dasyuridae). Records of the Australian Museum 16, 281–288.
| A new genus, species, and subspecies of marsupial mice (Family Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Woolley, P. (1966). Reproduction in Antechinus spp. and other dasyurid marsupials. Symposia of the Zoological Society of London 15, 281–294.
Woolley, P. (1974). The pouch of Planigale subtilissima and other dasyurid marsupials. Journal of the Royal Society of Western Australia 57, 11–15.
Woolley, P. A. (1990a). Reproduction in Sminthopsis macroura (Marsupialia: Dasyuridae). I. The female. Australian Journal of Zoology 38, 187–205.
| Reproduction in Sminthopsis macroura (Marsupialia: Dasyuridae). I. The female.Crossref | GoogleScholarGoogle Scholar |
Woolley, P. A. (1990b). Reproduction in Sminthopsis macroura (Marsupialia: Dasyuridae). II. The male. Australian Journal of Zoology 38, 207–217.
| Reproduction in Sminthopsis macroura (Marsupialia: Dasyuridae). II. The male.Crossref | GoogleScholarGoogle Scholar |
Woolley, P. A. (1991). Reproduction in Dasykaluta rosamondae (Marsupialia: Dasyuridae): field and laboratory observations. Australian Journal of Zoology 39, 549–568.
| Reproduction in Dasykaluta rosamondae (Marsupialia: Dasyuridae): field and laboratory observations.Crossref | GoogleScholarGoogle Scholar |
Woolley, P. A. (1992). New records of the Julia Creek Dunnart, Sminthopsis douglasi (Marsupialia: Dasyuridae). Wildlife Research 19, 779–783.
| New records of the Julia Creek Dunnart, Sminthopsis douglasi (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |